Swab Sampling Method for the Detection of Human Norovirus on Surfaces
Summary
A macrofoam based sampling methodology was developed and evaluated for the detection and quantification of norovirus on environmental hard surfaces.
Abstract
Human noroviruses are a leading cause of epidemic and sporadic gastroenteritis worldwide. Because most infections are either spread directly via the person-to-person route or indirectly through environmental surfaces or food, contaminated fomites and inanimate surfaces are important vehicles for the spread of the virus during norovirus outbreaks.
We developed and evaluated a protocol using macrofoam swabs for the detection and typing of human noroviruses from hard surfaces. Compared with fiber-tipped swabs or antistatic wipes, macrofoam swabs allow virus recovery (range 1.2-33.6%) from toilet seat surfaces of up to 700 cm2. The protocol includes steps for the extraction of the virus from the swabs and further concentration of the viral RNA using spin columns. In total, 127 (58.5%) of 217 swab samples that had been collected from surfaces in cruise ships and long-term care facilities where norovirus gastroenteritis had been reported tested positive for GII norovirus by RT-qPCR. Of these 29 (22.8%) could be successfully genotyped. In conclusion, detection of norovirus on environmental surfaces using the protocol we developed may assist in determining the level of environmental contamination during outbreaks as well as detection of virus when clinical samples are not available; it may also facilitate monitoring of effectiveness of remediation strategies.
Introduction
Human noroviruses are a leading cause of epidemic and sporadic acute gastroenteritis worldwide 1,2,3. The virus is extremely contagious and transmission occurs through direct person to person interaction or indirectly through contact with contaminated food, water or environmental surfaces. Noroviruses can be shed for extended periods and prolonged survival of the virus on environmental surfaces has been documented 1,2,3. During peak shedding, billions of virus particles are released per gram of feces, and vomit also contains a sufficient number of viral particles to cause infection 4,5,6,7,8,9,10. In addition, transfer of the virus between inanimate surfaces and human skin can occur easily 2,11,12. Hence, monitoring of environmental contamination may assist in outbreak investigations and in assessing the effectiveness of clean-up and disinfection procedures.
Several environmental sampling protocols have been described for the detection of rotavirus, coliphage MS2, feline calicivirus (FCV), and bacteriophage P22 13,14,15,16. However, the validation conditions described in these studies, including fast desiccation (<1 hr) and small surface areas (25 x 100 cm2), may not adequately represent field settings. In addition, the expected low contamination levels of environmental surfaces require protocols that are able to detect very few virus particles.
We developed a macrofoam-based surface sampling method for the detection and typing of norovirus. This method has been validated during several norovirus outbreaks. The protocol includes 1) how to collect swab samples from environmental surfaces (2) how to best maintain integrity of the samples during collection and shipping to the laboratory, and 3) laboratory testing and typing of norovirus.
Protocol
1. Swab Sampling in the Field
- Wear a clean pair of gloves.
- Measure the size of the sampling area without touching the surface using a measuring tape or ruler. Try to estimate the area as accurately as possible and fill out a report form (Supplementary Table 1).
- Check the swab kit for possible leakages and label sample transport bags and swab kits.
- Move the swab across the sampling area as follows: one stroke in horizontal direction, one stroke in vertical direction, and one stroke in a diagonal direction. Do not swab a surface area larger than 700 cm2.
- Place each swab into a tube and tighten the cap securely.
2. Storage and Transport of Swabs to the Laboratory
- Keep swabs at 4 °C for up to 48 h. If storage for longer periods is required, store the swabs at -20 °C (or -70 °C).
- Keep the tubes at 0-4 °C (i.e., use cold packs) in an insulated container during transport to the laboratory and ship within 24-48 h of collection.
3. Virus Concentration, Viral RNA Extraction and Purification
NOTE: All centrifugation steps use a table top centrifuge at 5,000 x g for 5 min at room temperature, unless stated otherwise. Be extra careful when working with the universal nucleic acid extraction (UNEX) buffer. Wear goggles or face shield.
- Label one 15 mL tube and one RNA Midi column for each sample. Include a negative extraction control in each experiment.
- To prepare the Lysis solution for 10 swabs, mix 25 ml of UNEX buffer with 25 mL of PBST (PBS pH 7.2 containing 0.02% Tween-80).
- Add 50 µL of coliphage MS2 suspension (106 PFU/µl) to 50 mL of Lysis solution.
- Place a swab in a 15 mL tube and add 5 ml Lysis solution. Mix and incubate for 10 min at room temperature.
- Add 5 ml 100% ethanol to each tube and vortex for 10 s.
- Carefully remove the swab from the 15 mL tube (it contains UNEX buffer) pressing it gently against the side of the tube to remove excess liquid and then discard the swab. The remaining volumes should be between 9-10 mL.
4. Midi Column Viral Nucleic Acid Extraction
- Carefully transfer 4.5 mL UNEX/ethanol mixture from step 3.6 onto a midi column, centrifuge and discard the filtrate.
- Load another 4.5 mL from the same mixture onto the same column, centrifuge, and discard the filtrate.
- For the first wash, add 3.5 ml of 70% ethanol onto the Midi column, centrifuge and discard the filtrate.
- For the second wash, add another 3.5 ml of 70% ethanol onto the Midi column. Centrifuge and discard the filtrate.
- For the dry-spin, centrifuge the Midi columns to remove all traces of ethanol (which can negatively affect your viral RNA recovery).
- Place the Midi column in a new 15 ml centrifuge tube. Add 250 µl elution buffer onto the Midi column; wait for 1 min before centrifuging to obtain the highest viral RNA recovery.
- Store the extracted nucleic acid at -70 °C or proceed immediately to step 5.
5. Concentration of Viral Nucleic Acid Using RNA Clean and Concentrator Kits
- Add 500 µL of RNA Binding Buffer to 250 µL of RNA eluted in step 4.7 above and vortex for 10 s.
- Add 750 µL 100% ethanol and vortex for 10 s.
- Label a spin column for each sample. Load the 750 µL sample onto a spin column.
- Centrifuge the spin columns at 12,000 x g for 1 min. Discard the flow-through.
- Load the remaining 750 µL onto a spin column and centrifuge at 12,000 x g for 1 min.
- For the pre-wash, add 400 µL RNA Prep buffer to each spin column and centrifuge at 12,000 x g for 1 min. Discard the flow-through.
- For the first wash, add 800 µL RNA Wash Buffer to each spin column and centrifuge at 12,000 x g for 1 min. Discard the flow-through.
- For the second wash, add 400 µL RNA Wash Buffer to spin column and centrifuge at 12,000 x g for 1 min. Discard the flow-through.
- For the dry-spin, centrifuge the spin column to remove all traces of wash buffer at 12,000 x g for 2 min.
- Carefully transfer spin column to a clean 1.5 mL microcentrifuge tube.
- Add 25 µL elution buffer onto the spin column and incubate for 1 min at room temperature. This will increase the recovery of viral RNA from the column.
- Collect RNA by centrifuging the spin column at 10,000 x g for 1 min.
- Either proceed directly to RT-qPCR (step 6) or store RNA at -70 °C.
6. Mutiplex RT-qPCR Detection of Genogroup I, and II Noroviruses, and Coliphage MS 2 (Supplementary Table 2)
- Clean working surfaces, pipettes, and centrifuges with RNase decontamination solution to reduce possible contamination.
- Thaw RT-qPCR reagents on ice. Thaw RNA on ice (in a separate area/room).
- Determine the number of reactions and add at least 10% more of them (e.g. if 10 reactions are needed, make master mix for 11).
- Vortex individual master mix components, except for 25x RT-qPCR enzyme, for 5 s. Carefully mix the enzyme by flicking the tube with a finger.
- Briefly centrifuge master mix components for 5 s.
- Prepare the master mix for the realtime RT-PCR detection of norovirus GI and GII according to the kit instructions and add norovirus-specific oligonucleotide primers and probes in a 1.5 ml microcentrifuge tube (Supplementary Table 3).
- Mix the master mix by pipetting 5-10 times up and down (vortexing is not recommended). Aliquot 22 µL of the master mix in each well of real time PCR 96-well plate.
- Vortex sample RNA for 5 sec, and collect by brief (5 s) centrifugation.
- Add 3 µL of sample RNA and GI and GII positive controls to the RT-qPCR plate (follow template from Table 2). Add 3 µL of nuclease-freewater in the no-template-control (NTC)wells.
- Seal the real-time plate with optical adhesive film.
- Carefully centrifuge the real-time plate at 1,300 x g for 1 min to remove any air bubbles or liquid drops that may be present in the wells.
- Set up a real-time PCR instrument and set-up the following thermocycling conditions: 1) RT step for 10 min. at 45 °C (2) activation of Taq polymerase, 10 min. at 95 °C and (3) 45 cycles of 15 s at 95 °C, and 60 s at 60 °C.
7. Quantification of Norovirus in Swab Samples
- Check the results of positive and negative controls to validate RT-qPCR results (Supplementary Table 3).
- Determine whether each standard curve meets the acceptable values given in Supplementary Table 2 and calculate the overall standard deviation for the standard curve using Equation 1.
(Equation 1) % efficiency = 100 x 10(average Ct Values-Intercept)/Slope. - If the PCR thermal cycler software does not calculate the slope for each standard curve, determine slope and R2values by regression using statistic software (e.g. Excel, SPSS, or SAS). In addition, calculate the % efficiency for standard curves following equation I
- Record the RNA copy number calculated by the thermal cycler software for all test samples based upon standard curves that meet the criteria specified in Supplementary Table 4. Rerun any samples with standard curves that do not meet the criteria or have false-positive controls. Check Ct values of coliphage MS2, which was added as an internal control to monitor PCR inhibition.
- Determine the total number of RNA copies per sample by the multiplying the RNA copy number calculated in step 7.2 by the ratio of the volume (25 to 50 µL) of total RNA eluent to that of the RNA (3 to 5 µL) used for the RT-qPCR reaction. Alternatively, calculate the RNA density by dividing the total RNA copy number by the object surface area (cm2) analyzed.
8. Genotyping of Real-time RT-PCR Positive Samples by Hemi Nested Conventional PCR Amplification
- Prepare the first round of master mix for each primer set of the RT-PCR assay (Supplementary Tables 2 , and 5).
- Add 5 µL of norovirus positive RNA to 20 µL of master mix.
- Run the RT-PCR under the following conditions: (1) RT step for 30 min at 42 °C (2) activation of Taq polymerase, 15 min at 95 °C, and (3) 40 cycles of 30 s at 95 °C, 30 s at 50 °C, and 60 sec at 72 °C. After 40 cycles, incubate for an additional 10 min at 72 °C.
- Prepare the second round of mater mix for each primer set of the RT-PCR assay (Supplementary Tables 2 and 5).
- Add 23 µL master mix and add 2 µL of first-round RT-PCR products (from first round) to each tube (ideally the first-round product should be diluted 1/10 in RNase-free water).
- Repeat step 8.3.
- Prepare a 2% agarose gel in 100 ml 1x Tris-acetate EDTA (40 mM Tris, 20 mM acetic acid, and 1 mM EDTA, at pH 8.3). After dissolving agarose by heating the mixture in a microwave oven, add 10 µL of nucleic acid stain per 100 mL of prepared gel after cooling the mixture to 60-70 °C, pour the gel and insert the combs. Let the gel settle for at least 30 min.
- Mix 15 µl of each RT-PCR product with 3 µL of 6x loading dye. Load 15 µL of each sample and electrophorese the agarose gel at 100 V for 1 h.
- Excise target RT-PCR fragments of appropriate size (330 bp for GI and 341 bp for GII) from the gel and purify RNA using a commercial gel extraction kit. The purified PCR product can now be used for Sanger sequencing.
Representative Results
Figure 1 presents a flowchart of the swab sampling protocol. This protocol consists of four main steps; 1) sample collection, 2) sample storage and transportation, 3) viral RNA purification and concentration and 4) RT-qPCR assay and genotyping.
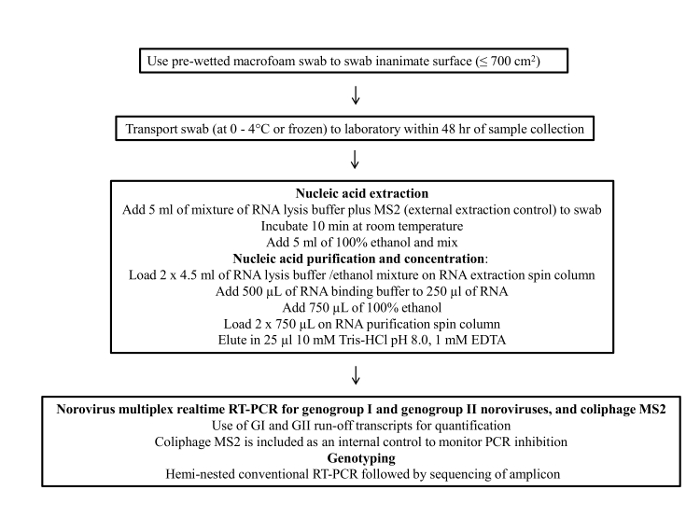
Figure 1: Flow chart of the final protocol for environmental surface sampling of norovirus Please click here to view a larger version of this figure.
Table 1 summarizes the results from 34 swab samples that were collected from a cruise ship that had reported cases of suspected norovirus gastroenteritis during a voyage. Swab samples were extracted and tested in duplicate for norovirus by multiplex real-time RT-qPCR. Seventeen (18.5%) samples tested positive including 8 samples from surfaces in cabins where passengers with norovirus symptoms had stayed and 9 from common areas on the ship. The number of genomic copies per sample were calculated from the Ct values (which ranged from 16 to 31) using a standard curve of a norovirus GII.7 RNA transcript. The median number of genome copies from swab samples in cabins was 3.6 log10(range: 2.4 – 4.5 log10 RNA copies), which was significantly higher than the genome copies from common areas (range: 1.2 – 2.1 log10 genome copies) (P <0.001). Four (23.5%) of the 17 positive swab samples were able to be genotyped, and all samples had identical GII.1 sequences.
| Location environmental swap collecteda | Sample Point Description | Average Ct value (# positive of the total number of samples tested) | Genotype | Norovirus RNA copy number per sampled areac |
| Atrium | Handrails | 34.3 (1/2) | GII | 16 |
| Cabin A | Toilet seat | 31.4 (2/2) | GII.b | 31,217 |
| Cabin A | Faucet | 37.5 (1/2) | GII | 491 |
| Cabin A | Door handle | 35.0 (2/2) | GII | 2,675 |
| Cabin A | Remote control | 38.6 (2/2) | GII.1b | 233 |
| Cabin B | Toilet seat | 33.5 (2/2) | GII.1b | 986 |
| Lido | Ice Cream Machine | 34.2 (2/2) | GII | 16 |
| Lido | Table Condiments | 35.2 (1/2) | GII | 15 |
| Lido | Table Top | 35.3 (1/2) | GII | 14 |
| Pizzeria | Counter surface | 35.7 (1/2) | GII | 14 |
| Main Galley | Fun-time Machine | 37.1(1/2) | GII | 64 |
| Vending Machine | Touchable Surfaces | 38.8 (1/2) | GII | 18 |
| Crew lounge | Keyboard Surface and Mouse | 36.8 (1/2) | GII | 80 |
| Cabin C | Faucet and door handle | 31.6 (2/2) | GII.1b | 26,458 |
| Cabin C | Telephone | 36.4 (2/2) | GII | 1,035 |
| Cabin C | Keyboard | 33.0 (2/2) | GII | 1,317 |
| Medical center | Clipboard | 36.0 (2/2) | GII | 113 |
| aCabin A, B and C has been occupied by individuals who had been clinically ill with viral gastoenteristis symptoms. | ||||
| bFour of the 17 GII-positive swab samples could be genotyped. | ||||
| cRNA copies were caluated based on a standard curve of norovirus GII.7 RNA transcripts. | ||||
| Note: above date were slightly modified from the original article6. | ||||
Table 1: Results from 34 swab samples that were collected from a cruise ship that had reported cases of suspected norovirus gastroenteritis during a voyage.
Figure 2 shows the relationship of Ct values determined by RT-qPCR and the ability to sequence these samples. In total, 127 out of 217 swab samples tested positive for GII norovirus by RT-qPCR. The samples displayed a wide range (12-40) of Ct values. In total, 29 (22.8%) of the RT-qPCR positive samples could be genotyped. Swab samples with Ct values below 27 and ranging between 28-31 were genotyped at rates of 100% and 72.2%, respectively. In contrast, only 22.0% and 1.6% of swab samples with Ct values of 32-36 and 37-40, respectively, produced hemi-nested amplicons that could be sequenced successfully.
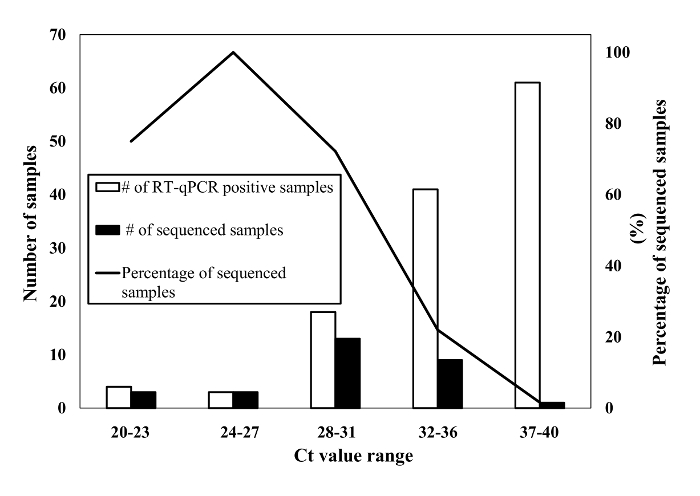
Figure 2: Results of RT-qPCR screening and sequencing of swab samples that had been collected during confirmed norovirus outbreaks. White bars represent the number of RT-qPCR positive swab samples virus. Human norovirus nucleic acids in those RT-qPCR positive samples were amplified using hemi-nested PCR assay and then sequenced for confirmation of genotyping. Black bars and line represent the number and the percentage of genotype confirmed swab samples, respectively. Please click here to view a larger version of this figure.
| Ct value range | Number of RT-qPCR positive samples | Number (%) of sequence confirmed samples a |
| 20-23 | 4 | 3 (75) |
| 24-27 | 3 | 3 (100) |
| 28-31 | 18 | 13 (72.2) |
| 32-36 | 41 | 9 (22.0) |
| 37-40 | 61 | 1 (1.6) |
| a) hemi nested PCR | ||
Table 2: RT-qPCR and genotyping results of swab samples.
| Sample Number | LOCATION | Description of surface | Surface area (cm2) | Date/time of sample collection | Clean (Y/N) | If cleaned, what disinfectant was used? | Detailed description of surface material and location |
| 1 | Room# 7-1302 | Toilet Seat | 700 cm2 | 6/26/2016; 9:00 am | No | not applicable | Toilet seat [top surfaces), and hard plastoc |
| 2 | Room #7-1330 | Telephone handle | 500 cm2 | 6/26/2016; 9:25 am | Yes | 1,000 ppm bleach | Hard plastic, rubber button |
Supplementary Table 1: Example of sample description form.
| genogroup / virus | Name oligonucleotide primer/probe | Sequence 5΄→3΄ | Reference |
| GI | Cog1F | CGY TGG ATG CGI TTY CAT GA | 17 |
| Cog1R | CTT AGA CGC CAT CAT CAT TYA C | 17 | |
| G1SKF | CTG CCC GAA TTY GTA AAT GA | 17 | |
| G1SKR | CCA ACC CAR CCA TTR TAC A | 17 | |
| Ring1E-probe | FAM – TGG ACA GGR GAY CGC – MGBNFQa | This study | |
| GII | Cog2F | CAR GAR BCN ATG TTY AGR TGG ATG AG | 17 |
| Cog2R | TCG ACG CCA TCT TCA TTC ACA | 17 | |
| Ring2-primer | TGG GAG GGC GAT CGC AAT CT | 17 | |
| G2SKR | CCR CCN GCA TRH CCR TTR TAC AT | 17 | |
| Ring2-probe | Cy5 or QUASAR 670 – TGG GAG GGC GAT CGC AAT CT – BHQ2b | 17 | |
| MS2 | MS2F | TGG CAC TAC CCC TCT CCG TAT TCA CG | 18 |
| MS2R | GTA CGG GCG ACC CCA CGA TGA C | 18 | |
| MS2P-probe | HEX – CAC ATC GAT AGA TCA AGG TGC CTA CAAGC – BHQ1c | 18 | |
| aGI TaqMan probe is 5’-labeled with 6-carboxyfluorescein (FAM) and 3’-labeled with MGBNFQ (Minor-groove Binding site) | |||
| bGII TaqMan probe is 5’-labeled with Cy5 or Quasar 670 and 3’-labeled with Black Hole quencher; Black Hole Quencher (BHQ) 2 used due to availability, BHQ 3 is preferred. | |||
| cMS2 TaqMan probe is 5’-labeled with HEX and 5’-labeled with BHQ1 | |||
Supplementary Table 2: Oligonucleotide primers and probes information.
| Component | Volume per reaction (µl) | Final concentration |
| 2x RT-PCR Buffer* | 12.5 | 1x |
| Nuclease-free water* | 1.08 | n/a |
| Detection Enhancer* | 1.67 | n/a |
| Cog 1F (10 µM) | 1 | 400 nM |
| Cog 1R (10 µM) | 1 | 400 nM |
| Ring 1E-probe (10 µM) | 0.5 | 200 nM |
| Cog 2F (10 µM) | 1 | 400 nM |
| Cog 2R (10 µM) | 1 | 400 nM |
| Ring 2-probe (10 µM) | 0.5 | 200 nM |
| MS2F (10 µM) | 0.25 | 100 nM |
| MS2R (10 µM) | 0.25 | 100 nM |
| MS2P-probe (10 µM) | 0.25 | 100 nM |
| 2x RT-PCR enzyme * | 1 | 1x |
| Master Mix volume | 22 | |
| * included in the real time RT-PCR kit | ||
Supplementary Table 3: Master mix for multiplex real-time GI/GII/MS2 norovirus RT-PCR
| Type of control | Result interpretation | ||
| Sample | Negative control | Should be negative | |
| Positive control | Should be positive | ||
| Negative sample | Sample is negative if each Ct value is undetectable for GI/GII | ||
| Positive sample | Ct values (GI/GII) of both replicates is < 38; this (arbitrarily) cut-off needs to be determined experimentally for each realtime PCR platform and kit | ||
| Tentative positive sample | Sample is tentatively positive if Ct value (GI/GII) of one replicate is <38 | ||
| Internal process control (MS2) | Samples with a threshold cycle (Ct) value of ≥32 for MS2 should be retested undiluted and 1/10 diluted. | ||
| Parameter | Acceptable value | ||
| Standard curve using norovirus RNA transcripts | R2 | >0.97 | |
| Efficiency | 90% to 115% | ||
Supplementary Table 4: Controls to include in each RT-qPCR test for detection of norovirus in environmental swab samples.
| Component | Final concentration | vol/rxn (µl) |
| 5x RT-PCR buffer | 1x | 5.00 |
| dNTP mix (10 mM) | 0.4 mM | 1.00 |
| Enzyme mix (RT and Taq) | 1.00 | |
| Forward primera | 0.5 µM | 0.50 |
| Reverse primer a | 0.5 µM | 0.50 |
| Rnase inhibitor (20 U/µl) | 20 U | 1.00 |
| Rnase-free water | 11.00 | |
| Total Volume | 20.00 | |
| aforward and reverse primer sets for GI and GII group are Cog1F + G1SKR, and Cog2F + G2SKR, respectively. | ||
| Second round RT-PCR | ||
| Component | Final concentration | vol/rxn (µl) |
| 5x RT-PCR buffer | 1x | 5.00 |
| dNTP mix (10 mM) | 0.4 mM | 1.00 |
| Enzyme mix (RT and Taq) | 1.00 | |
| Forward primerb | 0.5 µM | 0.50 |
| Reverse primerb | 0.5 µM | 0.50 |
| Rnase inhibitor (20 U/µl) | 20 U | 1.00 |
| Rnase-free water | 14.00 | |
| Total Volume | 23.0 | |
| bforward and reverse primer sets for GI and GII group are G1SKF + G1SKR, and Ring2 primer + G2SKR, respectively. | ||
| Note: above informaion was slightly modified from the original article19 | ||
Supplementary Table 5: Hemi-nested RT-PCR Master mix.
| Phase I | Field Sampling | 1. Check macrofoam swab kits (e.g., expiration date, tube leakages, and swab wetness) |
| 2. Swab surface (limit surface area to ≤100 inch2 (645 cm2)) | ||
| 3. Place swabs into the transport tubes, and tighten the caps securely to prevent leaking during shipping | ||
| 4. Place swab kit in a ziplock-bag | ||
| Phase II | Sample transport and storage | 1. Sampled swabs should be stored -70 °C (or -20 °C) |
| 2. Transporting swab samples to laboratory at 0-4 °C (cold-packs) in an insulated container and ship within 48 hr of collecting swabs | ||
| Phase III | RNA etraction | 1. Check lysis buffer (e.g., expiration data) |
| 2. Add MS2 as an internal control into lysis buffer before adding 100% ethanol | ||
| RNA purification and concentration | 3. Clean lab bench and small equipment using RNA RNase removal solution | |
| 4. Change gloves frequently during steps to avoid a RNA cross-contamination | ||
| Phase IV | RT-PCR Assay (QA/QC) | 1. Include quantified norovirus transcript RNAs (GI and GII) for each RT-qPCR assay to control for variations in Ct values for each PCR run |
| 2. Use absence of MS2 signal to monitor presence of PCR inhibitors |
Supplementary Table 6: Checklist for CDC's environmental sampling procedure.
Discussion
Noroviruses have a 50% human infectious dose between 18 and 103 virus particles20. Therefore, even low-level contamination of surfaces may pose a public health risk. Several aspects of the swab sampling protocol were evaluated including: 1) different swab materials, 2) storage condition swabs during transport, 3) viral RNA concentration, and 4) coliphage MS2 as internal extraction control.
Until recently, only the performance of swabs made from cotton, polyester, nylon and antistatic wipe) had been evaluated and proposed for field use13,14,15,21,22. Of these, cotton swabs have been recommended by the ISO 15216 protocol for detection of norovirus and hepatitis A virus from food preparation surfaces and fomites23. However, there are limited data on the testing performance of cotton swabs under field conditions with large high-touch surfaces such as doorknobs, computers, and toilet seats that have been frequently implicated in the transmission of noroviruses24,25. Additionally, the size of these environmental surfaces exceeds the capacity of fiber-tipped swabs. In our study, we demonstrated that macrofoam swabs, which have a larger swab head than the fiber-tip swabs, have higher recovery rates for human norovirus when sampling environmental surfaces with an area up to 700 cm2.
Noroviruses in solution are relatively unstable at ambient temperature and require at least refrigeration. Therefore, shipping to the laboratory should occur at 0-4 °C within 48 h after collection of the swabs. The volume of sample that can typically be tested in a PCR reaction is much smaller than that of the amount of sample after elution from the swab (3-5 μL vs. 5-10 mL) which, without concentration, significantly reduces the positivity rate. In environmental virology, polyethylene glycol (PEG) has been used successfully to concentrate viruses from large volumes of environmental waters. However, volumes up to 10 mL can be easily extracted and concentrated using Midi spin columns to volumes as low as 250 μL, and further concentrated 10-fold with high recovery using spin columns.
Although RT-qPCR is the gold standard for sensitive detection and quantification of norovirus, the sensitivity of these assays can be easily affected by the presence of PCR inhibitors that are co-extracted from the swab samples. Therefore, inclusion of an extraction control such as coliphage MS2 virus particles, in a multiplex PCR format that detects both norovirus as well as MS2, helps to monitor the presence of PCR inhibition and to assess variation among different experiments.
The test variables of a laboratory validation method are critical to determine the discriminatory power of a test method and are further translated into the method's likely field sampling performance. Taking real world field condition (e.g., delayed sampling, larger surface areas of up to 700 cm2) into account, the detection limit for norovirus was 3.4 log10(stainless steel) and 4 log10(toilet seat) copies norovirus per surface sampled6. Consequently, negative swab results are not definitive that no viral contamination has occured. Clinical and epidemiological information of norovirus illness always trumps environmental findings2,6.
Our data from a cruise ship showed that high-touch surfaces such as telephones, computer keyboards, and door handles tested positive for norovirus in cabins where people with reported viral gastroenteritis had stayed. These surfaces either became contaminated through direct contact or indirectly through droplets produced during toilet flushing or vomiting episodes6,7,8,9,10.
In conclusion, detection of norovirus on environmental surfaces using this newly developed swab protocol may assist in determining the level of environmental contamination during outbreaks, detect virus when clinical samples are not available and in monitoring of the effectiveness of cleaning practices. Fiber tipped swab (cotton and rayon) based surface sampling methods have been widely used to investigate other enteric viruses (e.g. rotavirus, and adenovirus)27,28,29. Considering the higher recovery efficiency for norovirus of macrofoam swabs over those fibers tipped swabs6, we expected that the macrofoam swabs will be able to detect other enteric viruses as well.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| Generic name for kits | ||||
| Macrofoam swab | Premoistened EnviroMax Swab kit | Puritan | 2588060PFUW | |
| RNA Lysis buffer | CDC UNEX buffer | Microbiologics | Cat No MR0501 | |
| RNA extraction spin column | Midi column | Omega Biotek | Cat No R6664-02 | |
| RNA purification spin column | Zymol RNA Clean and Concentrator kit | Zymo Research | Cat No R1016 | |
| Real time RT-PCR kit | AgPath kit One-Step RT-PCR Kit | Life Technologies | Cat No 4387391 | |
| Conventional RT-PCR kit | Qiagen one step RT-PCR kit | Qiagen kit | Cat No 210212 | |
| Gel extraction kit | Qiagen QIAquick gel extraction kit | Qiagen kit | Cat No 28704 or 28706 | |
| Coliphage MS2 | ATCC | Cat No 15597-B1 | ||
| RNA run-off transcripts | Bacteriophage MS2 (ATCC No. 15597-B1) can be cultivated using Escherichia coli (E.coli) Famp (ATCC No. 700891). | |||
| Realtime PCR platform | Applied Biosystems | Model ABI 7500 | GI and GII RNA run off transcripts were quantified spectrophotometrically at A260, diluted in diethyl pyrocarbonate-treated water to 1 × 106 copies/ μl, and stored at −80°C with 1.0 U /μl RNasin (Promega, Madison, WI). | |
| Optical 96-well reaction plate | Thermo Scientific | Cat No 4316813 | ||
| MicroAmp Clear Adhesive Film | Thermo Scientific | Cat No 4306311 |
Referências
- Isakbaeva, E. T., et al. Norovirus transmission on cruise ship. Emerg. Infect. Dis. 11, 154-158 (2005).
- Lopman, B. A., Gastañaduy, P., Park, G. W., Hall, A. J., Parashar, U. D., Vinjé, P. Environmental transmission of norovirus gastroenteritis. Curr. Opin. Virol. 2 (1), 1-7 (2011).
- Malek, M., et al. Outbreak of norovirus infection among river rafters associated with packaged delicatessen meat, Grand Canyon, 2005. Clin Infect Dis. 48 (1), 31-37 (2009).
- Atmar, R. L., et al. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 14 (10), 1553-1557 (2008).
- Glass, R. I., Parashar, U. D., Estes, M. K. Norovirus gastroenteritis. N. Engl. J. Med. 361 (18), 1776-1785 (2009).
- Park, G. W., et al. Evaluation of a New Environmental Sampling Protocol for Detection of Human Norovirus on Inanimate Surfaces. Appl. Environ. Microbiol. 81 (17), 5987-5992 (2015).
- Barker, J., Jones, M. V. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J. Appl. Microbiol. 99, 339-347 (2005).
- Tung-Thompson, G., Libera, D. A., Koch, K. L., de Los Reyes, F. L., Jaykus, L. A. Aerosolization of a Human Norovirus Surrogate, Bacteriophage MS2, during Simulated Vomiting. PloS one. 10, 0134277 (2015).
- Atmar, R. L., et al. Determination of the 50% human infectious dose for Norwalk virus. J. Infect. Dis. 209 (7), 1016-1022 (2014).
- Petrignani, M., van Beek, J., Borsboom, G., Richardus, J. H., Koopmans, M. Norovirus introduction routes into nursing homes and risk factors for spread: a systematic review and meta-analysis of observational studies. J. Hosp. Infect. 89 (3), 163-178 (2015).
- . Centers for Disease Control Prevention. Norovirus outbreak in an elementary school–District of Columbia, February 2007. MMWR. Morb. Mortal. Wkly. Rep. 56 (51-52), 1340-1343 (2008).
- Cheesbrough, J. S., Barkess-Jones, L., Brown, D. W. Possible prolonged environmental survival of small round structured viruses. J. Hosp. Infect. 35, 325-326 (1997).
- Julian, T. R., Tamayo, F. J., Leckie, J. O., Boehm, A. B. Comparison of surface sampling methods for virus recovery from fomites. Appl. Environ. Microbiol. 77, 6918-6925 (2011).
- Taku, A., et al. Concentration and detection of caliciviruses from food contact surfaces. J. Food. Prot. 65, 999-1004 (2002).
- Scherer, K., Ellerbroek, L., Schulenburg, J., Johne, R., Klein, G. Application of a swab sampling method for the detection of norovirus and rotavirus on artifically contaminated food and environmental surfaces. Food. Environ. Virol. 1 (42), 42-49 (2009).
- Herzog, A. B., et al. Evaluation of sample recovery efficiency for bacteriophage P22 on fomites. Appl. Environ. Microbiol. 78, 7915-7922 (2012).
- Vega, E., et al. CaliciNet: A Novel Surveillance Network for Norovirus Gastroenteritis Outbreaks in the United States. Emerging Infectious Diseases. 17 (8), 1389-1395 (2011).
- Rolfe, K. J., et al. An internally controlled, one-step, real-time RT-PCR assay for norovirus detection and genogrouping. J Clin Virol. 39 (4), 318-321 (2007).
- Kittigul, L., et al. Norovirus GII-4 2006b variant circulating in patients with acute Thailand during a 2006-2007 study. J. Med. Virol. 82 (5), 854-860 (2010).
- Teunis, P. F., et al. Norwalk virus: how infectious is it. J. Med. Virol. 80 (8), 1468-1476 (2008).
- Wollants, E., et al. Evaluation of a norovirus sampling method using sodium dodecyl sulfate/EDTA-pretreated chromatography paper strips. J. Virol. Methods. 122, 45-48 (2004).
- Weir, M. H., Shibata, T., Masago, Y., Cologgi, D., Rose, J. B. The Effect of Surface Sampling and Recovery of Viruses and Non-Spore Forming Bacteria on a QMRA Model for Fomites. Environ. Sci. Technol. 50 (11), 5945-5952 (2016).
- . Microbiology of food and animal feed-Horizontal method for determination of hepatitis A virus and norovirus in food using real-time RT-PCR. International Organization for Standardization (ISO). , (2013).
- Huslage, K., Rutala, W. A., Sickbert-Bennett, E., Weber, D. J. A quantitative approach to defining “high-touch” surfaces in hospitals. Infect. Control. Hosp. Epidemiol. 31 (8), 850-853 (2010).
- Wu, H. M., et al. A norovirus outbreak at a long-term-care facility: the role of environmental surface contamination. Infect. Control. Hosp. Epidemiol. 26 (10), 802-810 (2005).
- Ikner, L. A., Gerba, C. P., Bright, K. R. Concentration and recovery of viruses from water: a comprehensive review. Food Environ. Virol. 4 (2), 41-67 (2012).
- Gallimore, C. I., et al. Environmental monitoring for gastroenteric viruses in a pediatric primary immunodeficiency unit. J. Clin. Microbiol. 44 (2), 395-399 (2006).
- Ganime, A. C., et al. Dissemination of human adenoviruses and rotavirus species A on fomites of hospital pediatric units. Am J Infect Control. , (2016).
- Verani, M., Bigazzi, R., Carducci, A. Viral contamination of aerosol and surfaces through toilet use in health care and other settings. Am J Infect Control. 42 (7), 758-762 (2014).

