Establishing the Minimal Bactericidal Concentration of an Antimicrobial Agent for Planktonic Cells (MBC-P) and Biofilm Cells (MBC-B)
Summary
This protocol allows for a direct comparison between planktonic and biofilm resistance for a bacterial strain that can form a biofilm in vitro using a 96-well microtiter plate. Planktonic or biofilm bacteria are exposed to serial dilutions of the antimicrobial agent of choice. Viability is assayed by growth on agar plates.
Abstract
This protocol allows for a direct comparison between planktonic and biofilm resistance for a bacterial strain that can form a biofilm in vitro. Bacteria are inoculated into the wells of a 96-well microtiter plate. In the case of the planktonic assay, serial dilutions of the antimicrobial agent of choice are added to the bacterial suspensions. In the biofilm assay, once inoculated, the bacteria are left to form a biofilm over a set period of time. Unattached cells are removed from the wells, the media is replenished and serial dilutions of the antimicrobial agent of choice are added. After exposure to the antimicrobial agent, the planktonic cells are assayed for growth. For the biofilm assay, the media is refreshed with fresh media lacking the antimicrobial agent and the biofilm cells are left to recover. Biofilm cell viability is assayed after the recovery period. The MBC-P for the antimicrobial agent is defined as the lowest concentration of drug that kills the cells in the planktonic culture. In contrast, the MBC-B for a strain is determined by exposing preformed biofilms to increasing concentrations of antimicrobial agent for 24 hr. The MBC-B is defined as the lowest concentration of antimicrobial agent that kills the cells in the biofilm.
Introduction
Antibiotic resistance assays were initially developed to assay resistance of planktonic (free-swimming) cultures of bacteria. Since many bacterial infections involve biofilms (surface-attached cells), we were interested in developing a method to assay biofilm-specific antibiotic resistance. However, most antibiotic resistance assays are poorly suited for measuring the resistance of biofilms. For example, determining the minimal inhibitory concentration (MIC) is the gold standard for determining antibiotic resistance of planktonic bacterial cultures 1. This assay entails mixing a diluted planktonic culture with a dilution series of antibiotic. The concentration of antibiotic that inhibits the visible growth of the planktonic cells is the MIC. Since this assay relies on inhibition of growth, by definition, it cannot work with biofilm cultures, which requires examining the antibiotic sensitivity of cells in a pregrown biofilm. Instead of measuring growth inhibition, the MBC-B assay described here determines the concentration of antibiotic that kills cells already existing in a biofilm. Thus, this assay aims to mimic antibiotic treatments of established biofilm infections, and provide a more relevant view of the bacterial antibiotic resistance in vivo.
Since biofilms are generally more antibiotic resistant than planktonic cultures 2-4 , it was necessary to devise a method that directly relates the antibiotic resistance of a biofilm to that of a planktonic culture. Thus another goal of this method is to be able to directly compare the level of antibiotic resistance between planktonic and biofilm cells. The MBC-P and MBC-B assays described here make this possible because cells are cultured under similar conditions. We have utilized this method to study several genes that are important for biofilm-specific antibiotic resistance 5-8 .
Protocol
1. MBC-B
- Growing a biofilm (adapted from O'Toole9).
- Grow a culture of the wild-type strain of interest and mutant strain for 16 hr in a rich medium at 37 °C.
- Dilute the saturated overnight cultures 1:100 into fresh medium for antibiotic resistance assays. A standard medium for P. aeruginosa is M63 minimal medium supplemented with magnesium sulfate and arginine (see Table 1). This medium stimulates the formation of a more robust biofilm.
- Add 100 μl of the dilution per well in a 96-well microtiter dish (see Table 1). Since these assays are typically performed in triplicate for each strain, there should be 24 wells of each strain.
- Incubate the microtiter plate for 24 hr at 37 °C.
- Exposing the preformed biofilm to a concentration gradient of antibiotic
- Prepare a 10x dilution series of antibiotic for 7 wells. Example: for the antibiotic tobramycin, the final concentrations in the wells are 0.4, 0.2, 0.1, 0.05, 0.025, 0.0125, and 0.006 mg/ml 5-8 . From a stock of 25 mg/ml, prepare 10x dilutions of 4, 2, 1, 0.5, 0.25, 0.125, and 0.06 mg/ml. Leave on ice.
- Remove the spent supernatant (containing planktonic cells) using a multichannel pipette (see Table 1).
- Add 90 μl M63 (Mg/Arg) to all of the wells.
- Add 10 μl of each 10x antibiotic concentration in order to achieve the desired final concentrations. Add 10 μl water (no antibiotic control) to the final well for each replicate and strain.
- Incubate the microtiter plate for 24 hr at 37 °C.
- Refreshing the media and allowing for the detachment of live cells.
- Remove the spent supernatant (containing planktonic cells) using a multichannel pipette.
- Add 115 μl M63 (Mg/Arg) to all of the wells.
- Incubate the microtiter plate for 24 hr at 37 °C.
- Assay for live cells.
- Label two LB agar plates per 96-well microtiter plate.
- Sterilize the multiprong device (see Table 1) by dipping the prongs in 100% ethanol and passing the prongs across the open flame of a Bunsen burner. Repeat. Let the prongs cool slightly. Using the multiprong device, transfer ~3 μl (amount that is typically retained on the tips of the prongs) of planktonic culture from each well of the microtiter plate to the surface of an LB agar plate.
- Incubate the LB agar plates for 16 hr at 37 °C.
- Determine minimal bactericidal concentration of the antibiotic by identifying, by eye, the cut-off for bacterial growth (Figure 1).
2. MBC-P
- Preparing bacterial strains
- Grow a culture of the wild-type strain of interest and mutant strain for 16 hr in a rich medium at 37 °C.
- Dilute the saturated overnight cultures 1:100 into fresh medium for antibiotic resistance assays. A standard medium for P. aeruginosa is M63 minimal medium supplemented with magnesium sulfate and arginine (see Table 1).
- Add 90 μl of the dilution per well in a 96-well microtiter dish (see Table 1). Since these assays are typically performed in triplicate for each strain, there should be 24 wells of each strain.
- Exposing planktonic cells to a concentration gradient of antibiotic
- Prepare 10x dilution series of antibiotic for 7 wells. Example: for the antibiotic tobramycin, the final concentrations in the wells are 0.032, 0.016, 0.008, 0.004, 0.002, 0.001, and 0.0005 mg/ml. From a stock of 25 mg/ml, prepare dilutions of 0.32, 0.16, 0.08, 0.04, 0.02, 0.01, and 0.005 mg/ml. Leave on ice.
- Add 10 μl of each 10x antibiotic concentration in order to achieve the desired final concentrations. Add 10 μl water (no antibiotic control) to the final well for each replicate and strain.
- Incubate the microtiter plate for 24 hr at 37 °C.
- Assay for live cells.
- Label two LB agar plates per 96-well microtiter plate.
- Sterilize the multiprong device (see Table 1) by dipping the prongs in 100% ethanol and passing the prongs across the open flame of a Bunsen burner. Repeat. Let the prongs cool slightly. Using the multiprong device, transfer ~3 μl (amount that is typically retained on the tips of the prongs) of planktonic culture from each well of the microtiter plate to the surface of an LB agar plate.
- Incubate the LB agar plates for 16 hr at 37 °C.
- Determine minimal bactericidal concentration of the antibiotic by identifying, by eye, the cut off for bacterial growth (Figure 2).
Representative Results
MBC-P and MBC-B assays were carried out, comparing the sensitivity of PA14 wild type with PA14 ∆ndvB. Tobramycin was used as the antibiotic. Results corresponding to step 1.4.4 (Figure 1) and step 2.3.4 (Figure 2) are presented. PA14 and ∆ndvB were inoculated into the MBC-P and MBC-B assays in triplicate. After completing steps 1.0-1.4 of the MBC-B protocol and steps 2.0-2.3 of the MBC-P protocol, the viable cells were plated onto an LB agar plate. Concentrations of tobramycin (μg/ml) are indicated to the left of the cells. The MBC-B for PA14 is 100 μg/ml and the MBC-B for ∆ndvB is 12.5 μg/ml. The MBC-P for both PA14 and the ∆ndvB mutant is 8 μg/ml.
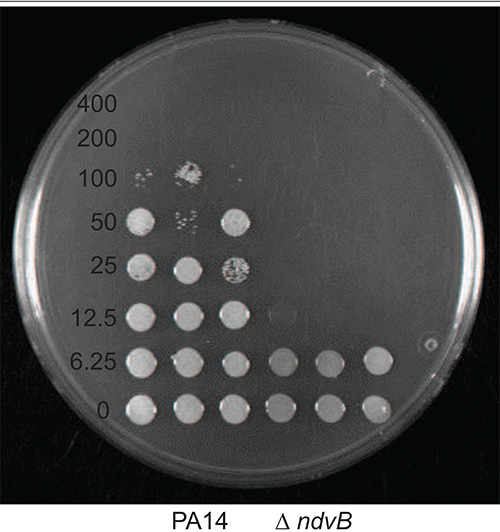
Figure 1. Representative results from an MBC-B assay with PA14 wild type vs. PA14 ∆ndvB and tobramycin. Values refer to the final concentration of tobramycin (μg/ml).

Figure 2. Representative results from an MBC-P assay with PA14 wild type vs. PA14 ∆ndvB and tobramycin. Values refer to the final concentration of tobramycin (μg/ml).
Discussion
Antibiotic resistance in planktonic cells is defined as an increase in the minimum inhibitory concentration (MIC) of an antibiotic due to a permanent change in the cells (e.g. a mutation). The mechanisms of biofilm-specific resistance or tolerance that have been identified to date are the result of the expression of wild type genes within biofilms. Thus, the classical definition of resistance does not apply to biofilms. However, another set of definitions has been presented: resistance mechanisms prevent the antibiotic from accessing its target, while tolerance mechanisms shut down the targets of antibiotics10. Biofilm-specific antibiotic resistance mechanisms encompass both of these definitions. The term “resistance" has been used throughout this protocol, but further experimentation must be carried out in order to determine if a gene is contributing to resistance or tolerance.
The MBC-P and MBC-B assays may be adapted for any biofilm-forming bacterial strain and any bactericidal antimicrobial agent. The two important criteria for adapting this assay to other bacteria and other antimicrobial agents are determining conditions that lead to biofilm formation and determining the appropriate range of concentrations for the antimicrobial agent using the wild type strain of interest. Proper biofilm-formation conditions can be determined using a JoVE biofilm formation protocol published by George O'Toole9. In order to determine an appropriate concentration range for the MBC-B assay, a good rule of thumb is to use 25 x MIC (minimal inhibitory concentration11) as the middle concentration of the range and adjust accordingly. For the MBC-P assay, use 1/10 of the values identified for the MBC-B assay. The goal is to define a range of concentrations that will allow for a clear difference between the MBC-B of the wild-type strain and a sensitive mutant strain. Thus, the top end of the range should be close to the MBC-B of the wild-type strain. We repeat this experiment at least three times, giving a minimum of 9 data points per strain.
The most critical step of these assays is to avoid contamination. To prevent contamination between strains, an empty column should be left between strains. Another critical step is to allow enough time for the metal prongs on the multiprong device to cool enough. This can be tested by flaming the prongs, timing cooling period and quickly touching the prongs with one's hands.
If detachment of the cells from the biofilm after exposure to antibiotics is an issue, the MBC-B method can be modified to include a sonication step. Skip step 1.3.3 (24 hr incubation after addition of fresh media). Instead, cover the wells of the 96-well microtiter plate after adding 115 μl of fresh media (step 1.3.2) with sterile adhesive plate seal and sonicate plate to dislodge biofilm cells from the walls of the wells. Use a water bath sonicator. Specific conditions (time, intensity) for the efficient removal of biofilm cells from the plastic must be determine beforehand.
A variation of the MBC-B was used to screen a library of 4,000 transposon-insertion mutants of P. aeruginosa for biofilm-specific antibiotic resistance mutants. A concentration of tobramycin (50 μg/ml) was chosen that would not kill wild type biofilm cells but would allow for the identification of mutants that were 3-fold more sensitive than the wild type biofilm. Instead of inoculating columns of wells with one specific strain of bacteria, each well was inoculated with a different mutant. The mutants were left to form a biofilm, exposed to 50 μg/ml tobramycin, left to recover from tobramycin exposure and then assayed for viability. Mutants that did not survive 50 μg/ml tobramycin exposure were retested under the same screening conditions. The MBC-P and MBC-B protocols were utilized to confirm the tobramycin-sensitive phenotypes of the mutants.
There are other methods to assay antibiotic resistance in biofilms, i.e. colony biofilms and biofilms grown in bioreactors12,13. These methods are considerably more laborious and limited in the number of strains that can easily be assayed. Thus, an advantage that the MBC-B has over these other methods is that it is high-throughput, allowing the researcher to conduct screens or to assay several strains, as well as several different antibiotic concentrations at one time.
| Name of the reagent | Company | Catalogue number | Comments (optional) |
| 1x M63 | Prepare as a 5x M63 stock by dissolving 15 g KH2PO4, 35 g K2HPO4 and 10 g (NH4)2SO4 in 1 L of water. This stock does not need to be autoclaved and can be stored at room temperature. Dilute 5x stock 1:5, autoclave, cool, then add the desired components. | ||
| KH2PO4 | Fisher | P285-500 | |
| K2HPO4 | Fisher | P288-500 | |
| (NH4)2SO4 | Sigma | A5132 | |
| Magnesium sulfate | Fisher | M63-500 | Add to 1 mM final concentration. Prepare as a 1 M stock in water and autoclave. |
| Tobramycin | Sigma | Prepare 50 mg/ml stock. Aliquot and store at -20 °C. | |
| Arginine | Sigma | A5131 | Add to 0.4% final concentration. Prepare as a 20% stock in water and filter sterilize. This alternative carbon/energy source can replace glucose and casamino acids |
| 96-well microtiter plates | Corning | 3595 | Sterile, flat-bottom, low evaporation |
| Tranferpette (multichannel pipette) | BrandTech | 2703610 | 8-channel, 20-200 μl |
| Multiprong device | Dan-Kar | MC48 | 48 prongs fit into ½ of a 96-well microtiter plate |
Table 1.
Declarações
The authors have nothing to disclose.
Acknowledgements
The author would like to thank Li Zhang, Xian-Zhi Li, Aaron Hinz, and Clayton Hall for editorial help with this manuscript. This assay was initially developed in the lab of George O’Toole, Geisel School of Medicine at Dartmouth. Research in Dr. Mah’s lab is supported by grants from the Natural Sciences and Engineering Research Council of Canada and Cystic Fibrosis Canada.
Materials
|
1x M63 |
Prepare as a 5x M63 stock by dissolving 15g KH2PO4, 35g K2HPO4 and 10g (NH4)2SO4 in 1 L of water. This stock does not need to be autoclaved and can be stored at room temperature. Dilute 5x stock 1:5, autoclave, cool, then add the desired components. |
||
|
KH2PO4 |
Fisher |
P285-500 |
|
|
K2HPO4 |
Fisher |
P288-500 |
|
|
(NH4)2SO4 |
Sigma |
A5132 |
|
|
Magnesium sulfate |
Fisher |
M63-500 |
Add to 1 mM final concentration. Prepare as a 1 M stock in water and autoclave. |
|
Tobramycin |
Sigma |
Prepare 50 mg/m stock. Aliquot and store at –20°C. |
|
|
Arginine |
Sigma |
A5131 |
Add to 0.4% final concentration. Prepare as a 20% stock in water and filter sterilize. This alternative carbon/energy source can replace glucose and casamino acids |
|
96-well microtiter plates |
Corning |
3595 |
Sterile, flat-bottom, low evaporation |
|
Tranferpette (multichannel pipette) |
BrandTech |
2703610 |
8-channel, 20-200 μl |
|
Multiprong device |
Dan-Kar |
MC48 |
48 prongs fit into ½ of a 96-well microtiter plate |
Referências
- Hoiby, N., Bjarnsholt, T., Givskov, M., Molin, S., Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 35 (4), 322-332 (2010).
- Mah, T. F., O’Toole, G. A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9 (1), 34-39 (2001).
- Mah, T. F. Biofilm-specific antibiotic resistance. Future Microbiol. 7 (9), 1061-1072 (2012).
- Mah, T. F., Pitts, B., Pellock, B., Walker, G. C., Stewart, P. S., O’Toole, G. A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 426 (6964), 306-310 (2003).
- Zhang, L., Mah, T. F. The Involvement of a Novel Efflux System in Biofilm-Specific Resistance to Antibiotics. J. Bacteriol. 190 (13), 4447-4452 (2008).
- Zhang, L., Hinz, A. J., Nadeau, J. P., Mah, T. F. Pseudomonas aeruginosa tssC1 Links Type VI Secretion and Biofilm-specific Antibiotic Resistance. J. Bacteriol. 193 (19), 5510-5513 (2011).
- Beaudoin, T., Zhang, L., Hinz, A. J., Parr, C. J., Mah, T. F. The Biofilm-Specific Antibiotic Resistance Gene, ndvB, is Important for Expression of Ethanol Oxidation Genes in Pseudomonas aeruginosa Biofilms. J. Bacteriol. 194 (12), 3128-3136 (2012).
- O’Toole, G. A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. (47), e2437 (2011).
- Lewis, K. Multidrug tolerance of biofilms and persister cells. Curr. Top. Microbiol. Immunol. 322, 107-131 (2008).
- Merritt, J. H., Kadouri, D. E., O’Toole, G. A. Growing and Analyzing Static Biofilms. Curr. Protoc. Microbiol. 1, 1B.1.1-1B.1.17 (2005).
- Ramey, B. E., Parsek, M. R. Chapter 1. Growing and analyzing biofilms in fermenters. Curr. Protoc. Microbiol. 1, 1B.3.1-1B.3.14 (2005).

