Protocols for Visualizing Steroidogenic Organs and Their Interactive Organs with Immunostaining in the Fruit Fly Drosophila melanogaster
Summary
We describe a protocol for dissection, fixation, and immunostaining of steroidogenic organs in Drosophila larvae and adult females to study steroid hormone biosynthesis and its regulatory mechanism. In addition to steroidogenic organs, we visualize the innervation of steroidogenic organs as well as steroidogenic target cells such as germline stem cells.
Abstract
In multicellular organisms, a small group of cells is endowed with a specialized function in their biogenic activity, inducing a systemic response to growth and reproduction. In insects, the larval prothoracic gland (PG) and the adult female ovary play essential roles in biosynthesizing the principal steroid hormones called ecdysteroids. These ecdysteroidogenic organs are innervated from the nervous system, through which the timing of biosynthesis is affected by environmental cues. Here we describe a protocol for visualizing ecdysteroidogenic organs and their interactive organs in larvae and adults of the fruit fly Drosophila melanogaster, which provides a suitable model system for studying steroid hormone biosynthesis and its regulatory mechanism. Skillful dissection allows us to maintain the positions of ecdysteroidogenic organs and their interactive organs including the brain, the ventral nerve cord, and other tissues. Immunostaining with antibodies against ecdysteroidogenic enzymes, along with transgenic fluorescence proteins driven by tissue-specific promoters, are available to label ecdysteroidogenic cells. Moreover, the innervations of the ecdysteroidogenic organs can also be labeled by specific antibodies or a collection of GAL4 drivers in various types of neurons. Therefore, the ecdysteroidogenic organs and their neuronal connections can be visualized simultaneously by immunostaining and transgenic techniques. Finally, we describe how to visualize germline stem cells, whose proliferation and maintenance are controlled by ecdysteroids. This method contributes to comprehensive understanding of steroid hormone biosynthesis and its neuronal regulatory mechanism.
Introduction
In multicellular organisms, a group of cells is endowed with a specialized function in their biogenic activity that is essential for the whole body. To fulfill their missions, each tissue or organ expresses a series of genes related to their functions and communicates with other tissues to orchestrate their activities in the context of development. To characterize such specialized cellular functions and inter-organ interactions, we need to specify a group of cells along with other types of cells being kept intact in the multicellular architecture.
One example of such specialized organs is a steroidogenic organ, where many biosynthetic enzymes mediate the conversion steps from cholesterol to active steroid hormones1. Most of these enzyme genes are specifically expressed in steroidogenic organs, and the biosynthesis pathway is tightly regulated by many external stimuli via humoral inputs and neuronal inputs. Once synthesized, steroid hormones are secreted into the hemolymph and are targeted to many tissues and organs for regulating the expression of a variety of genes2. Therefore, the action of a steroid hormone induces a systemic response to maintain homeostasis, growth, and reproduction.
To investigate the functions of steroid hormone biosynthesis and the pleiotropic actions of steroid hormones, Drosophila melanogaster can be utilized as a suitable model system. During the larval stages, the insect steroid hormone, ecdysteroid, is biosynthesized in a specialized endocrine organ called the prothoracic gland (PG)3. In the PG, several ecdysteroidogenic enzymes specifically catalyze the multiple conversion steps from cholesterol to ecdysone, which controls molting and metamorphosis at the appropriate developmental stages4. Therefore, a dynamic change in ecdysteroid titer is regulated by many signaling pathways in response to environmental cues. On the other hand, in the adult stage, ecdysteroid plays essential roles in physiology, including reproduction, sleep, memory, and lifespan5,6,7,8. It is known that ecdysteroid is actively biosynthesized in the ovary, regulating the progression of oogenesis6,7,8,9,10,11. Recently we have reported that the number of germline stem cells (GSCs) is affected by ecdysteroid and sex peptide signaling in response to mating stimuli12.
Powerful tools of D. melanogaster genetics and cell biology, including well-annotated genome information, binary gene expression systems, and transgenic RNAi techniques, have enabled us to identify genes essential to ecdysteroid biosynthesis in the PG and the ovary13,14,15. Once the ecdysteroidogenic genes are identified, the transcriptional regulation of these genes and the dynamic localizations of gene products can be examined in the biosynthesis pathway16. For this purpose, quantitative-reverse transcription-PCR, RNA in situ hybridization, and immunohistological analysis are conducted. The application of these techniques includes a challenging task; the elaborate dissection of the PG or the ovary. In particular, the PG of the fruit fly is relatively smaller than that of other insects (e.g. the silkworm and the blow fly), so one needs to practice the vital skill of fruit fly dissection for sampling. Furthermore, both ecdysteroidogenic organs receive innervations from the central nervous system (CNS)17,18,19,20. Thus, for accurate anatomical analyses, the ecdysteroidogenic organs should be kept intact along with the CNS and other organs, not to disrupt their neuronal connections.
Here we provide protocols for the dissection and visualization of steroidogenic organs in D. melanogaster. Learning the dissection technique is the key starting point for these experiments. In addition, one can successfully label the steroidogenic organs as well as their interactive organs with several antibodies and GAL4 driver lines. Taking advantage of these techniques, materials, and genetics, one can study the comprehensive mechanisms of steroid hormone biosynthesis.
Protocol
NOTE: The overall scheme of protocols is shown in Figure 1.
1. The Dissection of the Larval Ring Gland (RG)
NOTE: In D. melanogaster, which belongs to cyclorrhaphous Diptera, the PG is within a composite endocrine organ called the ring gland (RG, Figure 2D). Since it is unfeasible that the PG is surgically separated from other types of cells (discussed later), a practical target is to isolate an intact, undamaged RG by dissection.
- Preparation of larvae at the appropriate developmental stages
NOTE: To synchronize the developmental stages of D. melanogaster larvae, it is necessary to collect eggs within a narrow time window and to collect larvae at the appropriate times.- Collect eggs for 2 h on a grape-juice agar plate at 25 °C.
- Collect newly-hatched 1st instar larvae under a dissecting microscope with forceps and transfer them to a small vial containing mashed standard yeast/cornmeal fly food.
NOTE: The age of larvae is represented by "hours after egg laying (hAEL)", "hours after hatch (hAH)", or "hours after L3 ecdysis (hAL3E)". - At the appropriate time point, collect the staged larvae with a disposable plastic loop to avoid undesirable injury. To minimize a difference in developmental progression of the 3rd instar larvae, collect the 2nd instar larvae at 70 hAEL (48 hAH) and allow them to molt in 2 h intervals. Then, collect the 3rd instar larvae within 2 h after the L3 ecdysis; this procedure gives 0-2 hAL3E larvae.
- Transfer the staged larvae to a dish filled with phosphate buffered saline (PBS).
- Rinse the larvae with PBS to remove residual food from the body.
- Coarse dissection of larvae under a dissecting microscope
- Hold the mouth hook of a larva with forceps. Sever the larval body at the anterior one-third of the body length using micro scissors.
- Hold the cut end of the anterior larval body with one pair of forceps. Using the other pair of forceps, gently push the tip of the head toward the inside of the body; this procedure turns the larval body inside out.
NOTE: This step can be skipped for the dissection of 1st instar larvae. - Repeat steps 1.2.1-1.2.2 with additional larvae for 5-10 min.
NOTE: The number of larvae should be equal to or less than 20 to save time for dissection.
- Fillet dissection of larvae
NOTE: This method allows keeping the position of tissues to remain intact.- Leave larvae in water only for 1 h. The larvae are immobilized due to asphyxiation.
- Put a larva dorsal side up in a drop of PBS on a silicon-coated dish, with its dorsal side up.
NOTE: The dorsal side has 2 tracheal tubes running longitudinally. - Under a dissecting microscope, insert an insect pin into the anterior tip of the larva using forceps. Stretch the larval body to the posterior side and put a second pin into the posterior tip of the larva.
NOTE: In addition to insect pins, a needle made from a tungsten wire can be useful21. A needle can be embedded in a pipette tip through heating for use as a dissecting needle. - Under a dissecting microscope, make a small incision near the tail with micro scissors. From the incision, cut the dorsal cuticle longitudinally along the dorsal midline toward the head. Be careful not to damage tissues under the cuticle.
- Stretch the bodywall cuticle on each side and place 4 pins at each corner of the dissected bodywall.
- Remove a portion of anterior fat body with forceps, to expose the brain-RG complex be exposed on the surface. Remove a pair of salivary glands, if necessary.
- Fixation and staining of tissue with immunohistochemistry
- Put the anterior one-third of the larval bodies (step 1.2.2) into a 1.5 mL microtube filled with 500 µL the fix solution (4% paraformaldehyde in PBS). Fix less than 20 samples at one time, otherwise PBS attached to the fixed samples may cause an unfavorable dilution of the fixative. For fillet dissection, remove a drop of PBS and then add a drop of fix solution.
NOTE: For fix solution, either 3.7% formaldehyde or 4% paraformaldehyde may be used. - Incubate the dissected tissues in the fix solution for 30 min at room temperature (RT) or alternatively for 2 h at 4 °C.
- Replace the fix solution with 500 µL PBS and quickly rinse the samples 3 times. Wash the samples with 500 µL 0.3% PBT (PBS + 0.3% octylphenol ethoxylate) for 15 min on a rocker at RT. For fillet dissection, remove the insect pins and transfer the samples into a 1.5 mL microtube.
NOTE: For increasing the permeability of antibodies into tissues, the samples are treated with 500 µL 2.0% PBT for 1-2 h on a rocker at RT. - Replace PBT with 500 µL blocking solution (2% bovine serum albumin in PBT). Incubate the samples for 1.5 h on a rocker at RT.
- Replace the blocking solution with 50 µL the primary antibody solution, i.e. antibodies diluted in the blocking solution.
- Incubate the samples overnight on a rocker at 4 °C.
- Replace the primary antibody solution with 500 µL 0.3% PBT and quickly rinse the samples 5 times. Wash the samples 3 times with 500 µL 0.3% PBT for 15 min each on a rocker at RT.
- Replace 0.3% PBT with 50 µL the secondary antibody solution (dye-conjugated antibodies diluted in the blocking solution). For nuclear staining, 0.5 µL 4',6-diamidino-2-phenylindole (DAPI, 0.1 mg/mL stock solution) is added to 50 µL solution. Incubate the samples on a rocker for 2 h at RT or alternatively at 4 °C overnight.
NOTE: Keep the samples in the dark. - Replace the antibody solution with 500 µL 0.3% PBT and rinse 5 times. Wash the samples 3 times with 500 µL 0.3% PBT for 15 min each at RT.
- Put the anterior one-third of the larval bodies (step 1.2.2) into a 1.5 mL microtube filled with 500 µL the fix solution (4% paraformaldehyde in PBS). Fix less than 20 samples at one time, otherwise PBS attached to the fixed samples may cause an unfavorable dilution of the fixative. For fillet dissection, remove a drop of PBS and then add a drop of fix solution.
- Fine dissection of the brain-RG complex containing the PG and mounting
- Use a disposable pipet to transfer the immunostained samples to a dish filled with 0.3% PBT.
NOTE: To avoid inconvenient bubbles during dissection and mounting, 0.3% PBT can be replaced with PBS. - Under a dissecting microscope, hold the cuticle or mouth hook with one pair of forceps. Using a 27 G needle attached to a 1 mL syringe as a "knife", cut the anterior tip of the esophagus and eye discs to remove the brain-RG-eye disc complex from body cuticle.
- Separate the esophagus and gut from the brain-RG-eye disc complex with forceps. Since the esophagus passes through the brain above the ventral nerve cord (VNC), pull out the gut to the posterior side.
NOTE: To remove extra imaginal discs from the samples, cut the connections between the brain, eye discs, and leg discs with a needle knife. - Repeat the steps 1.5.1-1.5.4 for other samples.
NOTE: To prevent tissue debris from sticking to the samples, transfer each completed sample to a different clean dish filled with 0.3% PBT or PBS. - Transfer the samples to the center of a clean glass slide with a micropipette.
NOTE: For 2nd or 3rd instar larvae, the end of a micropipette tip should be truncated with a razor blade. - Under a dissecting microscope, align individual samples with their dorsal side up by using forceps.
NOTE: The RG is located on the dorsal side of the brain (Figure 2A). This alignment facilitates the specification of individual samples during imaging. - Tilt a glass slide and wipe off as much excess PBT as possible. Put a drop of mounting reagent on a side of the slide. Place the edge of a cover slip from the other side of the drop and put the cover slip on the samples slowly with forceps.
NOTE: This procedure prevents the samples from moving outside the cover slip. - Store the samples at 4 °C. Keep the samples in the dark.
- Use a disposable pipet to transfer the immunostained samples to a dish filled with 0.3% PBT.
2. The Dissection of the Ovary in Adult Females
- Preparation of adult females
- Feed female flies with standard yeast/cornmeal fly food for 3-4 days to fatten up the ovary. Maintain at 25 °C.
- Dissection of the adult female ovary
- Anesthetize female flies with CO2 gas and cut off their heads.
- Transfer fly bodies to a 3 cm dish filled with PBS.
- Under a dissecting microscope, hold the thorax on the dorsal side using forceps. Grasp the abdominal segment A5 or A6 with the other forceps and peel away the abdominal cuticle toward the posterior side. Wipe off the sticky cuticle from the tip of the forceps.
- Pinch an oviduct and take out the ovary from the body.
- Comb and spread the tips of the ovary with forceps.
NOTE: This operation improves the efficiency of immunostaining.
- Dissection of adult female brain, VNC and reproductive organs.
NOTE: This method allows the innervation of the ovary to be kept intact.- Anesthetize female flies with CO2 gas and transfer them to a silicon-coated dish filled with PBS.
- Under a dissecting microscope, hold the proboscis and peel away the head cuticle to expose the brain with forceps. Remove the trachea attached to the brain.
NOTE: The brain is a white and rounded structure. The brain connects to the VNC in the thorax. - Hold the thorax on the dorsal side and cut off the legs and wings with one pair of forceps. Using the other pair of forceps, peel away the thorax ventral cuticle from the bases of the legs. Once the VNC is exposed, remove the residual dorsal cuticle and muscles attached to the VNC using forceps. Be careful not to damage the connection between the brain and the VNC.
- Use forceps to peel away the abdominal cuticle and expose the ovary and their interactive organs including neurons, the crop, the gut, the oviduct, the uterus, and the accessary gland.
- Remove the residual tissues including the trachea and the fat body using forceps.
- Fixation and staining of tissue with immunohistochemistry
- Transfer approximately 8-10 pairs of the ovaries to a 1.5 mL microtube filled with 250 µL the fix solution (4% paraformaldehyde in PBS) and incubate the samples for 30 min at RT.
- Replace the fix solution with 500 µL PBS and quickly rinse the samples 3 times.
- Wash the samples 2 times with 500 µL 0.1% PBT for 5 min each at RT.
- Replace PBT with 50 µL blocking solution. Incubate the samples for 1 h on a rocker at RT.
- Replace the blocking solution with 50 µL the primary antibody solution.
- Incubate the samples overnight on a rocker at 4 °C.
- Replace the primary antibody solution with 500 µL 0.1% PBT. Wash the samples 3 times with 500 µL 0.1% PBT for 15 min each on a rocker at RT.
- Replace 0.1% PBT with 50 µL secondary antibody solution. For nuclear staining, add 0.5 µL DAPI to 50 µL the solution. Incubate the samples for 2 h on a rocker at RT.
- Replace the secondary antibody solution with 500 µL 0.1% PBT. Wash the samples 3 times with 500 µL 0.1% PBT for 15 min each on a rocker at RT.
NOTE: The washing procedure can be done at 4 °C overnight. Keep the samples in the dark.
- Mounting the samples onto glass slides
- Transfer the samples to a glass slide with 0.1% PBT using a micropipette.
NOTE: To avoid inconvenient bubbles during dissection and mounting, 0.1% PBT can be replaced with PBS. - For the ovary, separate the strings of the ovarioles from each other with forceps under a dissecting microscope. Do not injure the tips of the ovarioles containing the germaria. For the brain-VNC-reproductive organ complex, remove the residual cuticle and arrange the position on a glass slide.
- Wipe off as much excess PBT as possible and put a drop of mounting reagent in the center of the samples. Place a cover slip slowly using forceps.
- Store the samples at 4 °C. Keep the samples in the dark.
- Transfer the samples to a glass slide with 0.1% PBT using a micropipette.
3. Imaging with a Confocal Laser Scanning Microscope
- Observe the samples under the microscope and bring the steroidogenic organs in the field of view. Once the view is fixed, switch the imaging system to the image acquisition mode.
NOTE: The 10-20X objective lenses are used to obtain the images of the brain-RG complex or the whole ovary. Alternatively, the 40-63X objective lenses (water or oil immersion) are used for visualizing the subcellular localization of proteins in GSCs or the neuronal innervations of the PG and the ovary. - Set up the parameters of image acquisition on the attached software. Select the combination of fluorescence (e.g. GFP and RFP). By fast scanning procedure, adjust the laser power, the sensitivity of detectors (Gain/Offset), and the size of the pinhole.
NOTE: To acquire images at high quality, the scanning laser power and the sensitivity of detector (Gain) should be optimized for each sample. To outline the shape of the tissues, a transmitted-light image can be taken in addition to a fluorescent image. - Take Z stacks of the images. During a continuous fast scan, move the focal plane with the focus drive of the microscope and set the starting position and the end position. The number of slices and the interval distance between slices are adjusted accordingly.
- Select the scan speed, the scan method, and the bidirectional scan mode.
- "Start the real scans" for image acquisition.
- Save the image with the format of the attached software.
NOTE: The original image files contain the information of image acquisition parameters and scales. Exported images can be processed using an image processing software on the computer22.
Representative Results
We used the above protocols to visualize steroidogenic organs and their interactive organs in D. melanogaster larvae and adult females. The overall scheme of protocols is shown in Figure 1.
The RG, including the PG (Figure 2D), is smaller and more transparent than the brain and is located at the anterior-dorsal side of the brain (Figure 2A-C and 3A-E). To label the PG cells, several groups have generated various types of antibodies against ecdysteroidogenic enzymes (i.e., Neverland23, Spookier24, Shroud20, Phantom25, Disembodied25, and Shadow26). Among them, anti-Shroud (Sro) antibody is reliably used to label the PG by immunostaining (Figure 2C, 2E, and 3C). Alternatively, the binary gene expression system, GAL4/UAS system27, can be used to express fluorescent protein genes in PG cells. Through the promoter analysis of the ecdysteroidogenic gene phantom (phm), the PG-specific promoter can induce gene expression exclusively in the PG28. Therefore, the PG cells can be specifically visualized by expressing GFP or RFP under the control of phm-GAL4#22 (Figure 3D).
To visualize the neuronal connection between the PG and the brain, a group of neurons can be labeled with mCD8::GFP under the control of various GAL4 drivers and antibodies (Figure 2C, 2E, 3D, and 3E). The FlyLight database of GAL4 line collections is optimized for labeling neurons29. Among them, GMR45C06-GAL4 labels PG-projecting neurons (Figure 2C and E). The immunostaining of GFP-expressing larvae with anti-GFP antibody is effective in enhancing GFP signals. Furthermore, TRH-GAL4>mCD8::GFP larvae show the stomatogastric nervous system, in which serotonergic neurons project to not only the PG, but also to the proventriculus (PV, insect foregut) and the pharyngeal muscles (PM) (Figure 3D and E)20,30. Therefore, it is critical to maintain the position of the RG, the brain, and the other surrounding tissues during fillet dissections (Figure 3).
In adult females, ovarian ecdysteroid affects many aspects of oogenesis, such as GSC proliferation, cyst differentiation, egg chamber growth, and stress response11. As in the PG, ecdysteroidogenic enzymes in the ovary are also visualized with the specific antibodies described above12,31. As a downstream event upon which ecdysteroids act, the number of GSCs in the germarium is our focus (Figure 4). Although the germarium is an assembly of multiple cell types, GSCs are specified by immunostaining with two GSC marker antibodies, 1B1 and DE-cadherin32. To visualize the innervation of the ovary, the ovary is dissected along with the brain, VNC, gut, crop, uterus, and spermatheca (Figure 5). Neurons are visualized by mCD8::GFP under the control of nSyb-GAL4, a pan-neuronal driver (Figure 5B and D). Muscles around the ovary, uterus, and gut are stained with dye-conjugated phalloidin.
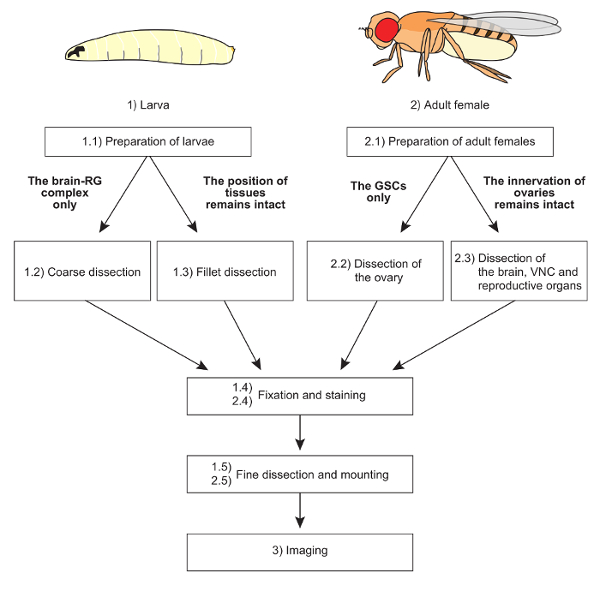
Figure 1: The overall scheme of protocols. Two distinct dissection methods are applicable to larvae and adult females, depending on the purpose of the experiments. The mounting methods are also devised according to sample conditions. Fixation, staining, and imaging techniques are basically common to all samples. Please click here to view a larger version of this figure.
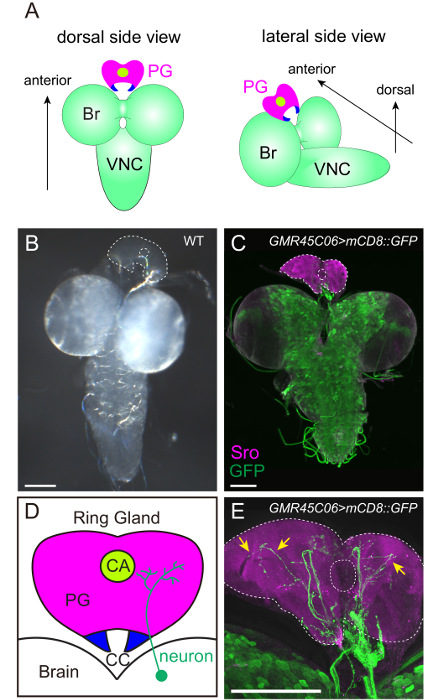
Figure 2: Visualization of the PG and the PG-projecting Neurons. (A and B) The brain-ring gland (RG) complex of the wild-type 3rd instar larva. In (B), the PG is outlined by dotted lines. The filamentous structure between the brain and the RG is the trachea. (C–E) The innervation of the PG was visualized with mCD8::GFP driven by GMR45C06-GAL4 in FlyLight collection. The PG and GFP-positive neurons were labeled with anti-Sro antibody (magenta) and anti-GFP antibody (green), respectively. The fluorescent image is merged with the transmitted-light image to specify the outline of tissues. In (D), the PG, the corpora allata (CA) and the corpora cardiaca (CC) are illustrated. The PG-projecting neurons are more prominent in the high-power image with 40X objective lens (arrows in E) than the low-power image with 10X objective lens (C). Scale bar = 100 µm. Please click here to view a larger version of this figure.
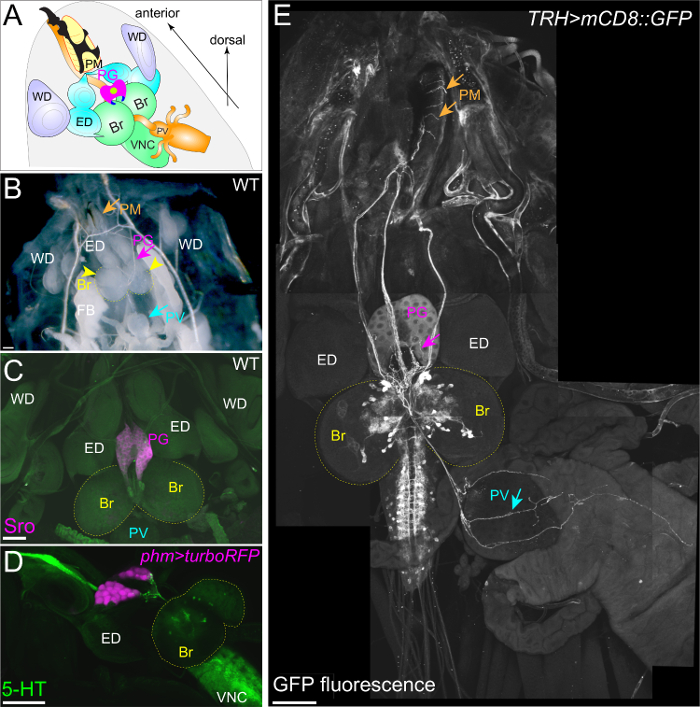
Figure 3: The Stomatogastric Nervous System Projects to the PG, the PV, and the PM. (A and B) The fillet dissection of a wild-type 3rd instar larva. The positions of the PG, brain (Br), ventral nerve cord (VNC), eye discs (ED), wing discs (WD), pharyngeal muscle (PM), and proventriculus (PV) remain intact. (C) The PG was exclusively labeled with anti-Sro antibody (magenta). (D) The PG was labeled with turboRFP driven by phm-GAL4#22. Serotonergic neurons were labeled with anti-5HT antibody (green). (E) The stomatogastric nervous system was visualized with mCD8::GFP driven by TRH-GAL4. Serotonergic neurons project to the PG, the PM, and the PV (arrows). The Scale bar = 100 µm. Please click here to view a larger version of this figure.
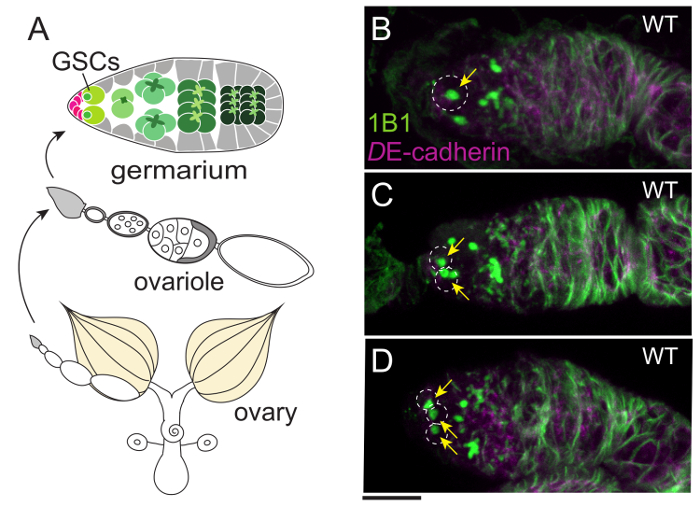
Figure 4: The Germaria of Wild-type Flies Containing One, Two, or Three GSCs. (A) An overview of the female reproductive organ. An ovary is composed of 16-20 ovarioles that are strings of egg chambers. The germarium, where GSCs are located, is at the tip of each ovariole. (B–D) One, two, or three GSCs are located in each germarium (white dotted circles) of the wild-type females. GSCs are stained with 1B1 antibody (green), which labels a spherical structure called the spectrosome (arrows) and a membranous cytoskeletal structure known as the fusome. The cell boundaries are visualized with anti-DE-cadherin antibody (magenta). The Scale bar = 10 µm. Please click here to view a larger version of this figure.
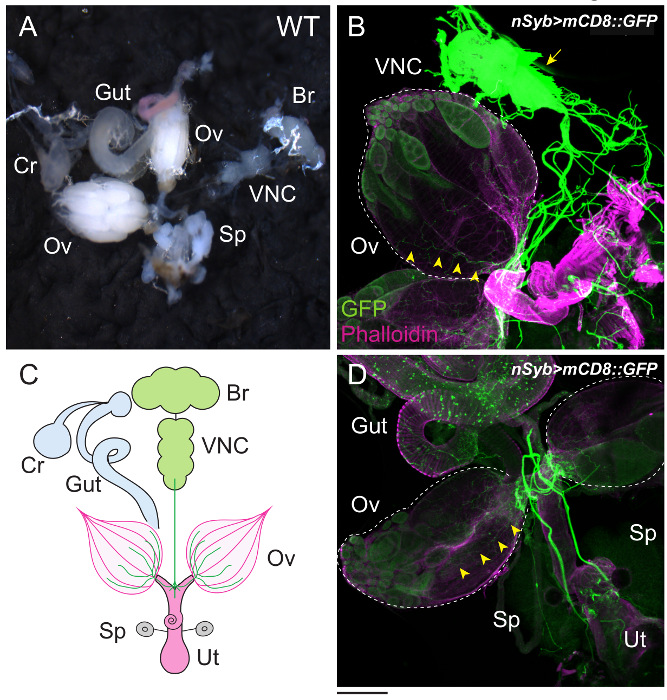
Figure 5: The Female Reproductive Organs and their Interactive Organs. (A) The ovary (Ov), gut (Gut), crop (Cr), brain (Br), ventral nerve cord (VNC), and spermatheca (Sp) were dissected under the microscope. (B–D) The innervation of the ovary was visualized with mCD8::GFP driven by nSyb-GAL4. GFP-positive neurons extend from the VNC (arrow), projecting to the surface of the ovaries (arrowheads). The ovaries are outlined by dotted lines. The samples were stained with anti-GFP antibody (green) and dye-conjugated phalloidin (magenta). Phalloidin associates with the filamentous actin of muscles around the ovaries (Ov) and uterus (Ut). The illustration is shown in C. The Scale bar = 200 µm. Please click here to view a larger version of this figure.
Discussion
We studied ecdysteroid biosynthesis and its regulatory mechanism in D. melanogaster, and devised a protocol for dissection and immunostaining. The timing of ecdysteroid biosynthesis is affected by environmental cues through neuronal inputs33, so it is essential to maintain the innervation of the ecdysteroidogenic organs along with the brain, VNC, and other tissues during dissection.
As described above, the D. melanogaster PG forms a complex endocrine organ RG with the corpora allata (CA) and the corpora cardiaca (CC) (Figure 2D). While the PG produces ecdysteroids, the CA and the CC produce juvenile hormones and adipokinetic hormone, respectively. This anatomical property has been an impediment to researchers studying ecdysteroidogenesis in D. melanogaster, as compared to other insects. However, in the past decades, fly genetics has allowed us to identify many ecdysteroidogenic genes that are specifically expressed in the PG4. These genes are also expressed in nurse cells or follicle cells of the ovary34,35. Now one can use a unique set of gene expression profiles in ecdysteroidogenic organs, making it possible to specify ecdysteroidogenic cells by immunostaining and transgenic techniques.
During dissection, it is essential to use fine forceps with sharp edges. Prior to dissection, the edges of the forceps can be sharpened with an Arkansas grinding stone to bring the edges together without any gaps. During dissection, debris or the residual tissues, such as the fat body, the gut, and the imaginal discs, should be removed as much as possible. In particular, pieces of the fat body easily stick to the tissue of interest. In addition, the mounting of the samples onto glass slides should also be performed carefully. A complex of tissues may be disarranged when a cover slip is placed on the samples in sticky mounting medium. Therefore, a sufficient number of samples must be dissected, and suitable samples must be chosen for imaging, such as those in which the RG or the ovary is on the uppermost side and the neuronal connections remain intact.
To perform successful immunostaining and to obtain the reproducible results, it is critical to maintain the same conditions for the sampling and handling procedures. The extent of immunostaining highly depends on the condition of individual animals at the time of sampling and fixation. For example, the age of the animals, the rearing temperatures, nutrient condition, and the dissection time should be carefully considered. To minimize handling errors of fixation in multiple experiments, we carefully keep almost equal number of samples to coordinate the dissection time and fixation reaction times. For the fixation solution, 4% paraformaldehyde or 3.7% formaldehyde is used. Because paraformaldehyde is quickly degraded over time, its powder is usually dissolved to make 10% stock solution in PBS, and aliquots are stored at -20 °C. The aliquots are unfrozen to make the fresh 4% fix solution prior to every dissection. Moreover, the extensive wash steps of samples reduce the background non-specific staining.
In the step of image acquisition, we follow the manufacturer's protocol for confocal microscopy. The microscopy system provides a user-friendly interface for researchers or students, such that the optimal sets of excitation/emission filters are automatically selected when the types of fluorophore are specified. While the microscopy system is easy to use, one needs to optimize the parameters of image acquisition on each sample or each focal plane, by changing the detector gain, scan speed, scan numbers, and the pinhole size.
One of the limitations of this technique is that immunostaining largely depends on the quality of antibodies. While antibodies against Noppera-bo, Neverland, and Shroud were successfully generated, we failed to generate several antibodies against other ecdysteroidogenic gene products, such as Ouija board36. To overcome this issue, the knock-in technique can be applied to insert a HA-tag in the gene locus of ouija board using the CRISPR/Cas9 system. By using such transgenic techniques in combination with immunostaining, at least 3-4 proteins can be simultaneously visualized in the PG or the ovary. In contrast to protein localization, however, a method to visualize endogenous ecdysteroids by immunostaining has not been established. Once anti-ecdysteroid antibody is available for immunostaining, the subcellular localizations of ecdysteroid biosynthesis and the transport of ecdysteroid can be investigated in the future.
Another limitation is that the temporal dynamics of ecdysteroidogenesis cannot be examined in fixed tissues. Considering that the ecdysteroid titer is fluctuated along the developmental stages, the activity of ecdysteroidogenic organs and the projecting neurons should be temporally regulated in response to various environmental stimuli. A recent study monitored the Ca2+ dynamics of the PG cells using live imaging techniques37. For future applications, we are currently developing a protocol for the live imaging of PG or cultured ovaries along with their projecting neurons. With respect to existing methods, this new protocol aims to visualize the activity of neurons and their target organs simultaneously. Once the activity of neurons can be monitored with the Ca2+ indicator GCaMP or Cameleon38, it can be manipulated with optogenetic or thermogenetic approaches at the appropriate times39. These methods contribute to a comprehensive understanding of steroid hormone biosynthesis and its neuronal regulatory mechanism.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank Reiko Kise and Tomotsune Ameku for their technical support for this work. We are also grateful to Kei Ito, Olga Alekseyenko, Akiko Koto, Masayuki Miura, the Bloomington Drosophila Stock Center, KYOTO Stock Center (DGRC), and the Developmental Studies Hybridoma Bank for stocks and reagents. This work was supported by grants to Y.S.N. from JSPS KAKENHI Grant Number 16K20945, The Naito Foundation, and Inoue Science Research Award; and by a grant to R.N. from MEXT KAKENHI Grant Number 16H04792.
Materials
| egg collection | |||
| tissue culture dish (55 mm) | AS ONE | 1-8549-02 | for grape-juice agar plates |
| collection cup | HIKARI KAGAKU | ||
| yeast paste | Oriental dry yeast, Tokyo | ||
| 100% grape juice | Welch Food Inc. | ||
| rearing larvae | |||
| small vials (12ml, 40×23.5 mm, PS) | SARSTEDT | 58.487 | |
| disposable loop | AS ONE | 6-488-01 | |
| standard fly food | the recepi us on the website of Blooington stock center. | ||
| dissection | |||
| dissecting microscope | Carl Zeiss | Stemi 2000-C | |
| dissecting microscope | Leica | S8 AP0 | |
| tissue culture dish (35 x 10 mm, non-treated) | IWAKI | 1000-035 | |
| Sylgard | TORAY | coarting silicon inside dishes | |
| Terumo needle (27G, 0.40 x 19 mm) | TERUMO | NN-2719S | A "knife" to cut the tissue |
| Terumo syringe, 1ml | TERUMO | SS-01T | |
| forceps, Inox, #5 | Dumont, Switzerland | ||
| insect pin (0.18 mm in diameter) | Shiga Brand | for fillet dissection | |
| micro scissors | NATSUME SEISAKUSHO CO LTD. | MB-50-10 | |
| fixation | |||
| ultrapure water | Merck Millipore | ||
| phosphate buffered saline (PBS) | |||
| Formaldehyde | Nacalai tesque | 16222-65 | |
| Paraformaldehyde | Nacalai tesque | 02890-45 | |
| Triton-X100 | Nacalai tesque | 35501-15 | |
| microtubes (1.5 ml) | INA OPTIKA | CF-0150 | |
| Incubation | |||
| As one swist mixer TM-300 (rocker) | As one | TM-300 | rocker |
| Bovine Serum Albumin | SIGMA | 9048-46-8 | |
| primary antibody | |||
| anti-Sro (guinea pig), 1:1000 | |||
| anti-GFP (rabbit), 1:1000 | Molecular Probes | A6455 | Shimada-Niwa ans Niwa, 2014 |
| anti-GFP (mouse mAb, GF200), 1:100 | Nakarai tesque | 04363-66 | |
| anti-5HT (rabbit), 1:500 | SIGMA | S5545 | |
| anti-Hts 1B1 (mouse) | Developmental Studies Hybridoma Bank (DSHB) | 1B1 | |
| anti-DE-cadherin (rat), 1:20 | DSHB | DCAD2 | |
| anti-nc82 (mouse), 1:50 | DSHB | nc82 | |
| secondary antibody | |||
| Goat anti-Rabbit IgG (H+L) Secondary Antibody, Alexa Fluor 488 conjugate | Life Technologies | A-11008 | |
| Goat anti-Mouse IgG (H+L) Secondary Antibody, Alexa Fluor 488 conjugate | Life Technologies | A-11001 | |
| Goat anti-Rat IgG (H+L) Secondary Antibody, Alexa Fluor 546 conjugate | Life Technologies | A-11081 | |
| Goat anti-Guinea Pig IgG (H+L) Secondary Antibody, Alexa Fluor 555 conjugate | Life Technologies | A-21435 | |
| Alexa Fluor 546 dye-conjugated phalloidin | Life Technologies | A-22283 | |
| Mounting reagents | |||
| Micro slide glass | Matsunami Glass Ind.,Ltd. | SS7213 | |
| Square microscope cover glass | Matsunami Glass Ind.,Ltd. | C218181 | |
| FluorSave reagent (Mounting reagent) | Calbiochem | 345789 | |
| Transfer pipette 1 ml (Disposable dropper) | WATSON | 5660-222-1S | |
| imaging | |||
| LSM700 laser scanning microscope system | Carl Zeiss | inverted Axio Observer. Z1 SP left | |
| image processing | |||
| LSM700 ZEN | Carl Zeiss | It is a special user interface based on the 64 bit Microsoft Windows7 operating system | |
| ImageJ | |||
| Fly stocks | |||
| w; GMR45C06-GAL4 | from Bloomington Drosophila Stock Center. (#46260) | ||
| UAS–GFP; UAS–mCD8::GFP | gifts from K. Ito, The University of Tokyo. | ||
| w[1118] | |||
| w; phantom-GAL4#22/UAS-turboRFP | |||
| w; UAS-mCD8::GFP; TRH-GAL4 | see in Ref29, Alekseyenko, O. V, Lee, C. & Kravitz, E. A.(2010) | ||
| w; UAS-mCD8::GFP | from Bloomington Drosophila Stock Center. (#32188) | ||
| yw;; nSyb-GAL4 | from Bloomington Drosophila Stock Center. (#51941) |
Referências
- Miller, W. L., Auchus, R. J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 32 (1), 81-151 (2011).
- Rousseau, G. G. Fifty years ago: The quest for steroid hormone receptors. Mol. Cell. Endocrinol. 375 (1), 10-13 (2013).
- Gilbert, L. I., Rybczynski, R., Warren, J. T. Control and biochemical nature of the ecdysteroidogenic pathway. Annu. Rev. Entomol. 47, 883-916 (2002).
- Niwa, R., Niwa, Y. S. Enzymes for ecdysteroid biosynthesis: their biological functions in insects and beyond. Biosci. Biotechnol. Biochem. 78 (8), 1283-1292 (2014).
- Kozlova, T., Thummel, C. S. Steroid regulation of postembryonic development and reproduction in drosophila. Trends Endocrinol. Metab. 11 (7), 276-280 (2000).
- Ishimoto, H., Kitamoto, T. Beyond molting-roles of the steroid molting hormone ecdysone in regulation of memory and sleep in adult Drosophila. Fly. 5 (3), 215-220 (2011).
- Ishimoto, H., Sakai, T., Kitamoto, T. Ecdysone signaling regulates the formation of long-term courtship memory in adult Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 106 (15), 6381-6386 (2009).
- Simon, A. F., Shih, C., Mack, A., Benzer, S. Steroid control of longevity in Drosophila melanogaster. Science. 299 (5611), 1407-1410 (2003).
- Buszczak, M., Freeman, M. R., Carlson, J. R., Bender, M., Cooley, L., Segraves, W. a Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development. 126 (20), 4581-4589 (1999).
- Carney, G. E., Bender, M. The drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genética. 154 (3), 1203-1211 (2000).
- Uryu, O., Ameku, T., Niwa, R. Recent progress in understanding the role of ecdysteroids in adult insects: Germline development and circadian clock in the fruit fly Drosophila melanogaster. Zoological Lett. 1, 32 (2015).
- Ameku, T., Niwa, R. Mating-Induced Increase in Germline Stem Cells via the Neuroendocrine System in Female Drosophila. PLOS Genet. 12 (6), e1006123 (2016).
- Danielsen, E. T., et al. A Drosophila Genome-Wide Screen Identifies Regulators of Steroid Hormone Production and Developmental Timing. Dev. Cell. 37 (6), 558-570 (2016).
- Ou, Q., Zeng, J., Yamanaka, N., Brakken-Thal, C., O’Connor, M. B., King-Jones, K. The Insect Prothoracic Gland as a Model for Steroid Hormone Biosynthesis and Regulation. Cell Rep. , (2016).
- Yamanaka, N., Rewitz, K. F., O’Connor, M. B. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu. Rev. Entomol. 58, 497-516 (2013).
- Niwa, Y. S., Niwa, R. Transcriptional regulation of insect steroid hormone biosynthesis and its role in controlling timing of molting and metamorphosis. Dev. Growth Differ. 58, 94-105 (2015).
- Monastirioti, M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev. Biol. 264 (1), 38-49 (2003).
- Siegmund, T., Korge, G. Innervation of the ring gland of Drosophila melanogaster. J. Comp. Neurol. 431 (4), 481-491 (2001).
- McBrayer, Z., et al. Prothoracicotropic Hormone Regulates Developmental Timing and Body Size in Drosophila. Dev. Cell. 13 (6), 857-871 (1979).
- Shimada-Niwa, Y., Niwa, R. Serotonergic neurons respond to nutrients and regulate the timing of steroid hormone biosynthesis in Drosophila. Nat. Commun. 5, 5778 (2014).
- Brady, J. A simple technique for making very fine, durable dissecting needles by sharpening tungsten wire electrolytically. Bull World Health Organ. 32 (1), 143-144 (1965).
- Abramoff, M. D., Magalhães, P. J., Ram, S. J. Image processing with ImageJ. Biophotonics Int. 11 (7), 36-42 (2004).
- Ohhara, Y., et al. Autocrine regulation of ecdysone synthesis by β3-octopamine receptor in the prothoracic gland is essential for Drosophila metamorphosis. Proc. Natl. Acad. Sci. USA. 112 (5), 1452-1457 (2015).
- Gibbens, Y. Y., Warren, J. T., Gilbert, L. I., O’Connor, M. B. Neuroendocrine regulation of Drosophila metamorphosis requires TGFbeta/Activin signaling. Development. 138 (13), 2693-2703 (2011).
- Parvy, J. P., et al. A role for βFTZ-F1 in regulating ecdysteroid titers during post-embryonic development in Drosophila melanogaster. Dev. Biol. 282 (1), 84-94 (2005).
- Parvy, J. -. P., et al. Forward and feedback regulation of cyclic steroid production in Drosophila melanogaster. Development. 141 (20), 3955-3965 (2014).
- Brand, A. H., Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118 (2), 401-415 (1993).
- Rewitz, K. F., Yamanaka, N., Gilbert, L. I., O’Connor, M. B. The Insect Neuropeptide PTTH Activates Receptor Tyrosine Kinase Torso to Initiate Metamorphosis. Science. 326 (5958), 1403-1405 (2009).
- Li, H. -. H., et al. A GAL4 driver resource for developmental and behavioral studies on the larval CNS of Drosophila. Cell Rep. 8 (3), 897-908 (2014).
- Alekseyenko, O. V., Lee, C., Kravitz, E. A. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLOS One. 5 (5), e10806 (2010).
- Domanitskaya, E., Anllo, L., Schüpbach, T. Phantom, a cytochrome P450 enzyme essential for ecdysone biosynthesis, plays a critical role in the control of border cell migration in in Drosophila. Dev. Biol. 386 (2), 408-418 (2014).
- Song, X., Zhu, C. -. H., Doan, C., Xie, T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 296 (5574), 1855-1857 (2002).
- Niwa, Y. S., Niwa, R. Neural control of steroid hormone biosynthesis during development in the fruit fly Drosophila melanogaster. Genes Genet. Syst. 89 (1), 27-34 (2014).
- Yoshiyama-Yanagawa, T., et al. The conserved Rieske oxygenase DAF-36/Neverland is a novel cholesterol-metabolizing enzyme. J. Biol. Chem. 286 (29), 25756-25762 (2011).
- Niwa, R., et al. Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the "Black Box" of the ecdysteroid biosynthesis pathway. Development. 137 (12), 1991-1999 (2010).
- Komura-Kawa, T., et al. The Drosophila Zinc Finger Transcription Factor Ouija Board Controls Ecdysteroid Biosynthesis through Specific Regulation of spookier. PLOS Genet. 11 (12), e1005712 (2015).
- Yamanaka, N., Marqués, G., O’Connor, M. B. Vesicle-Mediated Steroid Hormone Secretion in Drosophila melanogaster. Cell. 163 (4), 907-919 (2015).
- Riemensperger, T., Pech, U., Dipt, S., Fiala, A. Optical calcium imaging in the nervous system of Drosophila melanogaster. BBA-Gen. Subjects. 1820 (8), 1169-1178 (2012).
- Owald, D., Lin, S., Waddell, S. Light, heat, action: neural control of fruit fly behavior. Phil. T. Roy. Soc. B. 370 (1677), 20140211 (2015).

