RGD功能化水凝胶作为治疗应用的工具合成
Summary
We present a protocol for the synthesis of RGD-functionalized hydrogels as devices for cell and drug delivery. The procedure involves copper catalyzed alkyne-azide cycloaddition (CuAAC) between alkyne-modified polyacrylic acid (PAA) and a RGD-azide derivative. The hydrogels are formed using microwave-assisted polycondensation and their physicochemical properties are investigated.
Abstract
The use of polymers as biomaterials has provided significant advantages in therapeutic applications. In particular, the possibility to modify and functionalize polymer chains with compounds that are able to improve biocompatibility, mechanical properties, or cell viability allows the design of novel materials to meet new challenges in the biomedical field. With the polymer functionalization strategies, click chemistry is a powerful tool to improve cell-compatibility and drug delivery properties of polymeric devices. Similarly, the fundamental need of biomedicine to use sterile tools to avoid potential adverse-side effects, such as toxicity or contamination of the biological environment, gives rise to increasing interest in the microwave-assisted strategy.
The combination of click chemistry and the microwave-assisted method is suitable to produce biocompatible hydrogels with desired functionalities and improved performances in biomedical applications. This work aims to synthesize RGD-functionalized hydrogels. RGD (arginylglycylaspartic acid) is a tripeptide that can mimic cell adhesion proteins and bind to cell-surface receptors, creating a hospitable microenvironment for cells within the 3D polymeric network of the hydrogels. RGD functionalization occurs through Huisgen 1,3-dipolar cycloaddition. Some PAA carboxyl groups are modified with an alkyne moiety, whereas RGD is functionalized with azido acid as the terminal residue of the peptide sequence. Finally, both products are used in a copper catalyzed click reaction to permanently link the peptide to PAA. This modified polymer is used with carbomer, agarose and polyethylene glycol (PEG) to synthesize a hydrogel matrix. The 3D structure is formed due to an esterification reaction involving carboxyl groups from PAA and carbomer and hydroxyl groups from agarose and PEG through microwave-assisted polycondensation. The efficiency of the gelation mechanism ensures a high degree of RGD functionalization. In addition, the procedure to load therapeutic compounds or biological tools within this functionalized network is very simple and reproducible.
Introduction
水凝胶是由亲水性的交联的聚合物,它们是天然或合成的,并且其特征在于,一个独特的三维结构形成的三维网络。这些设备是在药物递送,组织工程,基因的载体和智能传感器1,2的生物医学领域越来越有吸引力。事实上,它们的高水含量,以及它们的流变学和机械性能使之适合候选模拟软组织的微环境,使它们有效工具水溶性细胞因子或生长因子的递送。其中最有希望采用的是作为可注射生物材料携带细胞和生物活性化合物。水凝胶可以通过保持并在生理相关的方式精确地递送干细胞调节信号提高细胞存活和控制干细胞的命运,如在体外和体内实验3,4-观察。这样做的主要优点是有可能保持接种(原位 )的区域内注射的细胞,减少细胞的叶面积和extravasates进入循环系统洪流,迁移遍布全身,失去靶目标5的量。三维水凝胶网络的稳定性是由于其交联位点,由聚合物链6之间的共价键或内聚力形成。
在此框架下,正交选择的化学品适用于聚合物链能够提高水凝胶演出7的多功能工具。事实上,用适当的化学基团的聚合物的改性可以帮助提供适当的化学,物理和机械性能,以提高细胞生存力以及它们在组织形成使用。以相同的方式,这些技术中以加载细胞或生长因子的凝胶基质中,使用了含RGD肽允许在细胞粘附和存活的改善。 RGD是由三肽精氨酸,甘氨酸和天冬氨酸,这是迄今为止的最有效和最经常使用的三肽,由于其处理多于一个细胞粘附受体的能力和对细胞锚定,行为和生存8,9-其生物学影响。在这项工作中,RGD官能水凝胶的合成进行了研究与设计网络的一个热情细胞微环境特征在于足够的生化特性的目的。
在水凝胶的合成使用微波辐射提供了一个简单的过程,以减少副反应,并在比常规热过程10更短的时间周期得到较高的反应速率和产率。此方法不需要纯化步骤和产量的无菌水凝胶由于聚合物之间的相互作用,并在反应系统11不存在有机溶剂。因此,它可以确保链接到聚合物网络,因为没有模RGD的高百分比ifications需要参与凝胶形成的聚合物的化学基团。羧基,由PAA与卡波姆和羟基,由PEG和琼脂糖,通过缩聚反应产生的水凝胶三维结构。所提到的聚合物可用于水凝胶在脊髓损伤的修复治疗12的合成。这些设备,如报告以前的作品13,14,显示出高生物相容性,以及类似于那些生活的许多组织和触变性质的机械和物理化学性质。此外,它们在原位保持局限性,在注射区域。
在这项工作中,PAA的羧基与炔部分修改( 图1),和一个RGD叠氮化物化合物合成用的结构(CH 2)N利用该三肽末端基团-NH 2的反应与制备化学化合物- N 3(<STRONG>图2)。随后,将改性的PAA通过CuAAC点击反应15-17( 图3)的RGD-叠氮化物衍生物进行反应。使用铜(I)催化剂的导致在这两个反应速率和区域选择性的重大改进。该CuAAC反应被广泛用于有机合成和在聚合物科学。它结合了高效率和高耐受性的官能团,并且它是通过使用有机溶剂的影响。高选择性,快速的反应时间和简单的纯化过程允许星形聚合物,嵌段共聚物或链接枝所需部分18的获得。此点击策略使得能够修改聚合物聚合后根据最后的生化应用定制的物理化学性质。所述CuAAC实验条件容易再现(反应不敏感,水,而可以最低限度发生铜氧化),和的性质形成三唑确保了产品的稳定性。使用铜金属可以被认为是一个临界点时,由于对细胞的潜在毒性作用,并在生物微环境,但透析用作纯化方法,以允许完全除去催化剂残留物的。最后,PAA改性RGD在水凝胶的合成( 图4),将所得网络的物理化学性质使用进行了研究,以检查这些系统如细胞或药物载体的潜在功能。
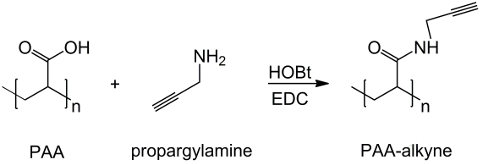
图1:PAA改性炔合成炔基官能PAA的方案。 “N”表示与羧基与炔丙基反应的单体。 请点击这里查看一个更大的版本这个数字。
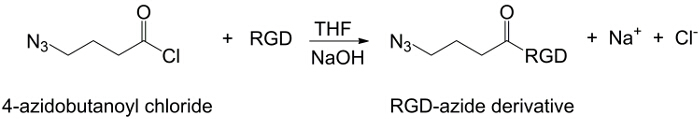
图2:。RGD- 叠氮合成 RGD-叠氮衍生物的合成请点击此处查看该图的放大版本。
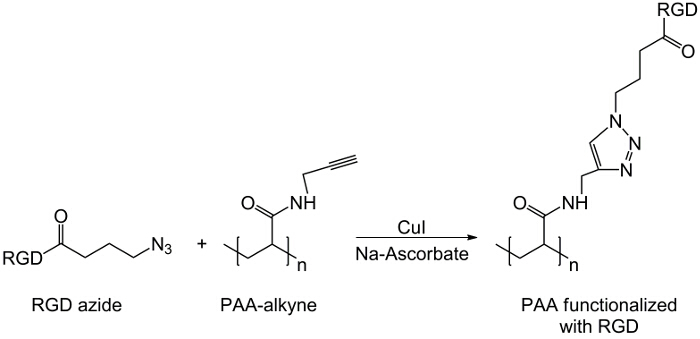
图3:点击反应 RGD-叠氮衍生物和炔PAA之间的点击反应方案。 请点击此处查看该图的放大版本。
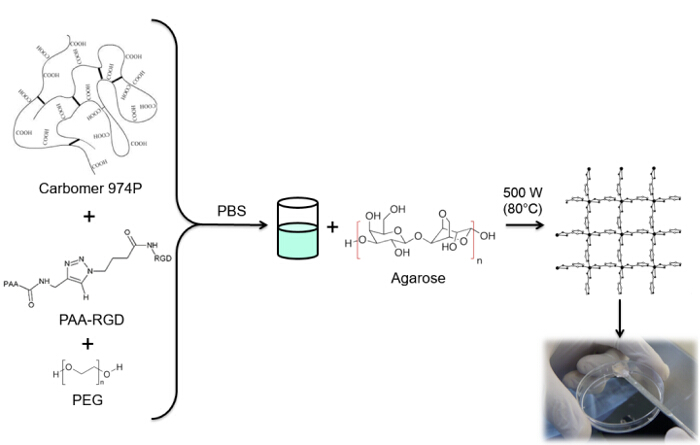
图4:水凝胶SYNThesis。RGD功能化水凝胶合成过程。 请点击此处查看该图的放大版本。
Protocol
Representative Results
Discussion
The PAA post-polymerization modification with alkyne moieties and the RGD functionalization with the azide group guarantee the formation of a stable bond between the polymer and the peptide. Indeed, triazole serves as a rigid linking unit among the carbon atoms, attached to the 1,4 positions of the 1,2,3-triazole ring and it cannot be cleaved hydrolytically or otherwise. In addition, triazole is extremely difficult to oxidize and reduce, unlike other cyclic structures such as benzenoids and related aromatic heterocycles<…
Declarações
The authors have nothing to disclose.
Acknowledgements
作者要感谢毛里齐奥教授马西的富有成果的讨论和小姐基娅拉阿莱格雷蒂语言编辑。作者的研究是由阪乔瓦尼Ricercatori 2010部(Ministero della Salute的GR-2010- 2312573)的支持。
Materials
| Poly(acrylic acid) solution average Mw ~ 100,000, 35 wt % in H2O | Sigma Aldrich | 523925 | CAS 9003-01-4 |
| Poly(ethylene glycol) 2,000 | Sigma Aldrich | 84797 | CAS 25322-68-3 |
| Carbomeer 974P | Fagron | 1387083 | |
| Agarose | Invitrogen Corp. | 16500-500 | UltraPure Agarose |
| RGD peptide | abcam | ab142698 | |
| 4-azidobutanoic acid | Aurum Pharmatech | Z-2421 | CAS 54447-68-6 |
| Oxalyl chloride | Sigma Aldrich | O8801 | CAS 79-37-8 |
| Propargylamine hydrochloride 95% | Sigma Aldrich | P50919 | CAS 15430-52-1 |
| Copper(I) iodide | Sigma Aldrich | 3140 | CAS 7681-65-4 |
| Sodium ascorbate | Sigma Aldrich | Y0000039 | CAS 134-03-2 |
| Phosphate buffered saline | Sigma Aldrich | P4417 | |
| Dialysis Membrane | Spectrum Laboratories, Inc. | 132725 | Spectra/Por 3 Dialysis Membrane Standard RC Tubing MWCO: 3,5 kD |
Referências
- Slaughter, B. V., Khurshid, S. S., Fisher, O. Z., Khademhosseini, A., Peppas, N. A. Hydrogels in Regenerative Medicine. Adv. Mater. 21 (32-33), 3307-3329 (2009).
- Rossi, F., Perale, G., Papa, S., Forloni, G., Veglianese, P. Current options for drug delivery to the spinal cord. Expert Opin. Drug Deliv. 10 (3), 385-396 (2013).
- Huebsch, N., et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9 (6), 518-526 (2010).
- Mothe, A. J., Tam, R. Y., Zahir, T., Tator, C. H., Shoichet, M. S. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 34 (15), 3775-3783 (2013).
- Khetan, S., et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12 (5), 458-465 (2013).
- Ashley, G. W., Henise, J., Reid, R., Santi, D. V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. U S A. 110 (6), 2318-2323 (2013).
- Rossi, F., van Griensven, M. Polymer Functionalization as a Powerful Tool to Improve Scaffold Performances. Tissue Eng. Part A. 20 (15-16), 2043-2051 (2014).
- Gould, S. T., Darling, N. J., Anseth, K. S. Small peptide functionalized thiol-ene hydrogels as culture substrates for understanding valvular interstitial cell activation and de novo tissue deposition. Acta Biomater. 8 (9), 3201-3209 (2012).
- Azagarsamy, M. A., Anseth, K. S. Wavelength-Controlled Photocleavage for the Orthogonal and Sequential Release of Multiple Proteins. Angew. Chem. Int. Edit. 52 (51), 13803-13807 (2013).
- Larrañeta, E., et al. Microwave-Assisted Preparation of Hydrogel-Forming Microneedle Arrays for Transdermal Drug Delivery Applications. Macromol. Mater. Eng. 300 (6), 586-595 (2015).
- Cook, J. P., Goodall, G. W., Khutoryanskaya, O. V., Khutoryanskiy, V. V. Microwave-Assisted Hydrogel Synthesis: A New Method for Crosslinking Polymers in Aqueous Solutions. Macromol. Rapid Comm. 33 (4), 332-336 (2012).
- Perale, G., et al. Multiple drug delivery hydrogel system for spinal cord injury repair strategies. J. Control. Release. 159 (2), 271-280 (2012).
- Rossi, F., Perale, G., Storti, G., Masi, M. A Library of Tunable Agarose Carbomer-Based Hydrogels for Tissue Engineering Applications: The Role of Cross-Linkers. J. Appl. Polym. Sci. 123 (4), 2211-2221 (2012).
- Frith, J. E., et al. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials. 34 (37), 9430-9440 (2013).
- Kolb, H. C., Finn, M. G., Sharpless, K. B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Edit. 40 (11), (2001).
- Sacchetti, A., Mauri, E., Sani, M., Masi, M., Rossi, F. Microwave-assisted synthesis and click chemistry as simple and efficient strategy for RGD functionalized hydrogels. Tetrahedron Lett. 55 (50), 6817-6820 (2014).
- Ossipov, D. A., Hilborn, J. Poly(vinyl alcohol)-based hydrogels formed by "click chemistry". Macromolecules. 39 (5), 1709-1718 (2006).
- Truong, V., Blakey, I., Whittaker, A. K. Hydrophilic and Amphiphilic Polyethylene Glycol-Based Hydrogels with Tunable Degradability Prepared by "Click" Chemistry. Biomacromolecules. 13 (12), 4012-4021 (2012).
- Hou, R. Z., et al. New synthetic route for RGD tripeptide. Prep. Biochem. Biotechnol. 36 (3), 243-252 (2006).
- Rossi, F., Chatzistavrou, X., Perale, G., Boccaccini, A. R. Synthesis and Degradation of Agar-Carbomer Based Hydrogels for Tissue Engineering Applications. J. Appl. Polym. Sci. 123 (1), 398-408 (2012).
- Mauri, E., Rossi, F., Sacchetti, A. Tunable drug delivery using chemoselective functionalization of hydrogels. Mater. Sci. Eng. C. 61, 851-857 (2016).
- Joaquin, A., Peppas, N. A., Zoldan, J. Hydrogel Polymer Library for Developing Induced Pluripotent Stem Cell Derived Cardiac Patches. Tissue Eng. Part A. 20, S55-S55 (2014).
- Rossi, F., et al. Tunable hydrogel-Nanoparticles release system for sustained combination therapies in the spinal cord. Colloids Surf. B Biointerfaces. 108, 169-177 (2013).
- Kolb, H. C., Sharpless, K. B. The growing impact of click chemistry on drug discovery. Drug Discov. Today. 8 (24), 1128-1137 (2003).
- Ossipov, D. A., Yang, X., Varghese, O., Kootala, S., Hilborn, J. Modular approach to functional hyaluronic acid hydrogels using orthogonal chemical reactions. Chem. Commun. 46 (44), 8368-8370 (2010).
- Anderson, S. B., Lin, C. C., Kuntzler, D. V., Anseth, K. S. The performance of human mesenchymal stem cells encapsulated in cell-degradable polymer-peptide hydrogels. Biomaterials. 32 (14), 3564-3574 (2011).
- Caron, I., et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials. 75, 135-147 (2016).
- Lee, J. W., Kim, H., Lee, K. Y. Effect of spacer arm length between adhesion ligand and alginate hydrogel on stem cell differentiation. Carbohyd. Polym. 139, 82-89 (2016).
- Liu, Y., Fan, Z., Wang, Y., Yu, L. Controlled Release of Low Molecular Protein Insulin-like Growth Factor-1 through Self-Assembling Peptide Hydrogel with Biotin Sandwich Approach. J.Biomed. Eng. 32 (2), 387-392 (2015).

