使用缺氧/SU5416模型对小鼠的肺高血压进行诱导和特征
Summary
该协议描述了小鼠肺高血压(PH)的诱导,基于暴露于缺氧和VEGF受体拮抗剂的注射。动物在协议启动3周后出现PH和右心室(RV)肥大。介绍了模型的功能和形态特征。
Abstract
肺高血压 (PH) 是一种病理生理状况,由超过 25 mm Hg 的呼吸道动脉压力定义,由右心导管评估。广泛的疾病可导致PH,其病因、基础病理学、临床表现、预后和治疗反应各不相同。尽管近年来取得了重大进展,但PH仍然是一种未治愈的疾病。了解基本机制可以为开发新疗法铺平道路。动物模型是实现这一目标的重要研究工具。目前,有几个模型可用于重述PH。该协议描述了两击鼠标 PH 模型。PH发育的刺激是缺氧和SU5416的注射,一种血管内皮生长因子(VEGF)受体拮抗剂。低氧/SU5416开始三周后,动物发展肺血管重塑模仿在人类PH中观察到的组织病理学变化(主要是第1组)。肺循环中的血管重塑导致右心室 (RV) 的重塑。详细描述了测量RV压力(使用开放胸法)、RV的形态分析(通过解剖和称重两个心室)和重塑术(通过评估血管重塑和心脏评估肺功能评估)的过程。该协议的优点是,在野生型和转基因小鼠中应用的可能性,相对容易和低成本的实施,以及感兴趣的疾病的快速发展(3周)。这种方法的局限性是小鼠不发展严重的表型和PH是可逆的返回诺莫夏。预防以及治疗研究可以很容易地在这个模型中实施,而没有必要的高级技能(而不是手术啮齿动物模型)。
Introduction
肺高血压 (PH) 是一种病理生理状况,由平均肺动脉 (PA) 压力在休息时超过 25 mm Hg 定义,由右心导管1,2评估。有各种各样的疾病,可导致PH。为了组织与PH相关的条件,开发了几种分类系统。目前的临床分类分类的多个PH相关疾病在5个不同的组1。这种区别很重要,因为不同组的患者的临床表现、病理学、预后和治疗反应都不同。表1总结了目前的分类,并辅之以每种疾病的基本组织病理学特征。
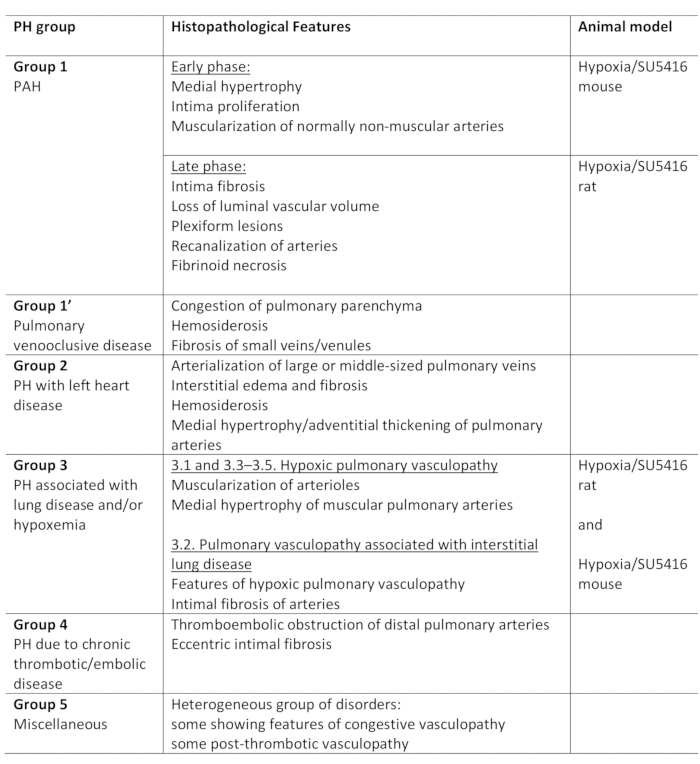
表1:PH临床分类概述,以及各组内的主要组织病理学特征。低氧/SU5416 协议的适用性,用于建模 PH。此表已从19 起修改。PH: 肺高血压, PAH: 肺动脉高血压
尽管在治疗PH相关疾病方面取得了重大进展,但PH仍然无法治愈,3年死亡率在20%至80%之间。这表明迫切需要了解PH的基本机制,然后,开发新的疗法,以防止,减缓进展,并治愈疾病。动物模型对于这个范围至关重要。目前,研究PH的各种模型都存在。有兴趣的读者会参考有关这个题目2,3,4,的优秀,评论。考虑到导致PH的各种疾病,很明显,人类PH的各种条件不能完全概括为一种动物模型。可用的动物模型可以分为 i) 单击, ii) 两击, iii) 淘汰, 和 iv) 过度表达模型3。在单击模型中,PH 是由单个病理刺激诱导的,而双命中模型将两种病理刺激与诱导更严重 PH 的目标相结合,从而更密切地模仿复杂的人类疾病。除了病因差异外,几种刺激导致PH建模差异,也取决于动物的种类和遗传背景4。
最常用的经典PH啮齿动物模型之一是慢性缺氧模型2。众所周知,低氧在人类和几种动物物种中诱发PH。缺氧的好处是PH的生理刺激(表1)。然而,虽然用于诱导啮齿动物PH的缺氧程度比人类严重得多,单一的侮辱(缺氧)只导致轻微的血管重塑。这不能模仿人类疾病的严重程度。增加第二次命中,一个额外的刺激诱导PH,显示了有希望的结果:注射化合物SU5416啮齿动物结合低氧刺激诱导更严重的PH表型2,5,6。,5,6SU5416是血管内皮生长因子(VEGF)受体-2的抑制剂。它阻断VEGF受体并导致内皮细胞凋亡。在缺氧条件下,这刺激了抗凋亡内皮细胞子集的增殖。此外,SU5416导致平滑的肌肉细胞增殖。这些效果的组合导致肺循环的病理血管重塑,并导致PA压力升高和右心室重塑2,5,7。,5,7该模型首先在大鼠6中描述,后来应用于小鼠,4、5、7。547与大鼠相比,小鼠模型表现出的血管重塑不太严重。此外,当回到诺莫夏时,PH在大鼠中继续进步,而在大鼠中则部分可逆。
以下协议描述了使用缺氧/SU5416 方法(规划、时间线、执行)在小鼠中建模 PH 的所有步骤。此外,本方案还描述了该模型的特征:功能性(通过侵入性测量右心室(RV)压力使用开放胸部技术)、形态测量(通过解剖和称重右心室和左心室),以及组织学(通过评估肺血管重塑、右心室心肌细胞肥大和纤维化)。
本协议中描述的所有步骤和方法都可以在任何经验级别上由调查人员轻松实施。虽然使用开放胸腔技术(此处描述)的 RV 的功能测量不是该领域的黄金标准方法,但它的优点是,即使经验较少的实验者也可以快速了解和准确再现。
Protocol
Representative Results
Discussion
该协议描述了如何通过结合两种病理刺激在小鼠的PH模型:慢性缺氧和SU5416注射(缺氧/SU5416)1818。为了试图将此鼠标模型与人类PH条件关联,不可避免地必须查看表1所示的当前PH分类。几乎所有形式的PH的特点是肺血管收缩和内皮细胞和平滑肌肉细胞的异常增殖。这导致肺动脉压力升高,从而增加右心室的后负荷。
每一次尝试描述PH动物模型应?…
Declarações
The authors have nothing to disclose.
Acknowledgements
这项工作得到了美国心脏协会(AHA-17SDG333370112和18IPA34170258)的资助,以及来自国家卫生研究院NIH K01 HL135474到Y.S.O.B的资助,并得到德国赫兹斯蒂夫通的支持。
Materials
| Acetic acid glacial | Roth | 3738.1 | |
| Acetone, Histology Grade | The Lab Depot | VT110D | |
| ADVantage Pressure-Volume System | Transonic | ADV500 | |
| Bouin's solution | Sigma | Ht10132 | |
| Cautery System | Fine Science Tools | 18000-00 | |
| Connection tubing and valves | |||
| Cotton-Tipped Applicators | Covidien | 8884541300 | |
| Coverslips, 24 x50 mm | Roth | 1871 | |
| Data Acquisition and Analysis | Emka | iox2 | |
| Direct Red 80 | Sigma | 365548-5G | |
| DMSO (Dimethyl Sulfoxide) | Sigma Aldrich | 276855 | |
| Dry ice | |||
| Dumont # 5 forceps | Fine Science Tools | 11251-10 | |
| Dumont # 7 Fine Forceps | Fine Science Tools | 11274-20 | |
| Embedding molds | Sigma Aldrich | E-6032 | |
| Eosin Solution Aqueous | Sigma | HT110216 | |
| Ethanol, laboratory Grade | Carolina Biological Supply Company | 861285 | |
| Fast Green FCF | Sigma | F7252-5G | |
| Fine scissors | Fine Science Tools | 14090-09 | |
| Goat Serum | invitrogen | 16210-064 | |
| Heating pad | Gaymar | T/Pump | |
| Hematoxylin 2 | Thermo Scientific | 7231 | |
| Hypoxic chamber | Biospherix | A30274P | |
| Induction chamber | DRE Veterinary | 12570 | |
| Intubation catheter (i.v. catheter SurFlash (20 G x 1") ) | Terumo | SR*FF2025 | |
| Iris scissors | Fine Science Tools | 14084-08 | |
| Isoflurane | Baxter | NDC-10019-360-40 | |
| Isoflurane vaporizer | DRE Veterinary | 12432 | |
| Mice (C57BL/6) | Charles River | ||
| Needles 25 G x 5/8" | BD | 305122 | |
| OCT | Tissue Tek | 4583 | |
| PBS (Phosphate Buffered Saline) | Corning | 21-031-CV | |
| Piric Acid- Saturated Solution 1.3 % | Sigma | P6744-1GA | |
| Pressure volume catheter | Transonic | FTH-1212B-4018 | |
| Retractor | Kent Scientific | SURGI-5001 | |
| Static oxygen Controller ProOx 360 | Biospherix | P360 | |
| SU 5416 | Sigma Aldrich | S8442 | |
| Surgical Suture, black braided silk, 5.0 | Surgical Specialties Corp. | SP116 | |
| Surgical tape | 3M | 1527-1 | |
| Syringe 10 ml | BD | 303134 | |
| Syringes with needle 1 ml | BD | 309626 | |
| Sytox Green Nuclein Acid Stain | Thermo Scientific | S7020 | |
| Tenotomy scissors | Pricon | 60-521 | |
| Toluol | Roth | 9558.3 | |
| Ventilator | CWE | SAR-830/P | |
| WGA Alexa Fluor | Thermo Scientific | W11261 | |
| Xylene | Roth |
Referências
- Galie, N., et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). European Heart Journal. 37 (1), 67-119 (2016).
- Stenmark, K. R., Meyrick, B., Galie, N., Mooi, W. J., McMurtry, I. F. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. American Journal of Physiology-Lung Cell Molecular Physiology. 297 (6), 1013-1032 (2009).
- Maarman, G., Lecour, S., Butrous, G., Thienemann, F., Sliwa, K. A comprehensive review: the evolution of animal models in pulmonary hypertension research; are we there yet. Pulmonary Circulation. 3 (4), 739-756 (2013).
- Gomez-Arroyo, J., et al. A brief overview of mouse models of pulmonary arterial hypertension: problems and prospects. American Journal of Physiology-Lung Cell Molecular Physiology. 302 (10), 977-991 (2012).
- Ciuclan, L., et al. A novel murine model of severe pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 184 (10), 1171-1182 (2011).
- Taraseviciene-Stewart, L., et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB Journal. 15 (2), 427-438 (2001).
- Vitali, S. H., et al. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: long-term follow-up. Pulmonary Circulation. 4 (4), 619-629 (2014).
- Breen, E. C., Scadeng, M., Lai, N. C., Murray, F., Bigby, T. D. Functional magnetic resonance imaging for in vivo quantification of pulmonary hypertension in the Sugen 5416/hypoxia mouse. Experimental Physiology. 102 (3), 347-353 (2017).
- Wang, Z., Schreier, D. A., Hacker, T. A., Chesler, N. C. Progressive right ventricular functional and structural changes in a mouse model of pulmonary arterial hypertension. Physiological Reports. 1 (7), 00184 (2013).
- Momcilovic, M., et al. Utilizing 18F-FDG PET/CT Imaging and Quantitative Histology to Measure Dynamic Changes in the Glucose Metabolism in Mouse Models of Lung Cancer. Journal of Visualized Experiment. (137), 57167 (2018).
- Guma, S. R., et al. Natural killer cell therapy and aerosol interleukin-2 for the treatment of osteosarcoma lung metastasis. Pediatric Blood Cancer. 61 (4), 618-626 (2014).
- Lattouf, R., et al. Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues. Journal of Histochemistry and Cytochemistry. 62 (10), 751-758 (2014).
- Penumatsa, K. C., et al. Transglutaminase 2 in pulmonary and cardiac tissue remodeling in experimental pulmonary hypertension. American Journal of Physiology-Lung Cell Molecular Physiology. 313 (5), 752-762 (2017).
- Wang, Z., et al. Organ-level right ventricular dysfunction with preserved Frank-Starling mechanism in a mouse model of pulmonary arterial hypertension. Journal of Applied Physiology. 124 (5), 1244-1253 (2018).
- van de Veerdonk, M. C., Bogaard, H. J., Voelkel, N. F. The right ventricle and pulmonary hypertension. Heart Failure Reviews. 21 (3), 259-271 (2016).
- Emde, B., Heinen, A., Godecke, A., Bottermann, K. Wheat germ agglutinin staining as a suitable method for detection and quantification of fibrosis in cardiac tissue after myocardial infarction. European Journal of Histochemistry. 58 (4), 2448 (2014).
- Pena, S. D., Gordon, B. B., Karpati, G., Carpenter, S. Lectin histochemistry of human skeletal muscle. Journal of Histochemistry and Cytochemistry. 29 (4), 542-546 (1981).
- Bueno-Beti, C., Hadri, L., Hajjar, R. J., Sassi, Y. The Sugen 5416/Hypoxia Mouse Model of Pulmonary Arterial Hypertension. Methods in Molecular Biology. 1816, 243-252 (2018).
- Colvin, K. L., Yeager, M. E. Animal Models of Pulmonary Hypertension: Matching Disease Mechanisms to Etiology of the Human Disease. Journal of Pulmonary and Respiratory Medicine. 4 (4), (2014).
- Benza, R. L., et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 122 (2), 164-172 (2010).
- Jacob, S. W., Rosenbaum, E. E. The toxicology of dimethyl sulfoxide (DMSO). Headache. 6 (3), 127-136 (1966).
- Jacob, S. W., Wood, D. C. Dimethyl sulfoxide (DMSO). Toxicology, pharmacology, and clinical experience. American Journal of Surgery. 114 (3), 414-426 (1967).
- Abraham, D., Mao, L. Cardiac Pressure-Volume Loop Analysis Using Conductance Catheters in Mice. Journal of Visualized Experiment. (103), 52942 (2015).
- Ma, Z., Mao, L., Rajagopal, S. Hemodynamic Characterization of Rodent Models of Pulmonary Arterial Hypertension. Journal of Visualized Experiment. (110), 53335 (2016).
- Townsend, D. Measuring Pressure Volume Loops in the Mouse. Journal of Visualized Experiment. (111), 53810 (2016).
- Penumatsa, K. C., Warburton, R. R., Hill, N. S., Fanburg, B. L. CrossTalk proposal: The mouse SuHx model is a good model of pulmonary arterial hypertension. Journal of Physiology. 597 (4), 975-977 (2019).

