Modification and Application of a Leaf Blower-vac for Field Sampling of Arthropods
Summary
Assessment of arthropod abundance in crops is critical for investigating population dynamics and species interactions. Here we describe the modification and application of a leaf blower-vac for suction sampling of arthropods in rice.
Abstract
Rice fields host a large diversity of arthropods, but investigating their population dynamics and interactions is challenging. Here we describe the modification and application of a leaf blower-vac for suction sampling of arthropod populations in rice. When used in combination with an enclosure, application of this sampling device provides absolute estimates of the populations of arthropods as numbers per standardized sampling area. The sampling efficiency depends critically on the sampling duration. In a mature rice crop, a two-minute sampling in an enclosure of 0.13 m2 yields more than 90% of the arthropod population. The device also allows sampling of arthropods dwelling on the water surface or the soil in rice paddies, but it is not suitable for sampling fast flying insects, such as predatory Odonata or larger hymenopterous parasitoids. The modified blower-vac is simple to construct, and cheaper and easier to handle than traditional suction sampling devices, such as D-vac. The low cost makes the modified blower-vac also accessible to researchers in developing countries.
Introduction
Repeated assessment of the abundance and diversity of phytophagous and entomophagous arthropods in crops is needed for ecological studies of population dynamics and species interactions, including the study of biological control. Rice is a major staple food, with a high potential for biocontrol by entomophagous arthropods1,2, but which can be disrupted by insecticides3. The diversity of arthropods in rice crops can be high, and arthropod species occupy various crop strata (e.g., ground, stem, canopy, flowers), differ in mode of movement (e.g., walking, jumping, flying) and foraging strategy (e.g., sessile sucking insects, hunting predators and flower visiting pollinators)4.
There is a wide range of arthropod sampling techniques, each with strengths and weaknesses. For instance, pitfall traps can be used to sample ground-dwelling arthropods, but provide activity-dependent relative population estimates5,6. Sweep nets can be used to sample fast-flying insects in the canopy7-9, but give relative estimates of arthropod abundance. The beat sheet method can be used to sample the plant-dwelling arthropod community and provides absolute estimates of arthropod abundance, but it cannot be used effectively in flooded crop fields such as rice paddies10.
Suction sampling, when conducted in combination with an enclosure covering a standardized area of the field, provides absolute estimates of the densities of plant-dwelling arthropods. This method can also be used in flooded rice. Samples can be stored for later processing and identification. The Dietrick vacuum (D-vac)11 is the first commercially developed suction sampler. Although D-vacs are still widely used12-14, they are relatively expensive, have a limited suction force15 and are relatively heavy, which makes them hard to handle in flooded rice fields16. Arida and Heong 16 developed a suction sampler using a petrol-driven leaf blower-vac, and this prototype was further refined by Domingo and Schoenly 17. Advantages of the blower-vac suction sampler as compared to the D-vac are that it is much cheaper and easier to handle.
Although the blower-vac suction sampling method has been used in many ecological studies18-23, the instructions for its modification and application have not been clearly described. Here we present a video-based, detailed description of the modification and application of a petrol-powered leaf blower-vac for suction sampling of arthropod populations in flooded rice fields. The modification is inspired by Arida and Heong 16 and Domingo and Schoenly 17, but the design has been further simplified compared to these original publications, facilitating construction and use.
Protocol
1. Modification of a Leaf Blower-vac for Suction Sampling
- Collect all parts listed in the Materials List.
- Connect all polyvinyl chloride (PVC) pipes with unplasticized polyvinyl chloride (U-PVC) glue (pipes 1-2-3-4-5 and 6-7).
- Drill three holes that are evenly distributed around the suction mouth of the machine.
- Connect the machine to the end 1 of PVC pipe 1-5 by inserting one screw into each of the three holes. Do not use glue to connect pipe end 1 to the machine because the connection should be reversible to clean the fan.
Note: In case the diameter of the suction mouth of the machine differs from the model described here, the diameter of pipe end 1 should be adjusted to fit the machine seamlessly. - Add 2 layers of thread seal tape to pipe ends 5 and 6.
- Put the piece of metal gauze between the hose and the mouth part (PVC pipes 6 and 7) to prevent the sampling net from being sucked into the machine. Use metal gauze with a mesh diameter between 0.5 mm and 0.5 cm.
- Connect the hose to pipe ends 5 and 6 with metal clamp hoops.
2. Prepare the Sampling Enclosure
- Remove the bottom from a plastic bucket (50 L, 40 cm bottom diameter). This size of the enclosure covers 2-4 rice hills in a transplanted rice field, depending on crop stage24.
- Attach a nylon mesh sleeve, with a length of 1 m, to the top of the bucket using a rubber band. For this sleeve, use a mesh size that is small enough to prevent the escape of the smallest target arthropods. Use a diameter less than 0.5 mm.
3. Field Application of the Modified Leaf Blower-vac for Suction Sampling
- Use two persons to operate the device in the field. One person operates the blower-vac and the other handles the bucket enclosure and the sampling nets.
- Start the machine.
- Insert a sampling net into the mouth part of the blower-vac and fix it with a rubber band. For the net, use a mesh size that is small enough to catch the smallest target arthropods, but does not create obvious airflow resistance. Use the light nylon material with a mesh diameter between 0.2-0.5 mm.
- Place the enclosure quickly over the plants at a random location in the field and push the bottom of the bucket firmly into the soil. Make sure that the sleeve is closed to prevent arthropods from escaping from the top of the enclosure.
- Remove all arthropods from inside the enclosure in a top-down spiraling way, for a standardized sampling duration. For rice crops in the vegetative and reproductive stages, use sampling durations of 1 and 2 min, respectively.
- After finishing the sample, remove the rubber band from the sampling net, quickly close the net and take it out of the mouth part of the blower-vac and close it with a knot, while keeping the machine running.
- Repeat steps 3.2 to 3.5 at random locations in the field for the next samples. The number of replications depends on the variation in the spatial distribution of arthropods in the field, the required accuracy of the estimate and the purpose of the study. Typically, six replicates will give a good impression of the arthropod community and species abundances.
Representative Results
A total of 295 arthropods were collected in 8 three-minute blower-vac samples from a rice crop in the ripening stage in Jiangxi Province, China, in September 2015. To determine the relationship between relative yield (proportion of the arthropods collected in the sample) and sampling duration, each sample was divided into six sub-samples of 30 seconds each. The mean number of individuals per sample was 36.9 ± 4.1 (mean ± SEM). A total of eight arthropod orders were found, with Hemiptera (28.8%), Araneae (27.5%) and Diptera (17.6%) being dominant (Figure 1). The relative yield, expressed as a percentage of the number of collected arthropods after three minutes, was 52.9% ± 5.1, 92.4% ± 1.9 and 97.3% ± 0.9 after 30 seconds, 2 and 2.5 minutes, respectively (Figure 2).
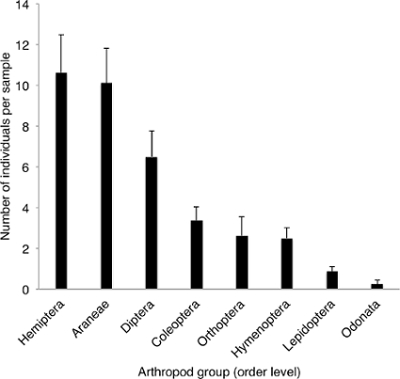
Figure 1. Species composition (at the order level) of arthropod samples in the suburbs of Nanchang city in Jiangxi Province, China. Eight samples were taken with a blower-vac of three-minutes each. Error bars represent the standard error of the mean. Please click here to view a larger version of this figure.
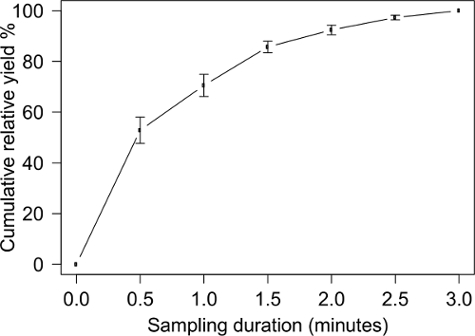
Figure 2. Cumulative relative yield as a function of total sampling duration based on sub-samples of 30 seconds each. The total number of arthropods collected over a sampling duration of three minutes is set at 100%. No arthropods were found upon visual inspection after the last blower-vac sample. Error bars represent standard errors of the mean of eight three-minute samples. Please click here to view a larger version of this figure.
Discussion
Suction sampling is one of many possible methods to sample arthropod communities in crops. For scientific research in rice systems, suction sampling is an appropriate option because the method provides absolute estimates of arthropod densities, it is non-destructive, and — in contrast to visual counts — allows collection and storage of samples for later processing. Compared to the commercially available D-vac, the blower-vac is smaller, lighter and easier to handle in (flooded) rice fields and also easier to combine with an enclosure. For example, a blower-vac weighs about 6 kg, whereas the backpack D-vac model, presented as the international standard for insect sampling, has a weight 12 kg11. More importantly, the sampling efficiency of the blower-vac is higher than the D-vac16,17, while the cost of the blower-vac is less. The modification of a leaf blower-vac into a suction sampler does not require special skills or equipment and takes less than an hour after all additional parts have been collected. The blower-vac described here is easier to construct and operate than versions described previously in the literature16,17, and the required parts (Table 1) are standard construction materials that are widely available. This makes the blower-vac also accessible to researchers with small budgets in developing countries.
The power and displacement of the engine determines the suction force of the blower-vac. Here we recommend a machine with a power between 0.7-1.2 kW and a displacement between 25-35 cc, which is adequate for sampling the plant-dwelling arthropod community in rice. The length of the flexible plastic hose and the diameter of the sucking mouth part (pipe 7) are critical for a good sampling performance. A hose that is too long will reduce the suction power, whereas a hose that is too short will be inconvenient to use during sampling. Similarly, a mouth part with too large a diameter will reduce the suction power, whereas a diameter that is too small will reduce the sampling efficiency due to the small surface. The sampling efficiency depends critically on the sampling duration. If sampling is conducted throughout the growing season, the sampling duration may have to be adjusted to the plant size, structure, and planting density to maintain a similar level of efficiency. Sampling efficiency should be checked by careful visual inspection of the enclosed area after sampling. If there are still arthropods present, the sampling duration must be increased. The recommended sampling durations for rice crops in the vegetative stage is 1 minute and in the reproductive and ripening stages it is 2 minutes.
Suction sampling with the blower-vac can be conducted in flooded fields, while alternative methods, such as pitfall and beat sheet sampling are not feasible in standing water. The blower-vac can also be used to sample the arthropod community on the water surface of flooded rice fields (e.g., predatory water bugs), as the machine is capable of sucking in some water. However, it is not recommended for sampling aquatic arthropods as the motor may stop running when the mouth part is inserted deeply into the water and the airflow is blocked. Apart from rice, the blower-vac can also be used in other crops and non-crop habitat, as long as the height and structure of the vegetation allows proper placement of the enclosure25.
Our blower-vac suction sampling method is non-destructive. Almost all arthropods collected in the sampling net survived, including those soft bodies such as mosquitos and damselflies. The application of this method, however, has some limitations and drawbacks. The blower-vac needs to be operated by two persons. Carrying the blower-vac in the field will result in some disturbance, and therefore this method may underestimate disturbance-sensitive species such as grasshoppers. Fast and abrupt placement of the enclosure in a relatively undisturbed area in the forward direction of movement may limit this potential bias. The loud noise of the blower-vac machine may also cause disturbance, and sampling at night in residential areas is not recommended. The method is not suitable for sampling highly mobile flying insects, such as predatory Odonata or larger hymenopterous parasitoids. As with any sampling method, the combination of the blower-vac with other methods, such as sweep net sampling or destructive harvesting of plants, can provide a more complete and balanced assessment of the arthropod community26.
Declarações
The authors have nothing to disclose.
Acknowledgements
The investigations were financially supported by the Division for Earth and Life Sciences of the Netherlands Organization for Scientific Research (grant 833.13.004), the Sci-Tech Landing Projection of Higher Education of Jiangxi Province (KJLD14030) and The Cultivation Plan for Young Scientists of Jiangxi Province (Jinggang star 20153BCB23014). We thank Daomeng Fu, Zhigang Li and Xiaolong Huang for their help in producing the movie.
Materials
| Machine | |||
| Leaf blower-vac | We used Oleo-Mac BV300, Made in Italy | Power: 1.0 kW, Displacement: 30.5 cc, Max air volume: 720 m³/h, Max air speed: 70 m/sec, Weight: 4.5 kg, Diameter of suction mouth: 113 mm | There are many different brands and models available. For comparable performance, the specifications concerning power and air speed should be similar to those presented here. |
| Additional parts for modification | |||
| PVC pipe 1 | Outer ø of end connected to the machine: 112 mm, Inner ø of end connected to PVC pipe 2: 110 mm | This is the cover of a ø 110 mm PVC pipe | |
| PVC pipe 2 | Outer ø: 110 mm, Length: 10 cm | Normal outer ø 110 mm PVC pipe; to connect PVC pipe 1 and 3 | |
| PVC pipe 3 | Inner ø of big end: 110 mm, Inner ø of small end: 50 mm | PVC ø 110 mm to ø 50 mm downpipe reducer | |
| PVC pipe 4 | Outer ø: 50 mm, Length: 5 cm | Normal ø 50 mm PVC pipe; to connect PVC pipe 3 and 5 | |
| PVC pipe 5 | Inner ø: 50 mm and 32 mm, Outer ø of small part: 38 mm | PVC ø 50 mm to ø 32 mm downpipe reducer | |
| Hose | Outer ø: 40 mm | Wire-fortified, flexible plastic hose | |
| Metal gauze | Mesh ø: 1 mm, ø: 60 mm | Prevent the sampling net from being sucked into the machine | |
| PVC pipe 6 | Outer ø of small end: 38 mm, Inner ø of big end: 63 mm | PVC ø 32 mm to ø 63 mm reducer | |
| PVC pipe 7 | Outer ø: 63 mm, Length: 25 cm | Normal outer ø 63mm PVC pipe | |
| U-PVC glue | U-PVC glue; to connect PVC parts | ||
| Metal clamp hoops (2) | Flexible between ø 35 mm – 51 mm | To connect the hose with the PVC pipes | |
| Thread seal tape | Width: 18mm | Seal the hose-PVC connections | |
| Screws (3) | Length: 25mm | To connect PVC pipe 1 with the suction mouth of the machine | |
| Sampling net and enclosure | |||
| Sampling net | Mesh size ø: 0.3 mm, Width of the mouth: 10 cm, Height: 30 cm | The sampling net has a conical shape. | |
| Bucket | Bottom ø: 40 cm, Volume: 50 L | Cut the bottom | |
| Nylon sleeve | Mesh size ø: 0.3 mm, Length: 1 m | To cover the bucket as enclosure | |
Referências
- Dale, D., Heinrichs, E. A. . Biology and management of rice insects. , (1994).
- Settle, W. H., et al. Managing tropical rice pests through conservation of generalist natural enemies and alternative prey. Ecology. 77 (7), 1975-1988 (1996).
- Heong, K. L., Schoenly, K. G., Haskell, P. T., McEwen, P. . Ecotoxicology. , 381-403 (1998).
- Schellhorn, N. A., Bianchi, F., Hsu, C. L. Movement of entomophagous arthropods in agricultural landscapes: links to pest suppression. Annu Rev Entomol. 59, 559-581 (2014).
- Zou, Y., Feng, J., Xue, D., Sang, W., Axmacher, J. C. A comparison of terrestrial arthropod sampling methods. J Resour Ecol. 3 (2), 174-182 (2012).
- Saska, P., et al. Temperature effects on pitfall catches of epigeal arthropods: a model and method for bias correction. J Appl Ecol. 50 (1), 181-189 (2013).
- Vander Werf, W., Evans, E. W., Powell, J. Measuring and modelling the dispersal of Coccinella septempunctata (Coleoptera : Coccinellidae) in alfalfa fields. Eur J Entomol. 97 (4), 487-493 (2000).
- Rashid, T., Johnson, D. T., Bernhardt, J. L. Sampling rice stink bug (Hemiptera : Pentatomidae) in and around rice fields. Environ Entomol. 35 (1), 102-111 (2006).
- Sarwshri, G. Aboveground arthropod pest and predator diversity in irrigated rice (Oryza sativa L.) production systems of the Philippines. J Trop Agr. 45 (1/2), 1-8 (2007).
- Wade, M. R., et al. Temporal variation in arthropod sampling effectiveness: the case for using the beat sheet method in cotton. Entomol Exp Appl. 120 (2), 139-153 (2006).
- Dietrick, E. J. An Improved Backpack Motor Fan for Suction Sampling of Insect Populations. J Econ Entomol. 54 (2), 394-395 (1961).
- Munyaneza, J. E., Crosslin, J. M., Upton, J. E., Buchman, J. L. Incidence of the beet leafhopper-transmitted virescence agent phytoplasma in local populations of the beet leaf hopper, Circulifer tenellus, in Washington State. J Insect Sci. 10 (1), 1-10 (2010).
- Finke, D. L., Denno, R. F. Intra-guild predation relaxes natural enemy impacts on herbivore populations. Ecol Entomol. 28 (1), 67-73 (2003).
- Koss, A. M., Snyder, W. E. Alternative prey disrupt biocontrol by a guild of generalist predators. Biol Control. 32 (2), 243-251 (2005).
- Elliott, N. C., et al. D-vac sampling for predatory arthropods in winter wheat. Biol Control. 38 (3), 325-330 (2006).
- Arida, G., Heong, K. Blower-Vac: a new suction apparatus for sampling rice arthropods. Int. Rice Res. New. 17, 30-31 (1992).
- Domingo, I., Schoenly, K. An improved suction apparatus for sampling invertebrate communities in flooded rice. Int. Rice Res. New. 23 (2), 38-39 (2012).
- Moorman, C. E., Plush, C. J., Orr, D. B., Reberg-Horton, C. Beneficial Insect Borders Provide Northern Bobwhite Brood Habitat. PLoS ONE. 8 (12), e83815 (2013).
- Bambaradeniya, C. N. B., et al. Biodiversity associated with an irrigated rice agro-ecosystem in Sri Lanka. Biodivers Conserv. 13 (9), 1715-1753 (2004).
- Whitehouse, M. E. A., Wilson, L. J., Fitt, G. P. A comparison of arthropod communities in transgenic Bt and conventional cotton in Australia. Environ Entomol. 34 (5), 1224-1241 (2005).
- Schoenly, K. G., et al. Fallowing did not disrupt invertebrate fauna in Philippine low-pesticide irrigated rice fields. J Appl Ecol. 47 (3), 593-602 (2010).
- de Kraker, J., van Huis, A., van Lenteren, J. C., Heong, K. L., Rabbinge, R. Identity and relative importance of egg predators of rice leaffolders (Lepidoptera : Pyralidae). Biol Control. 19 (3), 215-222 (2000).
- Hu, Y., et al. A comparative study on population development patterns of Sogatella furcifera between tropical and subtropical areas. J Asia-Pacif Entomol. 17 (4), 845-851 (2014).
- Schoenly, K. G., Domingo, I. T., Barrion, A. T. Determining optimal quadrat sizes for invertebrate communities in agrobiodiversity studies: A case study from tropical irrigated rice. Env Entomol. 32 (5), 929-938 (2003).
- Marcos, T., Mew, T. W., Borromeo, E., Hardy, B., et al. . Exploiting Biodiversity for Sustainable Pest Management. , 23-24 (2001).
- Kraker, d. J., Huis, v. A., Heong, K. L., Lenteren, v. J. C., Rabbinge, R. Population dynamics of rice leaffolders (Lepidoptera: Pyralidae) and their natural enemies in irrigated rice in the Philippines. Bull Entomol Res. 89 (5), 411-421 (1999).

