Summary
This paper describes a protocol for the synthesis of gold nanorods, based on the use of hydroquinone as reducing agent, plus the different mechanisms for controlling their size and aspect ratio.
Abstract
Gold nanorods are an important kind of nanoparticles characterized by peculiar plasmonic properties. Despite their widespread use in nanotechnology, the synthetic methods for the preparation of gold nanorods are still not fully optimized. In this paper we describe a new, highly efficient, two-step protocol based on the use of hydroquinone as a mild reducing agent. Our approach allows the preparation of nanorods with a good control of size and aspect ratio (AR) simply by varying the amount of hexadecyl trimethylammonium bromide (CTAB) and silver ions (Ag+) present in the “growth solution”. By using this method, it is possible to markedly reduce the amount of CTAB, an expensive and cytotoxic reagent, necessary to obtain the elongated shape. Gold nanorods with an aspect ratio of about 3 can be obtained in the presence of just 50 mM of CTAB (versus 100 mM used in the standard protocol based on the use of ascorbic acid), while shorter gold nanorods are obtained using a concentration as low as 10 mM.
Introduction
Gold nanoparticles (AuNPs) are one of the most widespread and promising nanostructures to be used in biomedical applications. Their use is essential in many point-of-care in vitro diagnostics products.1 They have been proposed as an effective tool for a number of other different applications: as a contrast agent in imaging studies,2 as a drug delivery system3 and as drugs for light-induced thermotherapy (or photothermal therapy).4 The great potential of AuNPs has driven, in the last twenty years, intense research on the development of new synthesis that is able to increase the control on the size and shape obtained.5 This is because different kinds of AuNPs are in fact more suitable than others for specific applications.
Among the different gold nanostructures, gold nanorods (AuNRs) have emerged as one of the most interesting systems. AuNRs are characterized by two plasmonic peaks associated with the oscillation of electrons along the longitudinal and the transverse axes, respectively.6 It is particularly important that the position of the most intense longitudinal peak can be tuned precisely between 620 and 800 nm, depending on the aspect ratio of the rods. This region matches the biological window,7 where the human tissues almost do not absorb light, allowing the development of a number of in vivo photonic applications involving AuNPs.
Despite a huge interest in this kind of nanostructures, the synthetic protocols for the preparation of AuNRs suffer from several limitations. In most of the cases, nanorods are prepared according to a two-step method developed by Sau and coworkers.8 In their protocol, nanorods are synthesized by reducing gold ions using ascorbic acid in presence of preformed gold seeds, silver ions and a large amount of hexadecyl trimethylammonium bromide (CTAB), a cationic linear surfactant.
The drawback of this protocol is that the reduction yield of gold ions is relatively low (about 20%) 9 and that a high amount of CTAB, an expensive reagent that accounts for more than half of the total cost for the reagents in the synthesis, is needed. The development of a new and more efficient synthetic route is thence considered to be an important need, allowing the spreading of biomedical approaches based on AuNRs.
In the first part of the present paper, we present an optimized protocol for the preparation of AuNR having an aspect ratio of about three. The synthesis is based on the use of hydroquinone as a mild reducing agent and it allows the preparation of AuNR with an almost quantitative reduction of gold ions, making use of a reduced amount of CTAB.10 This protocol for the preparation of the AuNRs is based on a two-step approach where gold seeds are used in a "growth solution".
In the second part, we show how to finely tune the size and aspect ratio of the obtained AuNR in two ways. The first way, similar to the standard protocol based on ascorbic acid, is to vary the amount of silver ions present in the "growth solution". The second way is based on the variation of the amount of CTAB that can be reduced down to a concentration of 10 mM (close to the critical micellar concentration reported by the supplier) to obtain well defined short nanorods.
Protocol
1. Synthesis of Gold Nanorods
Note: Use highly purified water throughout.
- Preparation of the gold seeds
- Dissolve 364.4 mg of hexadecyltrimethylammonium bromide (CTAB) in 5 ml water, under ultrasonication at 40 °C until the solution becomes clear. Let the CTAB solution cool down to room temperature.
- Separately, prepare 5 ml of tetrachloroauric acid (HAuCl4) in water (0.5 mM).
- Add the HAuCl4 solution to the CTAB solution under vigorous magnetic stirring, maintaining the temperature constant at 27 °C.
- Prepare 600 µl of sodium borohydride (NaBH4) solution in water (10 mM) at 4 °C. Add this solution to the mixture under vigorous stirring. Check if the solution's color immediately changes from yellow to brownish.
- Stir the suspension for 20 min before use. Store the seeds suspension for no longer than 24 hr at room temperature.
- Check the dimensions of the seeds using an UV-Vis spectrophotometer. Ensure that the seeds are small enough (about 2 nm) to be used in the preparation of gold nanorods by UV-visible spectroscopy.
Note: Spectrum must be similar to what is reported in Figure 1. Larger seeds identified by the presence of a plasmonic peak around 505-520 nm must not be used because they are likely to produce spherical nanoparticles.
- Preparation of the "growth solution" of gold nanorods.
- Dissolve 182.2 mg CTAB together with 22 mg hydroquinone in 5 ml water at 40 °C using ultrasonication. Cool down the solution to 27 °C.
- Prepare 200 µl of 4 mM silver nitrate (AgNO3) solution.
- Separately, prepare 5 ml of 1 mM solution of tetrachloroauric acid (HAuCl4).
- First add the silver nitrate solution prepared in step 1.2.2. Then, add the HAuCl4 solution prepared in step 1.2.3 to the solution of CTAB and hydroquinone prepared in step 1.2.1 under magnetic stirring.
- Immediately after, add under magnetic stirring 12 µl of the seeds suspension previously prepared according to the protocol reported at step 1.1 and let the reaction start. Check if the suspension changes color in about 30 min.
- Control the formation of the nanorods by checking the UV-visible spectrum of the suspension, as described in section 4, every 5 min. Proceed until the spectrum is stable. To allow the complete formation of the nanorods, leave the suspension under stirring for further 30 min (Figure 2).
- Divide the suspension in tubes (1 ml of suspension for each tube) and centrifuge at 10,000 x g for 10 min. Gold nanorods form a dark precipitate at the bottom of the tube.
- Resuspend the precipitate of each tube in 1 ml of water. Mix together the content of the tubes and store the suspension of gold nanorods at room temperature.
- Characterize the obtained nanorods by UV-visible spectroscopy and transmission electron microscopy as described in section 4 (Figure 3).
2. Tuning the Aspect Ratio of Nanorods by Varying the Concentration of Ag+ Ions
- Prepare a silver nitrate solution with a concentration of 4 mM, dissolving 3.4 mg AgNO3 in 5 ml of water.
- Prepare in three different vials the solution with CTAB and hydroquinone as described in section 1.2.1 and add respectively 100 µl, 150 µl or 200 µl of silver nitrate solution.
- Add the HAuCl4 solution prepared according to step 1.2.3 and proceed with the preparation of gold nanorods as described from point 1.2.5.
- Characterize the obtained nanorods by UV-visible spectroscopy and transmission electron microscopy. Vials with lower amounts of Ag+ will result in shorter nanorods (aspect ratio of 2 and 2.2 respectively) (Figure 4).
3. Tuning the Aspect Ratio of Nanorods by Varying the Concentration of CTAB
- Prepare different batches of gold nanorods with different concentrations of CTAB in the "growth solution". Use concentrations from 10 mM to 100 mM to produce gold nanorods having different size and aspect ratio. The concentrations of CTAB used in each experiment are summarized in Table 1 with the corresponding amount of milligrams used. Dissolve the different amounts of CTAB always with 22 mg of hydroquinone in 5 ml of water.
- Add 200 µl of silver nitrate solution (prepared according to step 1.2.2) and 5 ml of HAuCl4 solution (prepared according how described in step 1.2.3) in each vial under magnetic stirring.
- Add 12 µl of seeds suspension and observe the change in color of the final mixture.
- Stop the stirring when the suspension's color and the UV-visible spectrum are stabilized; the reaction time depends on the CTAB concentration in the growth solution.
- Centrifuge at 10,000 x g for 10 min and resuspend in water.
- Characterize the obtained nanorods by UV-visible spectroscopy and transmission electron microscopy. A lower concentration of CTAB will result in shorter nanorods while a higher concentration will give longer but larger nanorods. On contrary the aspect ratio of the nanorods will be higher in the range around 40-50 mM and will decrease both at lower and higher concentrations (Figure 5 and Figure 6).
4. Characterization of Gold Nanorods
- UV-visible Spectroscopy
- Dilute 100 µl of nanorods solution with 400 µl of water in a plastic micro-cuvette and acquire the UV-visible absorption spectrum (wavelength range between 400 and 840 nm) according to manufacturer's protocol.
- Collect UV-visible spectra (wavelength range between 400 and 840 nm) of the growth solution every 5 min in order to study the kinetics of the reaction.
- Transmission Electron Microscopy (TEM)
- Collect TEM images of each sample of nanorods, for the measurement of the size and of the aspect ratio of the obtained nanorods. Prepare the samples by placing a drop of suspension (4 µl) onto an ultra-thin Formvar-coated 200-mesh copper grids and leave to dry in air at 4 °C. Analyze the sample at TEM using an accelerating voltage of 200 kV according to manufacturer's protocol.
Representative Results
UV visible spectra of the gold seeds can be seen in Figure 1. UV visible spectra acquired at different times after the injection of the gold seeds are presented in Figure 2. UV visible spectra and transmission electron microscopic (TEM) images of the obtained gold nanorods are shown in Figure 3. UV visible spectra and transmission electron microscopic (TEM) images of gold nanorods with different aspect ratio obtained by varying the amount of silver ions are demonstrated in Figure 4 and CTAB in the growth solution in Figures 5 and 6. The UV visible spectra are used to observe the formation of the anisotropic gold nanoparticles and to obtain a rough indication of the aspect ratio. TEM images are used to determine the morphology of the nanostructures, to assess the precise aspect ratio of the AuNRs and to prove the crystal structure of gold.
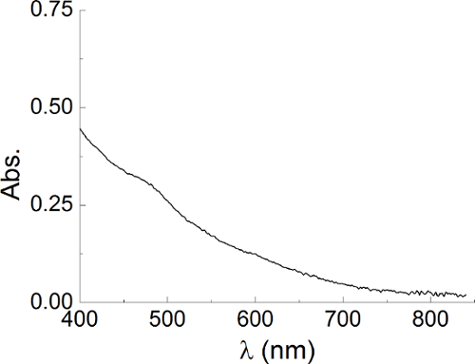
Figure 1. Gold seeds. UV-visible spectrum of gold seeds prepared according to the section 1.1. To prove that the dimension of the seeds is not too large, there must be no sign of the plasmonic peak in the region between 505 and 520 nm that characterizes plasmonic nanoparticles, so this figure demonstrates the presence of very small gold seeds. Please click here to view a larger version of this figure.
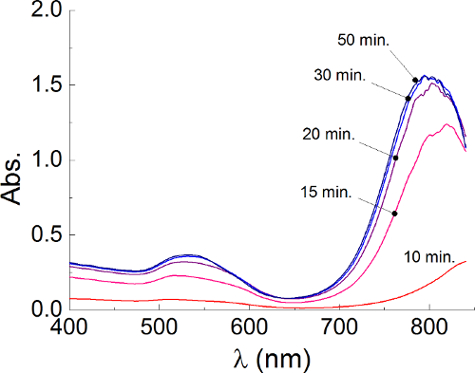
Figure 2. Reaction Kinetics. UV-visible spectra of gold nanorods acquired at different time since the injection of the gold seeds (CTAB 50 mM; Ag+ 200 µl). The spectra show a plasmonic peak that is initially very red shifted and that progressively moves toward lower wavelengths with time until it becomes stable suggesting that the reaction is complete after about 30 minutes from the seeds injection. Please click here to view a larger version of this figure.
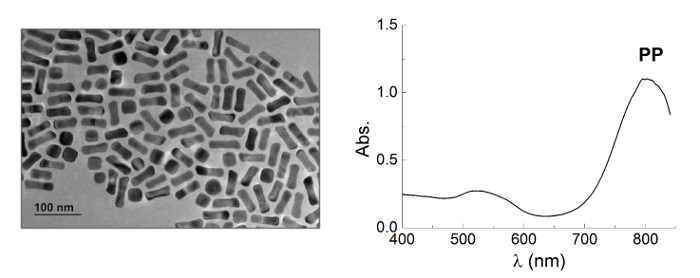
Figure 3. Gold Nanorods. TEM image (left) and UV-visible spectrum (right) of gold nanorods prepared according to the protocol 1.2. TEM image shows the elongated shape of the obtained nanoparticles, confirmed by the presence of the two plasmonic peaks in the UV-visible spectrum, associated with the oscillation of electrons along the longitudinal and the transverse axes. Scale bar of TEM image is 100 nm. Please click here to view a larger version of this figure.
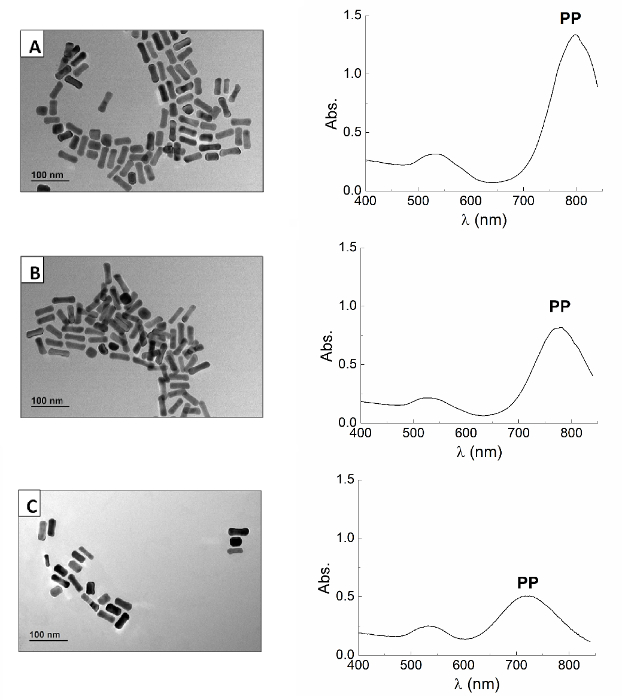
Figure 4. Gold Nanorods. TEM image (left) and UV-visible spectrum (right) of gold nanorods prepared according to the protocol 2 using 200 µl (A); 150 µl (B) and 100 µl (C) of Ag+ solution in the growth solution. As the TEM images show, the use of a higher amount of Ag+ in the growth solution results in longer nanorods. This is also demonstrated by the differences between the most intense plasmonic peak positions in the three batches of NRs. Scale bar of TEM images is 100 nm. Please click here to view a larger version of this figure.
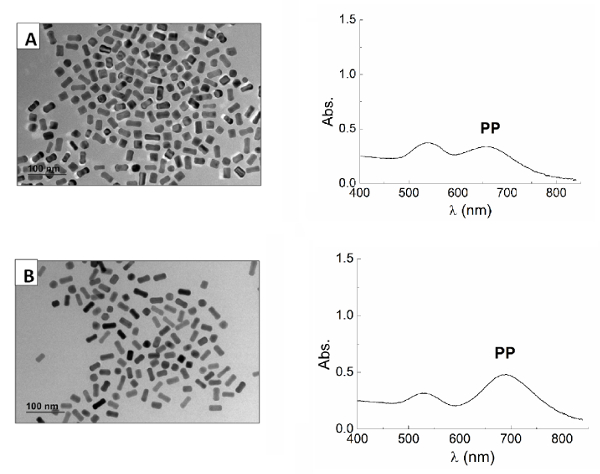
Figure 5. Gold Nanorods. TEM image (left) and UV-visible spectrum (right) of gold nanorods prepared according to the section 3 using lower concentrations of CTAB: 10 mM (A) and 20 mM (B) of CTAB in the growth solution. The use of a lower amount of CTAB in the growth solution results in shorter nanorods. Scale bar is 100 nm in all the pictures. Please click here to view a larger version of this figure.
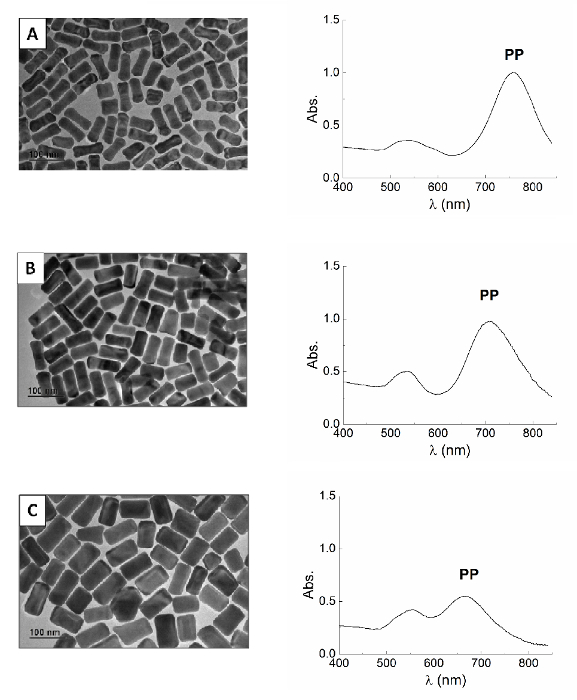
Figure 6. Gold Nanorods. TEM image (left) and UV-visible spectrum (right) of gold nanorods prepared according to the section 3 using higher concentrations of CTAB: 60 mM (A); 80 mM (B) and 100 mM (C) of CTAB in the growth solution. The use of a higher amount of CTAB in the growth solution results in nanorods that are longer but characterized by a lower aspect ratio. In fact, TEM images here reported show that the width of the rods increases; that causes the reduction of the aspect ratio. Scale bar is 100 nm in all the pictures. Please click here to view a larger version of this figure.
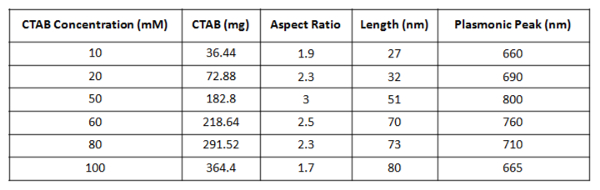
Table 1. CTAB concentration. Amounts of CTAB used for the preparation of gold nanorods with the different aspect ratio obtained.
Discussion
The protocol presented here applies hydroquinone, an aromatic molecule characterized by a weak reduction potential, to produce gold nanorods. There are two main advantages of the present protocol toward the most commonly employed synthetic route based on the use of ascorbic acid: the first is that hydroquinone is able to almost quantitatively reduce the gold ions allowing the production of higher amount of gold nanorods.11 The latter is given by the fact that it requires a lower amount of CTAB and a subsequent substantial reduction of costs. The present protocol is based on a two-step approach that deals with a separation of the nucleation step from the growth of the nanorods. We noticed that is extremely important that the dimension of the gold seeds used is kept around 3 nm as suggested by UV-visible spectroscopy.8 On the contrary, if larger seeds with a dimension of 5 nm or more are used, we inevitably obtain spherical nanoparticles.
The growth of gold nanorods can be easily followed by means of UV-visible spectroscopy. Rod shaped particles are characterized by spectra with two clear peaks corresponding to the two different dimensions of the rods. Moreover, this technique can be used to obtain a first estimation of the aspect ratio of the obtained rods according to the empirical law:
AR = 0.0078 • PP – 3.3
where AR is the empirical aspect ratio determined by TEM image analysis and PP is the position of the plasmonic peak relative to the longitudinal axis expressed in nanometers. The presence of the second plasmonic peak in the near infrared region of the spectrum is necessary to confirm the production of anisotropic particles. However, it must be noticed that the AR obtained thanks to this equation is just an empirical correlation of the experimental results obtained using TEM and UV-Visible spectroscopy and must be confirmed for each batch of AuNRs produced. After UV-Visible spectroscopy confirms the complete formation of AuNR, the suspension is centrifuged in order to remove the excess of CTAB present in the growth solution, and then the rods are suspended in pure water, where they appear to be stable for few months at room temperature. TEM analysis is also necessary for the complete characterization of the AuNR to obtain precise information about the length and the width.
The aspect ratio and the size of the obtained nanoparticles can be fine tuned in two ways. Similarly to what is commonly done in the synthesis of AuNRs based on ascorbic acid, the amount of silver ions in the growth solution is able to determine the formation of more or less elongated shapes. Ag+ induces a symmetry break of the forming nanorods once the seeds reached a size of about 5-6 nm.12 Thus, a higher amount of silver ions in the growth solution is able to induce the formation of longer AuNRs. When AuNRs with different aspect ratios are prepared through this approach, the length of nanorods can be tuned, but the width remains almost constant and is just slightly decreased when the longest rods (AR ≈ 3) are made. Another important parameter is the amount of CTAB used in the growth solution. The concentration of CTAB has been found to influence not only the aspect ratio but also the size of the nanorods. Interestingly, while the length of the obtained nanorods linearly depends on the concentration of CTAB, the aspect ratio behaves differently and a maximum is observed when CTAB is in the range between 40 and 60 mM. This corresponds to the fact that the width of the rods remains constant at low CTAB concentrations, but above 50 mM, the rod width starts to increase causing the reduction of the AR.
To summarize, we demonstrated how, by applying hydroquinone as reducing agent, it is possible to prepare nanorods using about half of the amount of CTAB compared to the common protocol based on reduction by ascorbic acid. Despite the fact that this approach is limited to the preparation of relatively short gold nanorods with an aspect ratio between 2 and 3, we expect that it could be easily adopted by other groups. This is because, even if it is based on a small modification of the standard ascorbic acid based approach, this method can drastically improve the AuNRs yield with a substantial reduction of costs. Moreover, it provides a good and reliable control of the size and the aspect ratio of the synthesized particles. Therefore, all of the advantages of this protocol could be useful for an easier and efficient diffusion of new medical applications of nanoparticles, because this more convenient synthetic route will help to develop the biomedical approach that makes use of nanorods in clinical practice with potential benefits for patients.
Declarações
The authors have nothing to disclose.
Acknowledgements
Funding for this research was provided by the Italian Ministry of Health under the frame of EuroNanoMed II (European Innovative Research & Technological Development Projects in Nanomedicine, project title: ”InNaSERSS”).
Materials
| Gold(III) chloride trihydrate | Sigma Aldrich | 520918 | |
| Hydroquinone | Sigma Aldrich | H17902 | |
| Silver Nitrate | Sigma Aldrich | 209139 | toxic |
| Sodium Borohydride | Sigma Aldrich | 480886 | |
| Hexadecyltrimethylammonium bromide (CTAB) | Sigma Aldrich | H5882 | Acute Tox. (oral). In this study we tested three different batches of CTAB (H5882) from Sigma Aldrich. Two of them were marked as made in China while one as made in India. In our experience only the batches marked as made in China were effective for the preparation of AuNR |
| Spectrophotometer | Thermo scientific | Nanodrop 2000C | |
| TEM | JEOL | 2100 |
Referências
- Zhou, W., Gao, X., Liu, D., Chen, X. Gold Nanoparticles for In Vitro Diagnostics. Chem Rev. 115 (19), 10575-10636 (2015).
- Bao, C., et al. Gold nanoprisms as optoacoustic signal nanoamplifiers for in vivo bioimaging of gastrointestinal cancers. Small. 9 (1), 68-74 (2013).
- Han, G., Ghosh, P., Rotello, V. M. Functionalized gold nanoparticles for drug delivery. Nanomedicine. 2 (1), 113-123 (2007).
- Choi, W. I., et al. Tumor regression in vivo by photothermal therapy based on gold-nanorod-loaded, functional nanocarriers. ACS Nano. 5 (3), 1995-2003 (2011).
- Langille, M. R., Personick, M. L., Zhang, J., Mirkin, C. A. Defining Rules for the Shape Evolution of Gold Nanoparticles . J. Am. Chem. Soc. 134 (35), 14542-14554 (2012).
- Lohse, S. E., Murphy, C. J. The Quest for Shape Control: A History of Gold Nanorod Synthesis. Chem. Mater. 25 (8), 1250-1261 (2013).
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotech. 19 (4), 316-317 (2001).
- Sau, T. K., Murphy, C. J. Seeded High Yield Synthesis of Short Au Nanorods in Aqueous Solution. Langmuir. 20 (15), 6414-6420 (2004).
- Ratto, F., Matteini, P., Rossi, F., Pini, R. Size and shape control in the overgrowth of gold nanorods. J. Nanopart. Res. 12, 2029-2036 (2010).
- Morasso, C., et al. Control of size and aspect ratio in hydroquinone-based synthesis of gold nanorods. J. Nanopart. Res. 17, 330-337 (2015).
- Vigderman, L., Zubarev, E. R. High-yield synthesis of gold nanorods with longitudinal SPR peak greater than 1200 nm using hydroquinone as a reducing agent. Chem. Mater. 25 (8), 1450-1457 (2013).
- Walsh, M. J., Barrow, S. J., Tong, W., Funston, A. M., Etheridge, J. Symmetry breaking and silver in gold nanorod growth. ACS Nano. 9 (1), 715-724 (2015).

