成像免疫突触动力学内皮平面细胞模型
Summary
Adaptive immunity is controlled by dynamic ‘immunological synapses’ formed between T cells and antigen presenting cells. This protocol describes methods for investigating endothelial cells both as understudied physiologic APCs and as a novel type of ‘planar cellular APC model’.
Abstract
适应性免疫是通过T细胞和抗原呈递细胞('的APC')之间动态相互作用调节被称为“免疫突触'。在这些贴心的细胞与细胞间的接口MHC /银TCR,F-肌动蛋白,粘连分离亚细胞簇和信号分子形成和重塑迅速。这些动力被认为是双方的效率和发展的免疫应答的质量的关键因素,因此,保护与病理性免疫。免疫突触生理装甲运兵车的当前的理解是可获得的成像分辨率不足的限制。尽管人造材料模型 (例如,平面脂质双层)提供了出色的分辨率和一直非常有价值的工具,它们是固有的非生理性和过于简单。血管和淋巴管内皮细胞已经成为“半职业的一个重要的外周组织(或基质)隔人装甲运兵车“。这些装甲运兵车(其中表达最专业的装甲运兵车的分子机器)都形成几乎平坦的细胞表面的独特的功能,且很容易转染的(例如,用荧光蛋白记者)。本文将描述一个基本的方法来实现的内皮细胞作为一种新颖的和生理'平面蜂窝APC模式“为改进的成像和基本的抗原性信令过程的询问。
Introduction
T淋巴细胞是其特征在于,以有效地识别肽抗原(Ag)材料的能力适应性免疫系统势必主要组织相容性复合体(MHC)的一个分支分子通过它们的T细胞受体(TCR)1。幼稚淋巴细胞组成迁移和扫描的专业抗原呈递细胞“(的APC;例如,树突细胞)的淋巴结内,而记忆/效应T细胞需要能够有效地测量极其广泛外周组织内APC和潜在的靶细胞。
在下面的APC的初始确认同源Ag的分,淋巴细胞逮捕他们的迁移,并开始形成一个专门的亲密细胞间的接口称为“免疫突触”(IS)。持续(即,30-60分钟)IS触点需要以扩增和维持信令2-7。新兴的研究确定了IS内,它是在连续的形成和快速řemodeling离散的亚细胞信号传导的微群 (例如,含有MHC /银TCR,F-肌动蛋白,黏附和信号分子)确定的强度和产生的免疫反应2-7质量。然而,动态信息这一过程和调控机制不完全理解8,9。这在很大程度上源于与APC的表面不规则拓扑和细胞-细胞相互作用平面的控制不良取向,问题的深刻限制所需时空成像相关联的技术挑战接近8-10(Figure1A)。
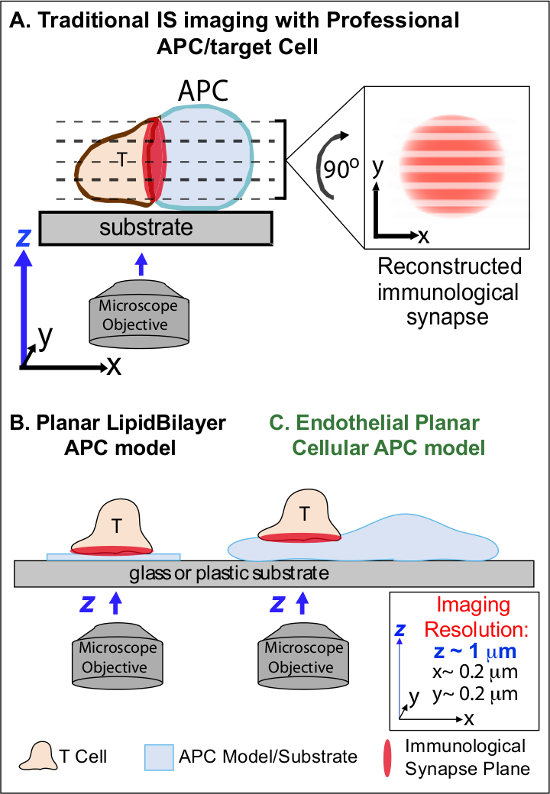
图1.生理平面成像免疫突触动力电池APC模型。该图说明免疫突触的T细胞和professio之间的传统影像最终APC(A)和T细胞与传统的平面脂双层APC模型(B)相比,这种新型内皮平面APC模式(C)。专职APC提供生理免疫突触,但提供取向不良的细胞-细胞接口(即相对于该最佳的xy成像平面;分辨率〜0.2微米),这显着地损害了空间(Z成象平面分辨率〜1微米)和时间(即由于需要通过所有的z成像平面)成像的分辨率反复扫描。双层车型有平面拓扑结构,提供最佳的时空成像分辨率,而且还高度简化,非生理性和刚性。这种内皮细胞模型结合脂质双层的平面拓扑与经典的APC的生理基板于生理环境提供最佳的空间和时间分辨率的成像。M /文件/ ftp_upload / 53288 / 53288fig1large.jpg“目标=”_空白“>点击此处查看该图的放大版本。
以前的工作已部分规避这些障碍通过开发平面衬底的模型(即,脂双层和抗体包被的表面上)提供最佳的时空分辨率(即,经由T细胞活化表面固定到一个单一的计划,是平行于最佳的xy成像平面)11-15日 (图1B)。这些模式促进了重要的见解控制抗原信号的T细胞,包括动态肌动蛋白/ TCR信号微群7,11-14的发现的亚细胞/分子动力学。然而,这种模型被固有地过于简化,以及刚性(排除的三维拓扑特征的发展/研究)( 图1B)。因此,尚不能确定如何与这样的调查结果PHYsiologic细胞与细胞免疫监视。
虽然仍然充分研究,血管及淋巴管内皮细胞正在成为一个大的( 即 ,更大数量比所有专业的装甲运兵车,由〜1000倍)的“半专业”的APC 16-18周边室。这些细胞表达MHC-I-,MHC-II-和多种共刺激分子(例如,CD40,LFA3,ICOSL,4-1BB,OX40L,TL1A,PD-L1,但不是CD80和CD86),并在战略上是位于血液组织界面,他们成为专业的定点功能16-18。以往的研究表明,内皮细胞可以有效地重新刺激效应/记忆,但不幼稚,T细胞19-25。因此,血管内皮细胞有可能在周围组织内的适应性免疫反应,如对T细胞的活化,分 化,存储器和公差16,17,26局部影响的效应阶段发挥独特的APC的作用。 CRI的角度讲,当在体外生长的细胞,内皮细胞形成几乎平坦的细胞表面,且很容易转染的(例如,用荧光蛋白记者)。这些特性非常适合拓扑动态过程中细胞间的相互作用19,27的高时空分辨率的成像。因此,内皮细胞可作为生理“平面细胞APC”的模式明显适合于驱动抗原识别和调节反应( 图1C)19,20的亚细胞/分子重塑机制的研究。
先前建立互补的成像技术(包括内皮细胞的细胞膜和胞浆内的荧光蛋白制造商的转染),用于粘附和跨内皮迁移27期间学习白细胞-内皮相互作用的细节,显示,白细胞由动态主动探测内皮的表面插入的亚微米级的D-回缩,肌动蛋白富圆柱形突起(〜在直径和深度200-1,000纳米)称为invadosome状突起(即,'ILPS')27,28。这些成像方法已被进一步扩大以及创造的协议采取的内皮APC功能优势,开发的第一个方法为T细胞与内皮细胞免疫突触的高时空分辨率成像报告19,20和进一步说明在此。从这个新的平面细胞APC模型推导出一个核心发现是,T细胞ILPS无论是在推动初期银检测和维持后续信号功能。事实上,多个ILPS(被稳定和应计响应初始钙通量)显示富集TCR和分子暗示的积极信号,例如PKC-Q,ZAP-70,磷酸和HS1的阵列。因此,ILPS似乎代表一个三维生理等效于TCR的信令微集群出现在平面双层模型。这种做法,因此,敏感地揭示/报道分子和建筑(以及隐含的生物力学)动力学没有其他检测。
本文所描述的方法应是有益的桥连专职APC和人工APC的基底模型之间的间隙,以提高我们的询问适应性免疫应答的基本机制的能力。而这里的重点是对CD4 + Th1型效应/记忆细胞的活化,这基本的方法可以容易地修改,以研究各种T细胞类型和AGS,如下面所讨论。
Protocol
Representative Results
Discussion
总体而言,这一协议描述方法研究内皮细胞作为一)充分研究生理装甲运兵车和ii)作为“平面细胞APC模式”一种新的类型。相对于前者,它已成为日益认识到,非造血外围设备(或“基质”)的APCs发挥关键的,非冗余的作用(即,相比于造血的APC)在成形适应性免疫应答16-18。在这样的“半专业”装甲运兵车,血管及淋巴管内皮细胞(这远多于专业装甲运兵车)均名列前茅赞赏<su…
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Peter T. Sage for his assistance in generating some of the representative images. This work was supported by an NIH R01 grant to C.V.C. (HL104006).
Materials
| BD Vacutainer stretch latex free tourniquet | BD Biosciences | 367203 | |
| BD alcohol swabs | BD Biosciences | 326895 | |
| BD Vacutainer Safety-Lok | BD Biosciences | 367861 | K2 EDTA |
| BD Vacutainer Push Button Blood Collection Set | BD Biosciences | 367335 | |
| RPMI-1640 | Sigma-Aldrich | R8758-1L | |
| Ficoll-Paque | Sigma-Aldrich | GE17-1440-02 | Bring to RT before use |
| FCS-Optima | Atlanta Biologics | s12450 | Heat inactivated |
| Penicillin-Streptomycin | Sigma-Aldrich | P4458-100ML | |
| Trypan blue | Sigma-Aldrich | T8154-20ML | |
| staphylococcal enterotoxin B | Toxin Technology | BT202RED | Stock solution 1mg/ml in PBS |
| toxic shock syndrome toxin 1 | Toxin Technology | TT606RED | Stock solution 1mg/ml in PBS |
| human IL-15 | R&D Systems | 247-IL-025 | Stock solution 50ug/ml in PBS |
| PBS | Life Technologies | 10010-049 | |
| Fibronectin | Life Technologies | 33016-015 | Stock solution 1mg/ml in H20 |
| HMVEC-d Ad-Dermal MV Endo Cells | Lonza | CC-2543 | Other Human Microvascular ECs can be used, i.e. HLMVECs |
| EGM-2 MV bullet kit | Lonza | CC-3202 | |
| Trypsin-EDTA | Sigma-Aldrich | T-4174 | Stock solution 10x, dilute in PBS |
| amaxa-HMVEC-L Nucleofector Kit | Lonza | vpb1003 | Required Kit for step 4 |
| IFN-g | Sigma-Aldrich | I3265 | Stock solution 1mg/ml in H20 |
| TNF-alpha 10ug, human | Life Technologies | PHC3015 | Stock solution 1mg/ml in H20 |
| phenol Red-free HBSS | Life Technologies | 14175-103 | |
| Hepes | Fisher Scientific | BP299-100 | |
| Calcium Chloride | Sigma-Aldrich | C1016-100G | Stock solution 1M in H20 |
| Magnesium chloride | Sigma-Aldrich | 208337 | Stock solution 1M in H20 |
| Human Serum albumin | Sigma-Aldrich | A6909-10ml | |
| Immersol 518 F fluorescence free Immersion oil | Fisher Scientific | 12-624-66A | |
| Fura-2 AM 20x50ug | Life Technologies | F1221 | Stock solution 1mM in DMSO |
| pEYFP-Mem (Mem-YFP) | Clontech | 6917-1 | |
| pDsRed-Monomer (Soluble Cytoplasmic DsRed) | Clontech | 632466 | |
| pDsRed-Monomer Membrane (Mem-DsRed) | Clontech | 632512 | |
| pEGFP-Actin | Clontech | 6116-1 | |
| Alexa Fluor 488 Phalloidin | Life Technologies | A12379 | |
| Formaldehyde solution 37% | Fisher Scientific | BP531-500 | Toxic, use fumehood |
| Triton X-100 | Sigma-Aldrich | X100-5ML | |
| Falcon 15mL Conical Centrifuge Tubes | Fisher Scientific | 14-959-70C | |
| Falcon 50mL Conical Centrifuge Tubes | Fisher Scientific | 14-959-49A | |
| Falcon Tissue Culture Treated Flasks T25 | Fisher Scientific | 10-126-9 | |
| Falcon Tissue Culture Treated Flasks T75 | Fisher Scientific | 13-680-65 | |
| Corning Cell Culture Treated T175 | Fisher Scientific | 10-126-61 | |
| Glass coverslips | Fisher Scientific | 12-545-85 | 12 mm diameter |
| Falcon Tissue Culture Plates 24-well | Fisher Scientific | 08-772-1 | |
| Delta-T plates | Bioptechs | 04200415B | |
| Wheaton Disposable Pasteur Pipets | Fisher Scientific | 13-678-8D | |
| 1.5 ml Eppendorf tube | Fisher Scientific | 05-402-25 | |
| ICAM1 mouse anti-human | BD Biosciences | 555509 | |
| HS1 mouse anti-human | BD Biosciences | 610541 | |
| Anti-Human CD11a (LFA-1alpha) Purified | ebioscience | BMS102 | |
| Anti-Human CD3 Alexa Fluor® 488 | ebioscience | 53-0037-41 | |
| Anti-MHC Class II antibody | Abcam | ab55152 | |
| Anti-Talin 1 antibody | Abcam | ab71333 | |
| Anti-PKC theta antibody | Abcam | ab109481 | |
| phosphotyrosine (4G10 Platinum) | Millipore | 50-171-463 | |
| Nucleofector II | Amaxa Biosystems | Required electroporator for step 4 | |
| Zeiss Axiovert | Carl Zeiss MicroImaging | ||
| Zeiss LSM510 | Carl Zeiss MicroImaging | ||
| Zeiss Axiovison Software | Carl Zeiss MicroImaging | ||
| NU-425 (Series 60) Biological Safety Cabinet | NuAIRE | Nu-425-600 | |
| Forma STRCYCLE 37 °C, 5% CO2 Cell culture Incubator | Fisher Scientific | 202370 | |
| Centrifuge 5810 | Eppendorf | EW-02570-02 | |
| Hemocytometer | Sigma-Aldrich | Z359629 | Bright-Line Hemocytometer |
| Isotemp Waterbath model 202 | Fisher Scientific | 15-462-2 |
Referências
- von Andrian, U. H., Mackay, C. R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343 (14), 1020-1034 (2000).
- Springer, T. A. Adhesion receptors of the immune system. Nature. 346 (6283), 425-434 (1990).
- Shaw, A. S., Dustin, M. L. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 6 (4), 361-369 (1997).
- Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N., Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 395 (6697), 82-86 (1998).
- Delon, J., Stoll, S., Germain, R. N. Imaging of T-cell interactions with antigen presenting cells in culture and in intact lymphoid tissue. Immunol Rev. 189, 51-63 (2002).
- Brossard, C., et al. Multifocal structure of the T cell – dendritic cell synapse. Eur J Immunol. 35 (6), 1741-1753 (2005).
- Dustin, M. L. The cellular context of T cell signaling. Immunity. 30 (4), 482-492 (2009).
- Balagopalan, L., Sherman, E., Barr, V. A., Samelson, L. E. Imaging techniques for assaying lymphocyte activation in action. Nat Rev Immunol. 11 (1), 21-33 (2011).
- Cebecauer, M., Spitaler, M., Serge, A., Magee, A. I. Signalling complexes and clusters: functional advantages and methodological hurdles. J Cell Sci. 123, 309-320 (2010).
- Oddos, S., et al. High-speed high-resolution imaging of intercellular immune synapses using optical tweezers. Biophys J. 95 (10), L66-L68 (2008).
- Grakoui, A., et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 285 (5425), 221-227 (1999).
- Bunnell, S. C., et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 158 (7), 1263-1275 (2002).
- Yokosuka, T., et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 6 (12), 1253-1262 (2005).
- Seminario, M. C., Bunnell, S. C. Signal initiation in T-cell receptor microclusters. Immunol Rev. 221, 90-106 (2008).
- Dustin, M. L. Supported bilayers at the vanguard of immune cell activation studies. J Struct Biol. 168 (1), 152-160 (2009).
- Martinelli, R., Carman, C. V., Bradshaw, R. A., Stahl, P. Lymphocyte Endothelilal Interactions. Encyclopedia of Cell Biology. , (2015).
- Choi, J., Enis, D. R., Koh, K. P., Shiao, S. L., Pober, J. S. T lymphocyte-endothelial cell interactions. Annu Rev Immunol. 22, 683-709 (2004).
- Marelli-Berg, F. M., Jarmin, S. J. Antigen presentation by the endothelium: a green light for antigen-specific T cell trafficking?. Immunol Lett. 93 (2-3), 109-113 (2004).
- Sage, P. T., et al. Antigen recognition is facilitated by invadosome-like protrusions formed by memory/effector T cells. J Immunol. 188 (8), 3686-3699 (2012).
- Kumari, S., et al. Actin foci facilitate activation of the phospholipase C-gama in primary T lymphocytes via the WASP pathway . eLife. , (2015).
- Marelli-Berg, F. M., et al. Major histocompatibility complex class II-expressing endothelial cells induce allospecific nonresponsiveness in naive T cells. J Exp Med. 183 (4), 1603-1612 (1996).
- Ma, W., Pober, J. S. Human endothelial cells effectively costimulate cytokine production by, but not differentiation of, naive CD4+ T cells. J Immunol. 161 (5), 2158-2167 (1998).
- Perez, V. L., Henault, L., Lichtman, A. H. Endothelial antigen presentation: stimulation of previously activated but not naive TCR-transgenic mouse T cells. Cell Immunol. 189 (1), 31-40 (1998).
- Epperson, D. E., Pober, J. S. Antigen-presenting function of human endothelial cells. Direct activation of resting CD8 T cells. J Immunol. 153 (12), 5402-5412 (1994).
- Shiao, S. L., et al. Human effector memory CD4+ T cells directly recognize allogeneic endothelial cells in vitro and in vivo. J Immunol. 179 (7), 4397-4404 (2007).
- Marelli-Berg, F. M., Okkenhaug, K., Mirenda, V. A two-signal model for T cell trafficking. Trends Immunol. 28 (6), 267-273 (2007).
- Carman, C. V., et al. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 26 (6), 784-797 (2007).
- Carman, C. V. Mechanisms for transcellular diapedesis: probing and pathfinding by ‘invadosome-like protrusions’. J Cell Sci. 122 ((Pt 17)), 3025-3035 (2009).
- Dustin, M. L., Tseng, S. Y., Varma, R., Campi, G. T. T cell-dendritic cell immunological synapses. Curr Opin Immunol. 18 (4), 512-516 (2006).
- Saito, T., Yokosuka, T. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr Opin Immunol. 18 (3), 305-313 (2006).
- Gomez, T. S., et al. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity. 24 (6), 741-752 (2006).
- Burbach, B. J., Medeiros, R. B., Mueller, K. L., Shimizu, Y. T-cell receptor signaling to integrins. Immunol Rev. 218, 65-81 (2007).
- Vicente-Manzanares, M., Sanchez-Madrid, F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 4 (2), 110-122 (2004).
- Ma, Z., Janmey, P. A., Finkel, T. H. The receptor deformation model of TCR triggering. Faseb J. 22 (4), 1002-1008 (2008).
- Ma, Z., Sharp, K. A., Janmey, P. A., Finkel, T. H. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol. 6 (2), e43 (2008).
- Groves, J. T. Bending mechanics and molecular organization in biological membranes. Annu Rev Phys Chem. 58, 697-717 (2007).
- Xu, C., et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 135 (4), 702-713 (2008).

