Droplet Digital TRAP (ddTRAP): Adaptation of the Telomere Repeat Amplification Protocol to Droplet Digital Polymerase Chain Reaction
Summary
We successfully converted the standard telomere repeat amplification protocol (TRAP) assay to be employed in droplet digital polymerase chain reactions. This new assay, called ddTRAP, is more sensitive and quantitative, allowing for better detection and statistical analysis of telomerase activity within various human cells.
Abstract
The telomere repeat amplification protocol (TRAP) is the most widely used assay to detect telomerase activity within a given a sample. The polymerase chain reaction (PCR)-based method allows for robust measurements of enzyme activity from most cell lysates. The gel-based TRAP with fluorescently labeled primers limits sample throughput, and the ability to detect differences in samples is restricted to two fold or greater changes in enzyme activity. The droplet digital TRAP, ddTRAP, is a highly sensitive approach that has been modified from the traditional TRAP assay, enabling the user to perform a robust analysis on 96 samples per run and obtain absolute quantification of the DNA (telomerase extension products) input within each PCR. Therefore, the newly developed ddTRAP assay overcomes the limitations of the traditional gel-based TRAP assay and provides a more efficient, accurate, and quantitative approach to measuring telomerase activity within laboratory and clinical settings.
Introduction
Telomeres are dynamic DNA-protein complexes at the ends of linear chromosomes. Human telomeres are composed of an array of 5'-TTAGGGn hexameric repeats which vary in length between 12–15 kilobases (kb) at birth1. Human telomerase, the ribonucleoprotein enzyme that maintains the telomeres, was first identified in HeLa cell lysates (cancer cell line)2. Together, telomeres and telomerase play a major role in a spectrum of biological processes such as genome protection, gene regulation, and cancer cell immortality3,4,5,6.
Human telomerase is comprised primarily of two key components, namely telomerase reverse transcriptase and telomerase RNA (hTERT and hTERC, respectively). The protein subunit, hTERT, is the catalytically active reverse transcriptase component of the telomerase enzyme. The RNA template, hTERC, provides telomerase with the template to extend and/or maintain telomeres. Most human somatic tissues have no detectable telomerase activity. The inability of DNA polymerase to extend the end of the lagging strand of DNA along with the lack of telomerase leads to the progressive shortening of telomeres after every round of cellular division. These phenomena lead to telomere shortening in most somatic cells until they reach a critically shortened length, whereby cells enter a state of replicative senescence. The maximal number of times a cell can divide is dictated by its telomere length and this block to continued cell division is thought to prevent progression to oncogenesis7. Cancer cells are able to overcome telomere-induced replicative senescence and continue to proliferate by utilizing telomerase to maintain their telomeres. Approximately 90% of cancers activate telomerase, making telomerase activity critically important in both cancer detection and treatment.
The development of the TRAP assay in the 1990s was instrumental in the identification of the necessary components of the telomerase enzyme, as well as for the measurement of telomerase in a wide range of cells and tissues, both normal and cancerous. The original gel-based PCR assay used radioactively labeled DNA substrates to detect telomerase activity. In 2006, the assay was adapted into a nonradioactive form using fluorescently labeled substrates8,9. By using fluorescently labeled substrates, users were able to visualize the telomerase extension products as bands on a gel by exposing it to the correct excitation wavelength. The sensitivity of the TRAP assay and its ability to detect telomerase activity in crude cell lysates has made this assay the most widely used method for telomerase activity detection. However, the TRAP assay has limitations. The assay is gel-based, making it difficult to perform the necessary replicates in moderate to high-throughput studies, and thus, proper statistical analysis is rarely achieved. Furthermore, the gel-based assay is difficult to quantify reliably due to the inability of detecting less than twofold differences in telomerase activity between samples. Overcoming these two limitations is critical for enzymatic activity assays such as the TRAP to move to clinical or industry settings for the detection of telomerase activity in patient samples or drug design studies.
Digital PCR was initially developed in 1999 as a means to convert the exponential and analog nature of PCR into a linear and digital assay10. Droplet digital PCR (ddPCR) is the most recent innovation of the original digital PCR methodology. Droplet digital PCR came about with the advent of advanced microfluidics and oil-in-water emulsion chemistry to reliably generate stable and equally sized droplets. Unlike gel-based and even quantitative PCR (qPCR), ddPCR generates absolute quantification of the input material. The key to ddPCR is the generation of ~20,000 individual reactions by partitioning samples into droplets. Following end-point PCR, the droplet reader scans each droplet in a flow-cytometer-like fashion, counting, sizing, and recording the presence or absence of fluorescence in each individual droplet (i.e., absence or presence of PCR amplicons in each droplet). Then, using Poisson’s distribution, input molecules are estimated based on the ratio of positive droplets to the total number of droplets. This number represents an estimate of the number of input molecules in each PCR. Furthermore, ddPCR is performed and analyzed on a 96-well plate which allows the user to run many samples, as well as perform biological and technical replicates for proper statistical analysis. As a result, we have combined the powerful quantification and moderate-throughput nature of ddPCR with the TRAP assay to develop the ddTRAP assay11. This assay is designed for users to study and robustly quantify absolute telomerase activity from biological samples11,12. The sensitivity of the ddTRAP allows the quantification of telomerase activity from limited and precious samples, including single-cell measurements. Furthermore, users can also study the effects of telomerase manipulations and/or drugs with absolute quantification of less than twofold changes (~50% differences). The ddTRAP is the natural evolution of the TRAP assay into the digital and higher-throughput nature of modern laboratory experiments and clinical settings.
Protocol
1. Buffer preparation and storage
- Prepare 50 mL of 1x stock RNase-/DNase-free NP-40 lysis buffer (10 mM Tris-HCl [pH 8.0], 1 mM MgCl2, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% [vol/vol] NP-40, 10% [vol/vol] glycerol, 150 mM NaCl, 5 mM β-mercaptoethanol, and 0.1 mM 4-benzenesulfonyl fluoride hydrochloride (AEBSF)). This buffer can be aliquoted and stored at -20 °C for future use. Avoid freeze/thaw cycles in order to obtain an optimal lysis of cells.
- Prepare 50 mL of 10x stock RNase-/DNase-free TRAP extension buffer (200 mM Tris-HCl [pH 8.0], 15 mM MgCl2, 630 mM KCl, 0.5% [vol/vol] Tween 20, and 10 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA)). This buffer can be aliquoted into 1 mL aliquots and stored at -20 °C for future use.
2. Cell lysis
- Preparation of the cells
- Thaw a frozen aliquot of NP-40 lysis buffer. Once thawed, place the lysis buffer on ice. Add phenylmethylsulfonyl fluoride (PMSF protease inhibitor) to the NP-40 lysis buffer to reach a final concentration of 0.2 mM.
NOTE: This should be done immediately prior to lysing the cells. - Remove any excess liquid from the collected cell pellets to ensure a dry cell pellet. Note that cell pellets, whether freshly collected or previously frozen (-80 °C or flash frozen in liquid N2), must not contain any leftover solutions from previous centrifugations as these solutions may interfere with downstream procedures.
- Grow, collect, and freeze both telomerase-positive and telomerase-negative cell lines (BJ fibroblasts, IMR-90 or U2OS) in order to use them as positive and negative controls. Use new control lysates every time the assay is performed to ensure assay reproducibility.
NOTE: It is advised that a large culture of telomerase-negative cells is grown and aliquoted prior to freezing to remove any tissue-culture-related artifacts that may be introduced during the long-term culture of cell lines to help ensure reproducible results of the negative control sample. - Place the cell pellets on ice. If the cells were frozen, allow them to thaw briefly on ice.
NOTE: Typical cell pellet sizes for the ddTRAP are between 500,000 and 1,000,000 cells. Smaller cell pellets may also be used if necessary, but the cell number must/ideally should be known. The ddTRAP can also be performed based on protein input using a BCA protein assay (input usually is 1 µg).
- Thaw a frozen aliquot of NP-40 lysis buffer. Once thawed, place the lysis buffer on ice. Add phenylmethylsulfonyl fluoride (PMSF protease inhibitor) to the NP-40 lysis buffer to reach a final concentration of 0.2 mM.
- Lysing of the cells
- Lyse the cells in the NP-40 lysis buffer. Maintain a cell equivalence of 25,000 cells/µL of buffer. For example, lyse a pellet (containing 1,000,000 cells) in 40 µL of NP-40 lysis buffer. Gently pipet the lysate up and down in order to break open the cells. Try to avoid making bubbles.
- Allow the cells to lyse on ice for 30 min. Make sure to gently vortex the lysate to prevent clusters of cell debris from forming. This can be done every 10 min and is meant to keep the lysate a homogenized mixture.
- Dilute the cell lysate (25,000 cells/µL) 1:20 in NP-40 lysis buffer, making the new cell equivalence 1,250 cells/µL. For example, dilute 5 µL of cell lysate into 95 µL of lysis buffer.
3. Telomerase extension reaction
- Prepare a master mix (Table 1) for the telomerase extension reaction (per reaction).
NOTE: It is best to prepare the extension reaction master mix during the cell lysis and store it on ice.- Pipet 48 µL of extension master mix into each PCR tube.
- Add 2 µL of the diluted (1,250 cells/µL) lysate to the extension reaction. The total volume should now be 50 µL with a final cell equivalence of 50 cells/µL.
- Perform the telomerase extension reaction (Table 2).
- Store the telomerase extension products at 4 °C for up to 3–5 days; however, it is optimal to use the extension products within the first 24 h.
4. Droplet digital PCR setup
- Prepare a master mix (Table 3) for the ddTRAP (per reaction).
NOTE: Once the reagents are at room temperature, do not place them or the master mix on ice. Placing the mix on ice may increase viscosity and lead to poor droplet formation. The DNA polymerase in the ddPCR supermix is a hot start and should be stable at room temperature. The ddPCR supermix may be stored at 4 °C after an initial thaw from -20 °C.- Pipet 19.8 µL of ddPCR master mix into each PCR tube.
- Add 2.2 µL of the extension reaction to each tube. Note that the total volume should now be 22 µL.
NOTE: The total amount of sample needed for a ddTRAP is 20 µL (cell equivalence of 100 cells). The extra volume is a precaution for pipet error or sample loss.
- Set up the droplet generation cartridge.
NOTE: The cartridge contains three different columns of wells that are labeled.- Load 20 µL of the reaction prepared according to step 4.1.2 into the sample well (middle well) in the cartridge. Avoid bubbles when pipetting the sample.
NOTE: If there are bubbles, gently tap the side of the cartridge so that these bubbles come to the top of the solution. - Load 70 µL of droplet generation oil into the oil well (left well).
NOTE: In order to avoid contamination, strictly use a separate set of pipettes and tips for ddPCR droplet generation steps. The order of loading the cartridge is important. The sample must be loaded prior to loading oil as oil is heavier and will fill the microfluidic chambers and lead to poor droplet formation. The minimum number of samples that can be run is eight. All wells of the cartridge must be loaded with the sample as any empty well will lead to a halt in the droplet generation from the droplet generator. If adequate samples are not available to load all eight wells within a cartridge, 20 µL of ddTRAP master mix (step 4.1) can be loaded into the remaining cartridges. - Secure the gasket in place by tethering it to the ends of the cartridge.
NOTE: The lack or improper placement of the gasket will prevent the generator from generating droplets. - Place the loaded and assembled cartridge into the droplet generator.
NOTE: The cartridge is recognized by a magnet in the generator, which will inform the user when the cartridge is placed properly.
- Load 20 µL of the reaction prepared according to step 4.1.2 into the sample well (middle well) in the cartridge. Avoid bubbles when pipetting the sample.
- Remove the cartridge once the droplets are generated and the cycle is complete (~60–90 s).
- Gently remove the gasket from the cartridge. Pipet the newly generated droplets (right well), using a multichannel pipette, into a 96-well PCR plate.
NOTE: The approximate volume for the newly generated droplet emulsion should be 40–43 µL. - Heat seal the plate with aluminum foil PCR plate seals once all the samples are loaded in the 96-well plate in order to prevent evaporation during the PCR steps.
- Gently remove the gasket from the cartridge. Pipet the newly generated droplets (right well), using a multichannel pipette, into a 96-well PCR plate.
- Load the 96-well plate into the thermocycler and perform the following PCR reaction (Table 4).
NOTE: All ramp rates between the temperature steps must be set to 2.5 °C/s in order to properly heat the reactions.
5. Detection of telomerase extension products
- Load the 96-well plate in the droplet reader.
NOTE: Make sure to orient the plate properly so that the A1 sample matches with that of the holder.- Open the software associated with the droplet reader. Double-click the first well A1 to open the sample/well editor screen.
- Click Experiment and select ABS from the drop-down menu.
NOTE: Experiment defines the type of assay to be used (i.e., absolute quantification or gene copy number assay). ABS stands for absolute quantification. - Select QX200 ddPCR Evagreen Supermix to ensure that the correct detection method is employed by the reader. Click Apply in the lower right-hand side of the well editor screen to save the user-defined settings to all of the highlighted wells.
NOTE: SUPERMIX defines the type of PCR mix and detection chemistry that will be read by the reader. - Click on TARGET in the well editor screen of the software in order to define the sample.
- Define the TYPE of sample by clicking the TARGET drop-down menu and selecting either unknown, reference, or NTC (no-template control).
NOTE: Unknown would refer to an experimental sample, reference could be a control sample of known telomerase activity, and an NTC is a critical control for the determination of assay validity in terms of contamination and background signal. - Label all the samples in the Sample Name section and click Apply to ensure the highlighted wells are edited appropriately.
- Click Run to run/read the plate. Select either Columns or Rows in the RUN OPTIONS screen when prompted to inform the machine about the orientation the plate should be read in.
6. Data analysis
- Determine the number of accepted droplets for each sample by clicking on individual wells. Double-click the individual wells or column/row headers to view and analyze the sample data.
NOTE: The most important criteria on whether or not to proceed with the analysis of a particular sample is the number of “accepted droplets”. For the ddTRAP, samples with 10,000 or more accepted droplets are valid for further analysis. Data can be provided to the user in many formats, including a .csv table, .jpg images, histograms, etc. There are many resources available for the in-depth analysis of ddPCR data, including the ddTRAP11,13. These resources offer a guide to analyzing ddTRAP data, from selecting thresholds11,13 to choosing samples for further analysis11,13, identifying false positives and negatives13, and general troubleshooting13. - Highlight the wells representing sample replicates and NTC samples. Analyze the samples in comparison to either NTC or negative control lines, such as BJ cells (as listed above in step 2.1.2).
- Manually set the threshold for the samples by clicking on the icon for setting thresholds on the bottom left of the screen. Set thresholds for each individual well or for multiple wells at a time (recommended).
NOTE: If background is detected in the NTC, it can be subtracted from all of the other samples to ‘normalize’ the signal and ensure that only the true positive signal is analyzed. NTC values are typically in the 0.2–1 molecule/µL range for the NP-40 lysis buffer system. Other buffer systems and components would need to be tested (for example, CHAPS buffers).
- Manually set the threshold for the samples by clicking on the icon for setting thresholds on the bottom left of the screen. Set thresholds for each individual well or for multiple wells at a time (recommended).
Representative Results
Using the ddTRAP, telomerase activity was measured in a cell panel consisting of the following cell lines (Figure 1): nonsmall cell lung cancer (H2882, H1299, Calu6, H920, A549, and H2887), small cell lung cancer (H82 and SHP77), and telomerase-negative fibroblasts (BJ). One million cell pellets were lysed in NP-40 buffer, and telomerase extension reactions were performed in biological triplicates. A common and highly recommended negative control is the “NTC”, the no-template control. This sample is generated by adding NP-40 lysis buffer (2 µL) to the telomerase extension reaction and proceeding with the extension products in an identical manner to other samples containing actual cell lysate. This sample allows the user to subtract the background signal, if any, to better quantify the telomerase activity. Although not shown in this figure, it is also possible to heat inactivate the lysate at 95° C for 5 min prior to the telomerase extension reaction as another negative control. This negative control is preferred if cell/sample abundance is not an issue.
By measuring the fluorescence intensity of every single droplet in the droplet emulsion, the droplet reader was able to estimate the concentration of input molecules (molecules/microliter) using the Poisson distribution (Figure 1A). In the case of the ddTRAP, these input molecules were telomerase extension products. The mechanism of the ddTRAP assay was as follows: telomerase extended the TS substrate. These extended substrates acted as the PCR templates in the ddPCR. Quantification of the PCR-amplified substrates provided a representation of telomerase enzymatic activity within a given cell line. Every droplet was plotted as shown in Figure 1A. Setting the threshold for a ddTRAP may be subjective; however, with the proper negative controls, the user can easily do so. In the example shown in Figure 1A, a threshold was set for all three biological replicates for SHP77, H2887, and NTC. Positive droplets had a fluorescence intensity around 6,000 fluorescence amplitude (FA) and formed a clear population at the top and separate from negative droplets around 1,100 FA. Therefore, the threshold may be set at ~2,000 FA in this experiment.
Once the data was collected and exported, it was possible to calculate the total telomerase extension products per cell equivalent between all the samples (Figure 1B). The signal from every well was an absolute concentration (molecules/microliter). By multiplying the concentration by 20 (input volume of the sample into ddPCR cartridges), the user may obtain the total number of molecules. This number can then be divided by the known cell equivalent (in our performance of the ddTRAP, we used 100 cells). This final value is in the units of telomerase extension products per cell equivalent as shown on the y-axis.
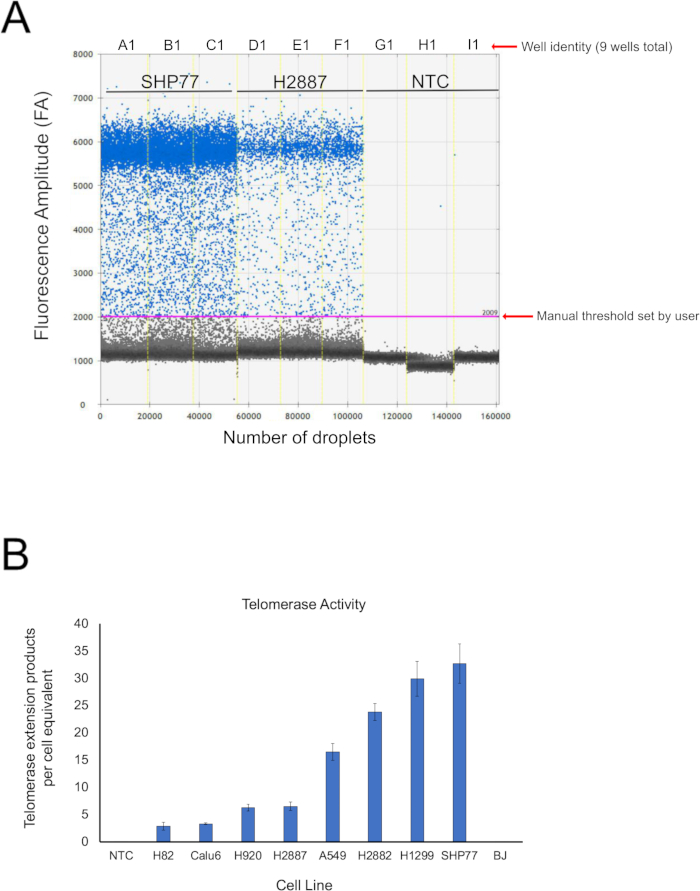
Figure 1: Telomerase activity in a lung cancer panel. (A) The 1D amplitude of droplet fluorescence intensity (fluorescence amplitude) for SHP77, H2887, and NTC. Wells for SHP77, H2887, and NTC were selected and a manual threshold was set at 2,000 FA. (B) Telomerase activity was estimated from the measured concentration of the nucleic acids detected following PCR and plotted in order to compare telomerase activity in lung cancer lines. FA = fluorescence amplitude units. Please click here to view a larger version of this figure.
Discussion
The measurement of telomerase activity is critical to a plethora of research topics including, but not limited to, cancer, telomere biology, aging, regenerative medicine, and structure-based drug design. Telomerase RNPs are low abundant, even in cancer cells, making the detection and study of this enzyme challenging. In this paper, we described the step-by-step procedures for the newly developed ddTRAP assay to robustly quantify telomerase activity in cells. By combining the traditional telomerase extension reaction with ddPCR, we were able to quantitatively detect telomerase activity (telomerase-extended products) in lung cancer cells.
The ddTRAP assay relies on the same theory as the TRAP assay. Cell lysates are obtained by lysing cells in a nonionic detergent (NP-40) lysis buffer to maintain enzyme activity and then used to perform a telomerase extension reaction of the “TS” substrate/primer. The novelty of the ddTRAP relies on the formation of droplets prior to PCR. The partitioning of the sample into droplets allows the assay to obtain absolute quantification of telomerase activity per cell.
Telomerase activity was measured in lung cancer cell lines, using the ddTRAP. One of the key limitations of the gel-based TRAP assay is the number of samples that can be processed at a time. Most gel combs can only accommodate up to 20 wells/samples. In contrast, the ddTRAP can run up to 96 samples at a time, significantly increasing the number of samples that can be processed at once. We assayed telomerase activity in eight cell lines. Most importantly, we ran each telomerase extension reaction in biological triplicates for a total of 24 extension reactions. We then were able to run each extension reaction as three technical replicates for PCR for a total of 72 samples on the ddPCR plate. Furthermore, the ddTRAP assay provides reproducible results with an interday CV (coefficient of variation) of 5.1% for 100 cell equivalents and an intraday CV of 8.61% for 100 cell equivalents. Interday and intraday represent biological replicates run on two different plates or the same plate, respectively, on HeLa cell lysates11. If 72 samples were processed in the traditional TRAP or any other gel-based assay, it would require much more hands-on time. This leads to higher costs of reagents, more valuable time needed from employees/students/trainees, a higher gel-to-gel variability, and a lack of reproducibility between users and laboratories. The ability to reliably and easily run replicates in the ddTRAP is a dramatic improvement over gel-based assays and allows many more samples to be processed efficiently, which aids in the statistical analysis of the data.
Finally, less variability between the samples in general in the ddTRAP and the possibility to perform the necessary replicates allows users to observe less than twofold changes between samples. The major limitation of most gel-based assays is the lack of proper quantification. Here, using the ddTRAP, we can detect the subtle differences in between the various lung cancer lines. The subtle differences can be critical when it comes to comparing drugs targeting telomerase activity and deciding whether or not to move forward with small molecule compounds from high-throughput screens.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge funding sources from the National Institutes of Health (NIH) (NCI-R00-CA197672-01A1). Small cell lung cancer lines (SHP77 and H82) were a generous gift from Drs. John Minna and Adi Gazdar from the UT Southwestern Medical Center.
Materials
| 1 M Tris-HCl pH 8.0 | Ambion | AM9855G | RNAse/DNAse free |
| 1 M MgCl2 | Ambion | AM9530G | RnAse/DNAse free |
| 0.5 M EDTA pH 8.0 | Ambion | AM9261 | RNAse/DNAse free |
| Surfact- Amps NP-40 | Thermo Scientific | 28324 | |
| 100% Ultrapure Glycerol | Invitrogen | 15514011 | RNAse/DNAse free |
| phenylmethylsulfonyl fluoride | Thermo Scientific | 36978 | Powder |
| 2-Mercaptoethanol | SIGMA-ALDRICH | 516732 | |
| Nuclease Free H20 | Ambion | AM9932 | RNAse/DNAse free |
| 2.5 mM dNTP mix | Thermo Scientific | R72501 | 2.5 mM of each dATP, dCTP, dGTP and dTTP |
| 2 M KCl | Ambion | AM9640G | RNAse/DNAse free |
| 100% Tween-20 | Fisher | 9005-64-5 | |
| 0.5 M EGTA pH 8.0 | Fisher | 50-255-956 | RNAse/DNAse free |
| Telomerase Substrate (TS) Primer | Integrated DNA Technology (IDT) | Custom Primer (HPLC Purified) | 5'- AATCCGTCGAGCAGAGTT-3' |
| ACX (Revers) Primer | Integrated DNA Technology (IDT) | Custom Primer (HPLC Purified) | 5'- GCGCGGCTTACCCTTACCCTTACCCTAACC -3' |
| Thin walled (250 ul) PCR grade tubes | USA Scientific | 1402-2900 | strips, plates, tubes etc. |
| QX200 ddPCR EvaGreen Supermix | Bio Rad | 1864034 | |
| Twin-Tec 96 Well Plate | Fisher | Eppendorf 951020362 | |
| Piercable foil heat seal | Bio Rad | 1814040 | |
| Droplet generator cartidges (DG8) | Bio Rad | 1863008 | |
| Droplet generator oil | Bio Rad | 1863005 | |
| Droplet generator gasket | Bio Rad | 1863009 | |
| 96-well Thermocycler T100 | Bio Rad | 1861096 | |
| PX1 PCR Plate Sealer | Bio Rad | 1814000 | |
| QX200 Droplet Reader and Quantasoft Software | Bio Rad | 1864001 and 1864003 | |
| ddPCR Droplet Reader Oil | Bio Rad | 1863004 | |
| Nuclease Free Filtered Pipette Tips | Thermo Scientific | 10 ul, 20 ul , 200 ul and 1000 ul |
Referências
- Frenck, R. W., Blackburn, E. H., Shannon, K. M. The rate of telomere sequence loss in human leukocytes varies with age. Proceedings of the National Academy of Sciences of the United States of America. 95 (10), 5607-5610 (1998).
- Morin, G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 59 (3), 521-529 (1989).
- de Lange, T. How telomeres solve the end-protection problem. Science. 326 (5955), 948-952 (2009).
- Shay, J. W., Wright, W. E. Role of telomeres and telomerase in cancer. Seminars in Cancer Biology. 21 (6), 349-353 (2011).
- Kim, W., Shay, J. W. Long-range telomere regulation of gene expression: Telomere looping and telomere position effect over long distances (TPE-OLD). Differentiation. 99, 1-9 (2018).
- Robin, J. D., et al. Telomere position effect: regulation of gene expression with progressive telomere shortening over long distances. Genes Development. 28 (22), 2464-2476 (2014).
- Shay, J. W., Wright, W. E. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 26 (5), 867-874 (2005).
- Norton, J. C., Holt, S. E., Wright, W. E., Shay, J. W. Enhanced detection of human telomerase activity. DNA Cell Biology. 17 (3), 217-219 (1998).
- Herbert, B. S., Hochreiter, A. E., Wright, W. E., Shay, J. W. Nonradioactive detection of telomerase activity using the telomeric repeat amplification protocol. Nature Protocols. 1 (3), 1583-1590 (2006).
- Vogelstein, B., Kinzler, K. W. Digital PCR. Proceedings of the National Academy of Sciences of the United States of America. 96 (16), 9236-9241 (1999).
- Ludlow, A. T., et al. Quantitative telomerase enzyme activity determination using droplet digital PCR with single cell resolution. Nucleic Acids Research. 42 (13), e104 (2014).
- Huang, E. E., et al. The Maintenance of Telomere Length in CD28+ T Cells During T Lymphocyte Stimulation. Scientific Reports. 7 (1), 6785 (2017).
- Ludlow, A. T., Shelton, D., Wright, W. E., Shay, J. W. ddTRAP: A Method for Sensitive and Precise Quantification of Telomerase Activity. Methods Molecular Biology. 1768, 513-529 (2018).

