Dietary Supplementation of Polyunsaturated Fatty Acids in Caenorhabditis elegans
PREPARAÇÃO DO INSTRUTOR
CONCEITOS
PROTOCOLO DO ALUNO
Polyunsaturated fatty acids are sensitive to heat, light and oxygen. Therefore, care must be taken when preparing fatty acid supplementation plates such that fatty acids are not exposed to excess heat and light. NGM media containing 0.1% Tergitol (NP-40) is autoclaved and partially cooled, after which fatty acid sodium salts are added with constant stirring. The plates are allowed to dry in the dark. Uptake of fatty acids by C. elegans cultured on these plates can then be monitored by gas chromatography.
1. Preparation of Fatty Acid Supplemented Media
- Measure out media components into an appropriately sized flask. Per 1 L, add 17 g Bacto-agar, 2.5 g tryptone, 3 g NaCl, 1 ml 5 mg/ml cholesterol dissolved in ethanol, and 10 ml 10% Tergitol dissolved in water.
- Add 80% of final desired volume of Millipore water and autoclave the media along with empty glass bottles (equal to the number of different fatty acid concentrations to be tested), as well as appropriate graduated cylinders.
- Cool media in a water bath set to 55 °C. While the media is cooling prepare the working stock solution of the fatty acid sodium salt by breaking open the glass vial, using safety precautions and ensuring glass particles do not mix in with the fatty acids. Weigh out enough fatty acid to make a 100 mM working stock.
- Bring the fatty acid solution to a final concentration of 100 mM with purified water. Fully dissolving the fatty acid sodium salt typically takes about 20-30 min. Purge the working stock of fatty acid with argon or nitrogen, because contact with an inert gas prevents oxidation. Cap the vial and store the working stock in the dark until media has cooled.
- (Optional) If RNAi media is desired, measure ampicillin and Isopropyl β-D-1-thiogalactopyranoside (IPTG) solutions here.
- When the agar has cooled to 55 °C add per 1 L: 1 ml of 1 M MgSO4, 1 ml of 1 M CaCl2, and 25 ml of phosphate buffer (108.3 g KH2PO4 and 35.6 g K2HPO4 per 1 L autoclaved). Add the filter sterilized ampicillin and IPTG solutions if making RNAi plates. For all types of plates, add sterile water to bring the final media volume to 1 L.
- Near a flame, transfer media to an autoclaved and appropriately sized graduated cylinder and then add warm sterile water to desired volume. Transfer media back to initial flask and mix by stirring.
- Near a flame, transfer media into a number of bottles equal to the number of concentrations to be tested by measuring with an autoclaved and appropriately sized graduated cylinder. Maintain the aliquoted media as liquid using a water bath until the fatty acid working stock has fully dissolved.
- Place one bottle on a stir plate and stir until the media is warm to the touch, but not hot. Make sure to leave yourself enough time to stir in the fatty acid stock solution and pour plates before the media begins to solidify. If stir plate has temperature control, set it to 55 °C.
- Dilute the 100 mM fatty acid working stock into the media for the final concentration desired. Stir media until the white precipitate is in solution (approximately 1 min). Media may still be slightly cloudy afterwards.
- (Optional) If RNAi media is desired add ampicillin to 0.1 mg/ml and IPTG to 2 mM final concentration.
- Pour media using an automated pipette aid and a sterile 25 ml pipette, adding 8 ml of media per 60 mm plate or 25 ml of media per 100 mm plate. Repeat fatty acid addition and plate pouring steps for the remaining bottles.
- After the agar has solidified, cover the fatty acid-supplemented plates with a box or store at room temperature in a well-vented drawer to protect from light oxidation.
- Seed E. coli OP50 onto plates two days after plates have dried. For 60 mm plates, pipette 300 µl of an overnight OP50 culture (grown at 37 °C). If RNAi plates were poured, seed with 300 µl of RNAi bacteria after the plates have dried for four days. Plate drying time may need to be adjusted due to differences in humidity in various lab environments.
- Incubate the plates in a dark environment at room temperature while the bacterial lawn is drying.
2. Inducing Germ Cell Destruction by Supplementation of DGLA
- C. elegans grown on plates containing 0.3 mM DGLA (20:3n-6) become sterile due to the destruction of germ cells in both larval stage and adult nematodes.
- Prepare a synchronized population of L1 larvae by treating gravid hermaphrodites with alkaline hypochlorite solution(for a 10 ml solution: 0.5 ml of 5M NaOH, 2.5 ml of household bleach, and 7 ml H2O). Gently rock the gravid hermaphrodites in this solution until the adult worms dissolve. Eggs will be preserved, and can be pelleted by low speed centrifugation.
- Wash egg preparation 3 times in M9 buffer (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 ml 1 M MgSO4, H2O to 1 L. Add MgSO4 after autoclaving). To provide enough oxygen, resuspend the eggs in a 15 ml plastic tube to a final volume of 5 ml of M9 buffer and rock on a shaker overnight.
- L1 larvae should be added to the supplemented plates two days after seeding with OP50, or one day after plating the RNAi bacteria. Incubate worms at 20 °C for three days or until they reach the adult stage.
- Adult worms can be scored for sterility using a dissecting microscope with high enough magnification to visualize embryos developing in the uterus. Successful germ cell loss will appear as a clear uterus void of eggs.
- Worms can also be scored by fixing and staining with the nucleic acid dye diamindinophenylindole (DAPI), which facilitates the visualization of nuclei in germ cells and developing embryos. A rapid DAPI stain is achieved by picking worms into a drop of M9 buffer on a watchglass9.
- Flood watch-glass with 1 ml of a 0.2 ng/m. DAPI in 95% ethanol solution, let sit for approximately 5 min.
- Pick worms into a drop of VectaShield on a slide, and then cover with a coverslip.
- Seal coverslip and store at 4 °C in the dark overnight for optimum staining, however slides can also be viewed immediately. Score for presence or absence of germ cell nuclei using a fluorescence microscope equipped with a UV lamp and filter.
3. Confirming Fatty Acid Uptake by Gas Chromatography
Overall fatty acid composition of C. elegans can be determined by producing fatty acid methyl esters (FAMEs) which are then separated and quantified using gas chromatography4.
- Collect 500-1,000 adult worms by washing them off of the plates with water and transferring to a silanized 13 mm x 100 mm glass screw top tube.
- Let worms settle by gravity, and then remove as much water as possible with a glass Pasteur pipette.
- Wash worms once with water, and then again remove as much water as possible.
- FAMEs are formed by adding 1 ml of 2.5 % H2SO4 in methanol, and then heating at 70 °C for 1 hr in a water bath.
- Remove the tubes from the water bath and cool for 1 min.
- Extract FAMEs by adding 1.5 ml water and 0.25 ml of hexane.
- Recap tubes and shake vigorously.
- Centrifuge tubes in a tabletop clinical centrifuge for 1 min at maximum speed to separate hexane from aqueous solvent.
- Transfer hexane (top layer) to a GC vial insert within a GC vial, being careful not to transfer any of the aqueous phase. For GC analysis, 1-2 μl of FAMEs in hexane is injected onto a polar capillary gas chromatography column suitable for FAMEs analysis. The Agilent 7890 GC injector is set at 250 °C, with a flow rate of 1.4 ml/min, and the GC oven is programmed for an initial temperature of 130 °C, which is held for 1 min. Subsequently, the temperature is ramped 10 °C/min until 190 °C, and then ramped again at 5 °C/min until 210 °C and held for an additional 1 min.
- To ensure that uptake of DGLA has occurred, analyze FAMEs by flame ionization detection (FID) or mass spectrometry (MS) detection, using authentic standards for the identification of the C. elegans fatty acids.
Dietary Supplementation of Polyunsaturated Fatty Acids in Caenorhabditis elegans
Learning Objectives
Supplementation of the C. elegans diet is limited by the ability of the bacterial food source to uptake and incorporate fatty acid into the bacterial membrane. To determine the ability of E. coli OP50 to assimilate various fatty acids into its membranes, OP50 was plated onto media with no supplement, 0.1 mM and 0.3 mM concentrations of stearic acid (18:0), sodium oleate (18:1n-9), and sodium DGLA (20:3n-6). Plates were dried at room temperature for 2 days in the dark, and incubated at 20 °C for 3 days. Bacterial lawns were collected by gently scraping the lawn into water with a flame-sterilized spatula. Bacteria were pelleted by centrifugation, and treated with 2.5% H2SO4 in methanol to produce fatty acid methyl esters, which were analyzed by GC/MS following the methods listed in the Procedure, step 3. The results demonstrate that unsaturated fatty acids (oleate and DGLA) incorporate into OP50 in higher amounts than the saturated fatty acid stearic acid (Figure 1A).
Additionally, L1 stage N2 larvae were grown on the same batch of supplemented plates and harvested after three days growth at 20 °C. Worms were washed off of the plates and fatty acids in total worm preps were analyzed by GC/MS. The change in supplemented fatty acids is graphed in Figure 1B. These studies demonstrate that supplementation of saturated fatty acids does not change the relative amount of saturated fatty acids in worm tissues, while supplementation of unsaturated fatty acids increased the relative amounts of unsaturated fatty acids in C. elegans lipids. Taken together, the data shown in Figure 1A and Figure 1B demonstrate that the relative accumulation of supplemented fatty acids in C. elegans correlates directly with the relative accumulation of fatty acids in the dietary E. coli.
We have previously shown that dietary DGLA causes sterility in C. elegans10. Figure 2 illustrates the dose response of DGLA induction of sterility in C. elegans. The concentration of DGLA in worm lipids in which 50% of the population will be sterile is approximately 12%. Interestingly, the response to DGLA can be altered by genetic mutations in C. elegans. A recent finding is that the insulin growth factor-dependent stress pathways can suppress the DGLA-induced germ cell destruction8. Supplementing the diet of worms containing deleterious mutations in either the daf-2 insulin/IGF receptor, daf-2(e1370), or the daf-16/FOXO transcription factor, daf-16(mu86), illustrates the usefulness of this method to unravel genetic pathways that influence the physiological effects of dietary fats. Synchronized L1 larvae were pipetted onto DGLA supplemented media. After 3-4 days of growth, worms were scored for sterility, as determined by the absence of eggs in the uterus of adult worms. DGLA supplemented daf-2(e1370)mutants were fertile, with little to no induced germ cell loss compared to wild type (N2) worms at both 0.15 mM and 0.3 mM supplementations (Figure 3).In contrast, DGLA supplemented worms with inactive FOXO (daf-16(mu86)) displayed a higher percentage of sterile worms compared to wild type when fed on plates containing 0.15 mM DGLA (Figure 3).
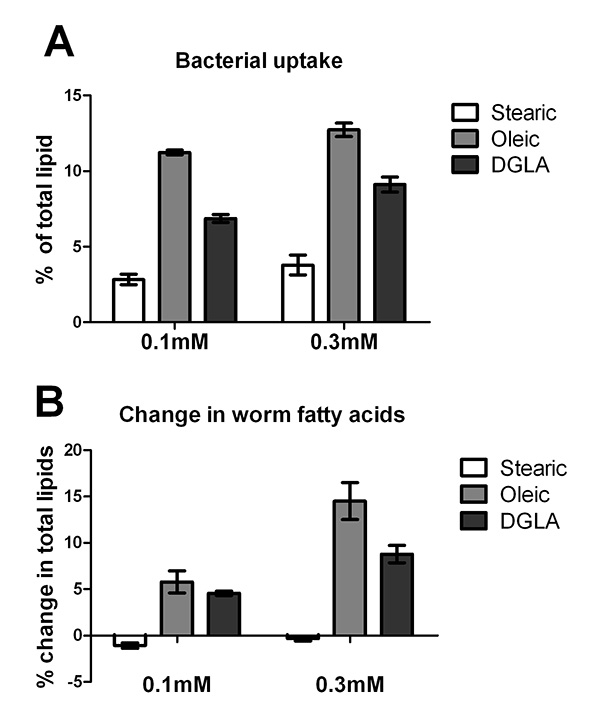
Figure 1. Uptake and incorporation of supplemented fatty acids by E. coli OP50 and C. elegans. A. E. coli OP50 was grown on plates containing 0.1 mM or 0.3 mM stearic acid, sodium oleate, or sodium DGLA as well as un-supplemented plates. After five days of growth on plates at 20 °C, E. coli were harvested and fatty acid methyl esters were generated for analysis by GC/MS. Because OP50 does not produce oleic acid or DGLA, and produces only trace amounts of stearic acid, the percentage of each supplemented fatty acid in the E. coli lipids reveals the ability of OP50 to incorporate the supplemented fatty acid. Error bars are SD. B. Change in C. elegans fatty acids in young adults grown for three days, starting at L1 stage, on E. coli plates containing 0.1 mM or 0.3 mM stearic acid, sodium oleate, or DGLA. The values for change in stearic acid and DGLA were obtained by subtracting the relative amount of 18:0 or 20:3 in worms grown on supplemented plates from those of worms grown on unsupplmented plates. To monitor uptake of oleic acid, the sum of oleic acid plus downstream C20 PUFAs (20:3, 20:4n-6, 20:4n-3, and 20:5) were calculated in supplemented and unsupplemented plates, because incorporated oleic acid is further desaturated and elongated. Error bars are SD. Click here to view larger image.
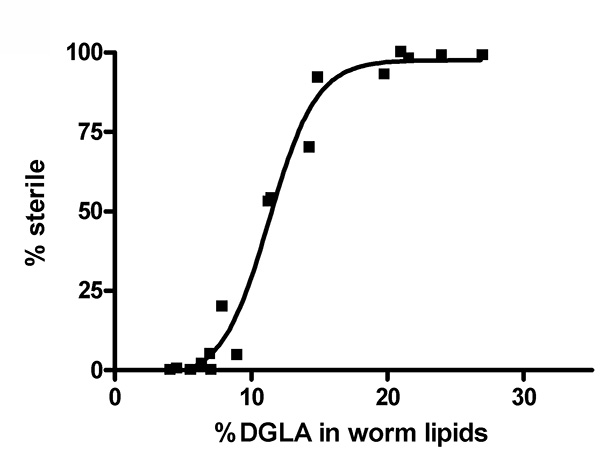
Figure 2. Increasing concentrations of DGLA in worm lipids correlate with increasing sterility in C. elegans. Wild type (N2) worms were treated with various concentrations of DGLA. The % DGLA in total worm lipids and the % of the population that is sterile is plotted for is plotted for 17 data points from five independent feeding experiments using dietary DGLA concentrations ranging from 0-0.3 mM DGLA. Click here to view larger image.
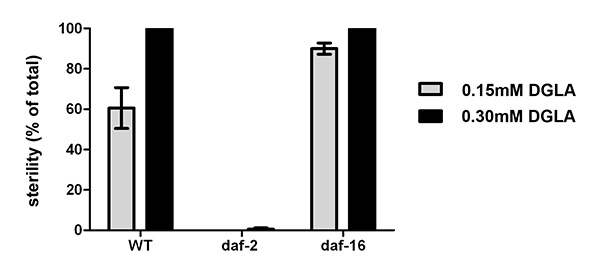
Figure 3. Physiological effects of supplementing C. elegans with DGLA. Starved L1 larval wild type, daf-2(e1370), or daf-16(mu86) were plated onto un-supplemented, 0.15 mM or 0.3 mM DGLA supplemented media and grown to the adult stage. At least 150 individual worms were then scored for sterility. Thedaf-2(e1370) mutants were almost completely fertile, even at 0.3 mM DGLA, while thedaf-16(mu86) mutants display an increased number of sterile worms compared to wild type at 0.15 mM DGLA. Error bars are SEM. Click here to view larger image.
List of Materials
| Bacto-Agar | Difco | 214010 | |
| Tryptone | Difco | 211705 | |
| NaCl | J.T. Baker | 3624-05 | |
| Tergitol | Sigma | NP40S-500mL | |
| Cholesterol | Sigma | C8667-25G | (5 mg/mL in ethanol) |
| MgSO4 | J.T. Baker | 2504-01 | |
| CaCl2 | J.T. Baker | 1311-01 | |
| K2HPO4 | J.T. Baker | 3254-05 | |
| KH2PO4 | J.T. Baker | 3246-05 | |
| Sodium dihomogamma linolenate | NuCHEK | S-1143 | |
| Warm sterile Millipore water | |||
| Sterile water for collecting worms | |||
| Nuclease-free Water for DGLA stock solution | Ambion | AM9932 | |
| Ampicillin | Fisher Scientific | BP1760-25 | 100 mg/ml in water (for RNAi plates) |
| Isopropyl-beta-D-thiogalactopyranoside (IPTG) | Gold Biotechnology | 12481C100 | 1 M in water (for RNAi plates) |
| HSO4 | J.T. Baker | 9681-03 | |
| Methanol | Fisher Scientific | A452-4 | |
| Hexane | Fisher Scientific | H302-4 | |
| diamindinophenylindole (DAPI) | Sigma | D9542 | |
| VectaShield | Vector Laboratories | H-1000 | |
| Glass Flask | Corning | 4980-2L | |
| Autoclaveable Glass bottles with stirbars | Fisherbrand | FB-800 | |
| Autoclaveable Glass Graduated Cylinder | Fisherbrand | 08-557 | |
| Stir Plate | VWR | 97042-642 | |
| Waterbath at 55+ °C | Precision Scientific Inc. | 66551 | |
| Screwcap Brown Glass Vial | Sun SRI | 200 494 | |
| Argon gas tank | |||
| Automated Pipette aid | Pipette-Aid | P-90297 | |
| Sterile Serological Pipettes (25 ml) | Corning | 4489 | |
| Bunsen Burner | VWR | 89038-534 | |
| Dissection microscope | Leica | TLB3000 | |
| Silanized glass tube | Thermo Scientific | STT-13100-S | for FAMEs derivitization |
| PTFE Screw caps | Kimble-Chase | 1493015D | |
| Clinical tabletop centrifuge | IEC | ||
| GC Crimp Vial | SUN SRi | 200 000 | |
| GC Vial Insert | SUN SRi | 200 232 | |
| GC Vial cap | SUN SRi | 200 100 | |
| Gas Chromatograph | Agilent | 7890A | |
| Mass Spectrometry Detector | Agilent | 5975C | |
| Column for gas chromatography | Suppelco | SP 2380 | 30 m x 0.25 mm fused silica capillary column |
Preparação do Laboratório
Fatty acids are essential for numerous cellular functions. They serve as efficient energy storage molecules, make up the hydrophobic core of membranes, and participate in various signaling pathways. Caenorhabditis elegans synthesizes all of the enzymes necessary to produce a range of omega-6 and omega-3 fatty acids. This, combined with the simple anatomy and range of available genetic tools, make it an attractive model to study fatty acid function. In order to investigate the genetic pathways that mediate the physiological effects of dietary fatty acids, we have developed a method to supplement the C. elegans diet with unsaturated fatty acids. Supplementation is an effective means to alter the fatty acid composition of worms and can also be used to rescue defects in fatty acid-deficient mutants. Our method uses nematode growth medium agar (NGM) supplemented with fatty acidsodium salts. The fatty acids in the supplemented plates become incorporated into the membranes of the bacterial food source, which is then taken up by the C. elegans that feed on the supplemented bacteria. We also describe a gas chromatography protocol to monitor the changes in fatty acid composition that occur in supplemented worms. This is an efficient way to supplement the diets of both large and small populations of C. elegans, allowing for a range of applications for this method.
Fatty acids are essential for numerous cellular functions. They serve as efficient energy storage molecules, make up the hydrophobic core of membranes, and participate in various signaling pathways. Caenorhabditis elegans synthesizes all of the enzymes necessary to produce a range of omega-6 and omega-3 fatty acids. This, combined with the simple anatomy and range of available genetic tools, make it an attractive model to study fatty acid function. In order to investigate the genetic pathways that mediate the physiological effects of dietary fatty acids, we have developed a method to supplement the C. elegans diet with unsaturated fatty acids. Supplementation is an effective means to alter the fatty acid composition of worms and can also be used to rescue defects in fatty acid-deficient mutants. Our method uses nematode growth medium agar (NGM) supplemented with fatty acidsodium salts. The fatty acids in the supplemented plates become incorporated into the membranes of the bacterial food source, which is then taken up by the C. elegans that feed on the supplemented bacteria. We also describe a gas chromatography protocol to monitor the changes in fatty acid composition that occur in supplemented worms. This is an efficient way to supplement the diets of both large and small populations of C. elegans, allowing for a range of applications for this method.
Procedimento
Fatty acids are essential for numerous cellular functions. They serve as efficient energy storage molecules, make up the hydrophobic core of membranes, and participate in various signaling pathways. Caenorhabditis elegans synthesizes all of the enzymes necessary to produce a range of omega-6 and omega-3 fatty acids. This, combined with the simple anatomy and range of available genetic tools, make it an attractive model to study fatty acid function. In order to investigate the genetic pathways that mediate the physiological effects of dietary fatty acids, we have developed a method to supplement the C. elegans diet with unsaturated fatty acids. Supplementation is an effective means to alter the fatty acid composition of worms and can also be used to rescue defects in fatty acid-deficient mutants. Our method uses nematode growth medium agar (NGM) supplemented with fatty acidsodium salts. The fatty acids in the supplemented plates become incorporated into the membranes of the bacterial food source, which is then taken up by the C. elegans that feed on the supplemented bacteria. We also describe a gas chromatography protocol to monitor the changes in fatty acid composition that occur in supplemented worms. This is an efficient way to supplement the diets of both large and small populations of C. elegans, allowing for a range of applications for this method.
