Injecting Gryllus bimaculatus Eggs
Summary
Here we present a protocol to inject cricket eggs, a technique which serves as a foundational method in many experiments in the cricket, including, but not limited to, RNA interference and genomic manipulation.
Abstract
Altering gene function in a developing organism is central to different kinds of experiments. While tremendously powerful genetic tools have been developed in traditional model systems, it is difficult to manipulate genes or messenger RNA (mRNA) in most other organisms. At the same time, evolutionary and comparative approaches rely on an exploration of gene function in many different species, necessitating the development and adaptation of techniques for manipulating expression outside currently genetically tractable species. This protocol describes a method for injecting reagents into cricket eggs to assay the effects of a given manipulation on embryonic or larval development. Instructions for how to collect and inject eggs with beveled needles are described. This relatively straightforward technique is flexible and potentially adaptable to other insects. One can gather and inject dozens of eggs in a single experiment, and survival rates for buffer-only injections improve with practice and can be as high as 80%. This technique will support several types of experimental approaches including injection of pharmacological agents, in vitro capped mRNA to express genes of interest, double-stranded RNA (dsRNA) to achieve RNA interference, use of clustered regularly interspaced short palindromic repeats (CRISPR) in concert with CRISPR-associated protein 9 (Cas9) reagents for genomic modification, and transposable elements to generate transient or stable transgenic lines.
Introduction
The ability to modify the genome or influence gene expression in organisms is the basis for the design of many types of experiments testing functional causality. It is also critical for the comparative and evolutionarily-relevant work that genomic and non-genomic modification techniques be available in organisms outside traditional genetic laboratory animal model systems (e.g., Mus musculus, Danio rerio, Drosophila melanogaster, and Caenorhabditis elegans). Whether it is the desire to understand organismal diversity1 or one's adherence to Krogh's principle, that for every biological question there is an organism best suited to its solution2,3, the ability to modify genomes or influence the gene expression is essential for modern experimental designs.
The cricket Gryllus bimaculatus is an emerging model system. Used for the last century in neuroethology experiments4, the last two decades have witnessed an increased experimental interest in the cricket, particularly focused on the evolution and development of this organism5. The cricket is a hemimetabolous insect that branches basally to well-studied holometabolous insects, such as D. melanogaster and Tribolium castaneum6. Due to its useful position on the evolutionary tree, scientists are interested in asking modern, sophisticated experimental questions in this insect, which has led to a growing interest in adapting molecular tools for the use in G. bimaculatus.
Injections of molecular reagents into cricket eggs can be used for genomic modification experiments as well as non-genomic manipulations of gene expression in embryos. For example, transgenic G. bimaculatus carrying eGFP insertions have been created using the transposase piggyBac7,8. Investigators have successfully created knockout G. bimaculatus using Zinc-finger nucleases (ZFNs) and transcription activator-like (TAL) effector nucleases (TALENs) to introduce double-stranded breaks in specific genomic regions9. Though ZFNs and TALENs allow site-specific targeting in animals beyond the big four model systems, these reagents have quickly been surpassed by the CRISPR/Cas9 system, which is simpler to use, more efficient, and highly flexible10. CRISPR has been used in G. bimaculatus to produce knock-out11 as well as knock-in lines12,13 In addition to genomic modification, dsRNA can be injected into eggs to knock down mRNA expression in developing embryos, allowing investigators to understand the role of specific transcripts throughout development14,15. Some limited details on how to inject cricket eggs have been published previously12.
Here, we describe a detailed protocol for injecting early G. bimaculatus eggs. This protocol is effective and easily adaptable to various laboratory settings, injection materials, and possibly to other insects. While additional details for designing and implementing genomic modification and knockdown experiments have been published elsewhere12,13, these approaches will ultimately rely on the injection protocol detailed here.
Protocol
1. Hardware Setup and Preparation of Materials
NOTE: Please see Table 1 and Table of Materials for preparation of solutions, reagent, and equipment details.
- Set up a dissecting microscope in order to see eggs and guide the injection needle. (Figure 1A shows a dissecting microscope equipped with fluorescence.) Fluorescence capability is advantageous but not necessary.
- Position a 3-axis micromanipulator to maneuver the injection needle into place (Figure 1A).
- Set up a microinjector with tubing and a needle holder to inject small amounts of material (Figure 1A). Optionally use a foot pedal which is not shown in the figure.
- Design and create a stamp to create wells for eggs (Figure 1C) by printing the inverse of the desired pattern, either with a 3D printer or a laser engraver.
NOTE: The 3D printed sample stamp shown is 4.5 cm x 5 cm with 128, 3 cm x 1 cm x 1 mm protrusions for creating individual wells. Details on how to make a variety of customizable molds have been published elsewhere16. - Prepare 10x Injection Buffer, HEPES buffered saline (HBS), and a stock solution of Tetramethylrhodamine Dextran dye and store at 4 °C.
- Prepare experimental solutions to be injected, such as dsRNA, CRISPR/Cas912, transposable elements, in vitro capped mRNA7,8, pharmacological agents17, ZFNs, or TALENS9.
- Place double-stick tape on several layers of standard lab tape on a glass microscope slide in order to create a platform 1 mm or so above the glass slide, which will be used for holding needles for the assessment.
- Prepare a cricket container approximately 40 cm x 60 cm in size with a lid possessing a mesh-covered opening for air circulation.
- Add food, water, and sheltering materials.
- Add several dozen healthy male and female adult crickets. Identify adult cricket by the presence of wings.
NOTE: A range of post-imaginal ages may increase egg yields. The density of crickets should be relatively high in order to be able to collect enough eggs for an experiment, but not so crowded that cannibalism occurs. For example, approximately two dozen healthy crickets, with a roughly equal male to female ratio, in a container of the size noted above should be sufficient to collect about 200 eggs in a one to two h period. Crickets can be kept at room temperature, but development and behavior will be optimal if crickets are maintained in a warm (23.5–26 °C), humid (40 – 60% relative humidity) incubator.
2. Making Needles for Injection
- Use Borosilicate Glass Capillaries with filament (see the Table of Materials for size details). Prepare the pull needles on the same day, or up to a few days before injections are completed.
- Place a capillary glass into the puller, center the glass on the filament, and tighten the knobs to secure the glass in place.
- Set the puller temperature near the glass ramp temp (-5 °C to +10 °C).
- Use a single-step, high-heat protocol to pull a long taper with a small opening but avoid melting the ends closed.
NOTE: For example, when using the micropipette puller detailed in the Table of Materials equipped with a box filament, use the following parameters for glass with a ramp temperature of 478: Heat 478; Pull 45; Vel 75; Del 120. Optimal conditions for producing a needle with the shape shown in Figure 2B should be determined empirically by each user. - Bevel needle tips in preparation for the injections.
- Wet the spinning grinder plane of the beveler (Figure 2A) with dripping water. Use either reverse osmosis (RO) or diethylpyrocarbonate (DEPC)-treated distilled water.
- Place the pulled needle into the beveler and turn to a 20° angle.
- Lower the needle until it just touches the grinding plane and bevel for 2 to 3 min.
- Assess the bevel by placing the beveled needle on double-stick tape that has been adhered to a glass microscope slide.
- Place the slide holding the needle on the stage of a compound microscope equipped with a camera and image acquisition software.
- Acquire an image of the needle tip using a 20x objective.
- Use the imaging software to measure the interior lumen diameter of the needle just proximal to the beveled opening (Figure 2C). The optimal size is 10 µm + 2 µm.
- Discard needles that have an opening below 8 µm and above 12 µm.
- Store the needles in a container with a lid to avoid getting dusty. Use a strip of wax or similar material to elevate the needles off the bottom of the container to prevent breaking the tips (Figure 2D).
3. Egg Collection and Preparation
- Maximize the number of eggs laid by depriving crickets of any moist laying material (water vials, egg dishes) overnight or for at least 8–10 h before attempting to collect eggs.
NOTE: Since females have the ability to internally store sperm, a differently timed procedure may be necessary if carefully timed fertilization is experimentally essential18. - Make 40 mL of 1% agarose in water and pour into a 10 cm petri dish.
- Place an egg-well stamp on the surface of the agarose before it solidifies.
- Remove the stamp, once the agarose is solidified, to reveal the wells (Figure 1C).
- Fill the agarose well plate with HBS containing 1% Penicillin/Streptomycin.
- Place the lid on the dish, wrap in parafilm and store at 4 °C.
NOTE: Dishes can be stored for a few days at 4 °C but should be warmed up to room temperature before use to avoid condensation. - Make an egg collection dish for crickets to lay eggs in by filling a 35 mm petri dish with white playground sand (Figure 1B).
NOTE: The sand specified in the Table of Materials is of the appropriate grain size. If the sand grains are too large, the sand will damage the eggs. - Cover the egg dish with a square of paper towel cut to be approximately 18 cm x 18 cm, and place on the top of dish with sand. Through this paper towel cover the filled dish with tap water.
- Tilt the petri dish and gently squeeze the top to remove excess water.
- Tuck the corners of the paper towel square under the dish and place the egg dish in the inverted lid, which will help keep the paper towel cover in place.
- Place the egg dish into the cricket bin and allow the adult females to oviposit eggs for one to two h.
NOTE: The relatively short time here ensures that all the eggs will be similar in the stage of development at the time of injection. If injection before cellularization is important, injections should occur within 14 h of egg laying19. - Meanwhile, allow the agarose dish (from step 3.6) to warm to the room temperature in preparation for holding eggs for injection.
- Remove egg collection dish, now containing freshly laid eggs, from the cricket bin, and remove the paper towel cover. Place a strainer with a pore size of 0.5–1 mm over a beaker of at least 500 mL capacity (Figure 1B).
- Rinse the contents of the egg-laying dish (sand and eggs) into the strainer under gently running tap water letting the sand grains fall through the strainer mesh into the water in the beaker below but leaving the cricket eggs in the strainer basket.
- Fill a container with RO water and place it in a tray. Invert the strainer over the container and tap it against the dish to dislodge the eggs into the water. The eggs will sink to the bottom of the container.
- Cut the tip off a P1000 pipette tip with scissors to make an opening approximately 3 mm in diameter. Place this tip on a P1000 pipettor and use it to transfer the eggs from the container to the agarose egg mold dish, transferring as little water as possible.
- Use plastic tweezers to line up eggs in agarose wells filled with HBS plus 1% Penicillin/Streptomycin. Each egg will sink to the bottom of an individual well. Cover with the petri dish lid until ready to inject.
4. Prepare the Microinjector
- Attach the microinjector to a compressed air or gas source (this protocol was optimized using either compressed air or N2).
NOTE: If using a portable air tank, fill to at least 75 psi. The tank will lose air pressure throughout the injections and may need to be refilled; injections become difficult below 40psi. - Turn on the microinjector. Set the injection time to 0.17 s and the pressure to 10–15 psi.
NOTE: The exact pressure needed will depend on the opening of the needle and must be determined empirically for every round of injection and/or new needle used. - Ensure that the BALANCE knob of the microinjector is turned to 0 (typically fully counter-clockwise) and that the microinjector is pressurized and in the injection mode.
5. Injections
- Filter about 500 µL of 10x Injection Buffer using a 1 mL syringe and a 0.45 µm syringe filter.
- Mix injection reagents (the details that follow are for the injection of dsRNA prepared as described in12).
NOTE: It may be advisable to design and perform several controls, especially when one is first learning this technique. Poking eggs without injecting, injecting buffer + dye alone, or injecting buffer + dye + a control reagent (such as eGFP dsRNA) are all good tests of how disruptive various elements of the injection protocol might be. See Figure 3 for the survivability of a number of different controls. - Start with a 4 μL dsRNA aliquot.
- Add 0.5 μL of the filtered 10x Injection Buffer to the dsRNA aliquot.
- Add 0.5 μL of 50 mg/mL Tetramethyl rhodamine Dextran dye stock solution. If working on a dissecting scope without fluorescence, use Phenol Red instead.
- Keep all materials on ice for the duration of the injection period.
- Load the injection solution into an injection needle using 20 μL loading tips and a p10 pipet.
- Draw up a 1.5 μL injection solution and insert the loading tip into the wide end of the injection needle.
- Eject solution as far down into the needle as possible and eliminate air bubbles by flicking gently; be careful not to break the needle.
- Place the dish of eggs under the dissecting microscope and select a low magnification of around 10x (1x magnification viewed through 10x eye objectives).
- Insert the needle into the injection housing and tighten.
NOTE: Take care that the needle is properly and firmly inserted into the housing. To do this, pull the wide end of the needle back out of the metal housing, bringing the silicon tubing in the housing with it. Adjust the silicon tubing so that the glass end of the needle protrudes just beyond the silicon. If this is not done, the silicon tubing may get pinched between the glass and the metal housing, blocking the end of the needle. - Carefully insert the injection housing with an attached needle into the micromanipulator. Be aware of the sharp end of the needle and be careful that it is not bumped into surfaces and broken.
- While looking at both the eggs and the needle through the microscope, move the needle near the eggs in the top left corner of the dish grid.
- Lower the needle until the tip enters the HBS plus 1% Penicillin/Streptomycin buffered solution in the dish.
- Center the needle in the field of vision and move the egg dish so that the needle is a few mm closer to the edge of the dish than the eggs.
- Do not obstruct the view of the grid of eggs with the needle.
- Set the microscope to the filter appropriate for Rhodamine so it is possible to observe the fluorescence in the needle and focus on the tip of the needle.
- On the microinjector, slowly turn the BALANCE knob clockwise until the injection solution starts to leak out of the needle into the suspension solution. The balance number on the screen of the microinjector will begin to increase, though the numerical value is not important. Next, turn the knob back counterclockwise slightly just until the dye stops leaking out of the needle.
NOTE: It is critical to set this balance each time a new needle is used. Without the balance set appropriately, the microinjector will continue to inject for some time after the injection is triggered. It is even possible that a single push of the injection button with an unbalanced needle will result in the expulsion of the entire contents of the loaded needle. - With the needle centered in the field of view, move the egg dish so that the needle is aimed at the egg to be injected first. It is recommended that injections start in one corner of the grid of arranged eggs, for example, the upper left corner.
- Adjust the magnification to about 50x; at this magnification, a single egg will fill most of the field of view.
- Use both the micromanipulator and one’s hand on the egg dish to move the needle into position for the injection.
- Advance the needle using the micromanipulator and insert the tip of the needle into the first egg to be injected.
- Insert the needle at 20–30% egg length from the posterior (blunter) end of the egg (Figure 1E), perpendicular to the long axis of the egg.
- Inject solution either with the injection foot pedal or the injection button on the microinjector. A small bolus of fluorescent material inside the egg will indicate a successful injection (Figure 1D).
NOTE: It is not uncommon for some yolk to be ejected from the egg during injection (Figure 1F), and this does not necessarily indicate that the injected egg will be inviable. - Retract the needle from the egg. If the egg is unintentionally lifted out of its well upon retraction of the needle, use small forceps to push the egg back into its well while retracting the needle.
- If the needle clogs during injection, remove the needle from the egg and, keeping the needle submerged in the HBS plus 1% Penicillin/Streptomycin solution covering the eggs, clear the clogged needle using either the Limpar function or by pushing the inject button.
- Continuing to use the same needle, repeat the injection procedure for as many eggs as possible. At first, this may be only about 20 or 30 eggs but will increase to over 100 with practice.
- If the needle becomes clogged and cannot be cleared, discard the needle, fill a new needle and begin again (i.e., return to step 5.7).
- Store the dish of injected eggs in a warm incubator (23.5–26 °C) that is slightly humid (to minimize the evaporation) until the embryos have reached the desired stage of development for the analysis.
- At least every 24 h, remove eggs that are no longer viable (Figure 3A,B), and replace the suspension solution with fresh HBS plus 1% Penicillin/Streptomycin.
- Two to three days after injection, transfer the eggs by gentle pipetting or with plastic forceps to paper towels moistened with HBS plus 1% Penicillin/Streptomycin in a lidded petri dish to continue development in the warm, humid incubator.
- Monitor the eggs, and remove eggs that are no longer viable each day (Figure 3A,B).
Representative Results
Crickets readily lay eggs in the moist material, and providing adequate material, such as moist sand or dirt, induces them to lay a large number of eggs. This is especially effective if crickets are first deprived of egg-laying material for 8–10 h. Eggs laid in clean sand can be easily separated, collected (Figure 1B) and placed into custom-designed egg wells for injection (Figure 1C). A dissecting microscope, a microinjector, and a micromanipulator (Figure 1A) can be used to inject individual eggs with a variety of materials (Figure 1D). Eggs are injected near the posterior end, at a point approximately 20–30% along the anterior-posterior axis (Figure 1E). Distinguishing the more pointed anterior end from the more blunted posterior end can be difficult, as these differences are often subtle (Figure 1E). Rolling an egg from side to side often helps to reveal which end is which. After removal of the injection needle, egg yolk is sometimes ejected (Figure 1F), though this does not appear to compromise the health of the developing embryo inside.
One of the most critical steps in this protocol is the preparation of beveled needles. After pulling long, slender needles they must be beveled to a 20° angle and have an opening diameter of 10μm (± 2 μm). If the diameter is too large, the injection solution will leak out of the needle, and these needles will create large holes in eggs, decreasing survival rates. Narrower needles increase survivability, but if they are too narrow, they tend to clog easily, which makes injections slow and frustrating. With a needle diameter of 10 μm ± 2 μm, it is possible to inject upwards of 100 eggs with a single needle without having the needle clog.
Needles can be beveled with a micro grinder or beveller (Figure 2A). These pieces of equipment can be obtained with a microscope attached, or one could add a dissecting scope on an independent stand for models that do not include the microscope. A small micromanipulator holds the needle, which can be tilted to the desired angle and lowered to the surface of a rotating grinding plane, which is moistened with dripping water. An example of a pulled, but unbeveled needle can be seen in Figure 2B, and a needle with a 20° bevel is shown in Figure 2C. Needles must then be stored safely to avoid damage and to be kept clean (Figure 2D).
Setting the balance and injection pressure appropriately on the microinjector should allow one to inject a comparable amount of material into each egg injected with the same needle. Optimization of injection and balance pressures will be necessary when changing to a needle with a different diameter. This optimization will maximize the consistency of injection pulses.
The survival rate is one important parameter for assessing the success of these experiments. Most dead eggs can easily be identified by sight as follows: the yolk and embryonic tissue within the egg begin to clump unevenly (Figure 3A) and eventually most of the yolk will migrate to one side of the egg (Figure 3B). When assessed after 4–6 days (equivalent to stages 8–15)19, greater than 85% of uninjected eggs survived while eggs injected with experimental reagents generally had a lower survival rate (Figure 3C). Controlling for all aspects of the injection itself, including the needle puncture, the introduction of dye and buffer, or the presence of vehicle or dsRNA, is required in order to understand the true effects of the experimental manipulation attempted (Figure 3C). Depending on the experimental manipulation, a higher level of lethality may be expected, and one may need to alter the timing of the assay or the timing of injection in order to assess any non-lethal effects of the manipulation.
The way one assesses the phenotypic results of injection depends on what was injected. For example, phenotypic changes as a result of specific mRNA knockdowns may be obvious at the gross anatomy level. For example, the gene Enhancer of zeste (E(z)) normally suppresses Hox genes including Ultrabithorax (Ubx). Injecting dsRNA for E(z) knocks down E(z) function and releases Ubx suppression, resulting in ectopic Ubx expression. This leads to the transformation of five pairs of ventral appendages (antenna, maxilla, labium, T1 and T2 leg) into T3 leg-like appendages, due to the derepression of Ubx in those segments20. In a second example, it is possible to use GFP expression to assay the successful insertion of a transgene. For example, when a cDNA encoding an eGFP-tagged Histone 2B gene under the control of a G. bimaculatus Actin promoter was inserted into the cricket genome using piggyBac transposase, it became possible to visualize each nucleus in the egg using fluorescence to excite the eGFP-tagged His2B protein (Figure 4C,D). In this case, it was possible to monitor the movement of nuclei over time7.
| Name of Solution | Components | Comments/Description |
| 10x Injection Buffer Solution | In 1 L H2O add: 0.8 g NaCl, 0.09 g Na2HPO4, 0.04 g KH2PO4, 2.98 g KCl | Store stock at 4 °C. Filter a small amount immediately before use with a 1 mL syringe equipped with a 0.45 µm syringe filter. Repeat as needed to filter approximately 500 µL of Injection Solution. Keep filtered Injection Solution on ice for the duration of the experiment. |
| HEPES buffered saline | In 1 L H2O, add: 8.77 g NaCl, 5.2 g HEPES, | pH to 7.0 and store at 4 °C. |
| Tetramethyl Rhodamine Dextran (10,000 MW) dye stock solution | Rhodamine dye from Thermofisher | This dye is often sold as a lyophilized powder. In this case, make up a stock solution of 50 mg/mL with water. Centrifuge at 12,000 x g for 5 min to remove any insoluble particles. Collect the supernatant after centrifugation, avoiding any particulate matter at the bottom of the tube, aliquot and freeze at -20 °C. Stock aliquots in use can be kept at 4 °C for one to two weeks. Avoid freeze-thawing aliquots multiple times. If needles are clogging due to dye, the dye can also be filtered through Whatman #2 filter paper. |
Table 1: Solution recipes and notes.
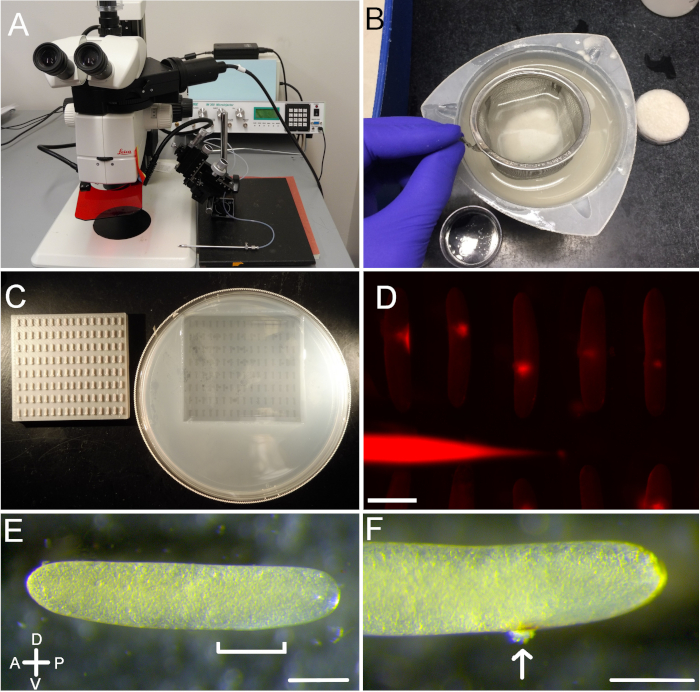
Figure 1: Egg injection protocol overview. (A) A typical set up that can be used for egg injections. (B) Eggs are separated from sand via a sieve. (C) Eggs are held for injection in small wells made by placing a mold into warm, liquid agarose. (D) Fluorescence in the needle and in five injected eggs can be seen through the fluorescent dissecting microscope. (E) An uninjected egg. The anterior end (slightly pointed; left) and posterior end (more blunted; right) are distinguishable from each other. The region most suitable for injection, near the posterior end, is shown with the bracket. (F) An egg two days after injection. The injection site is visible as a slight discoloration, and a small amount of expelled yolk is evident (arrow). Scale bars = 1 mm (D); 0.5 mm (E and F). Please click here to view a larger version of this figure.
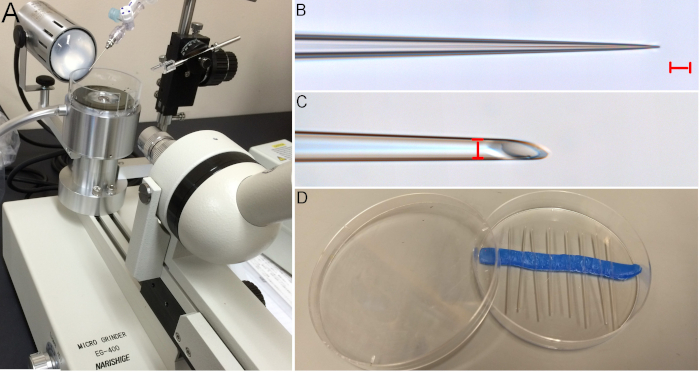
Figure 2: Needles are beveled using a micro grinder or beveller. (A) An example of a micro grinder with a circular, spinning grinding surface moistened by dripping RO water (water drips from the syringe shown in the top of the image). (B) Before beveling, pulled needles are long and narrow. (C) After beveling to 20°, a sharp injection needle is created. Note the 10 µm diameter of the inner lumen. (Red scale bars = 10 µm). (D) Pulled, beveled needles can be stored in a Petri dish with a lid and held in place using dental wax. Please click here to view a larger version of this figure.
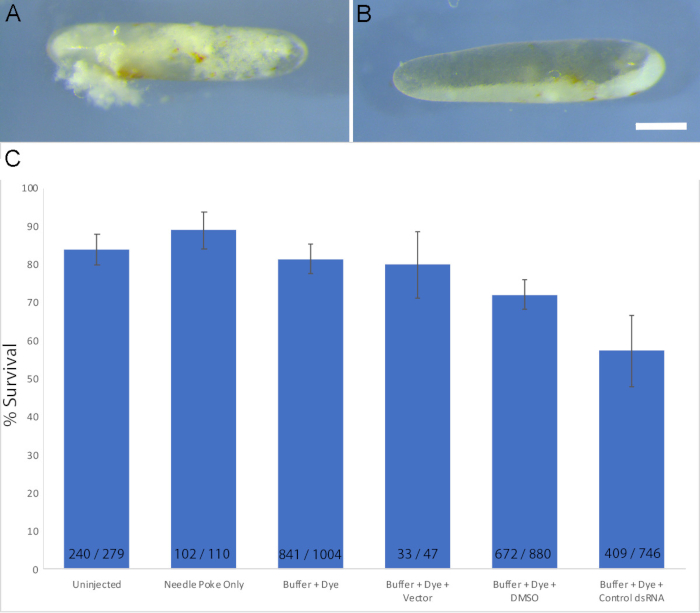
Figure 3: Injections can decrease the rate of survival. (A) Dead and dying eggs are obvious upon visual inspection. (B) Over time, the material will migrate to one side of the egg. (C) Comparison of survival rates four to six days after egg laying for a variety of conditions, including: uninjected eggs (n = 6 experiments); eggs punctured with a 9 µm needle but uninjected (n = 2 experiments); eggs injected with buffer and dye (n = 13 experiments); eggs injected with buffer, dye, and plasmid vector (n = 2 experiments); eggs injected with buffer, dye, and DMSO (n = 11 experiments); and eggs injected with buffer, dye, and control dsRNA (n = 22 experiments). The type of control dsRNA used included dsRNA against eGFP or dsRed. The numbers at the base of each bar note the total number of surviving eggs / total eggs used for each condition. Error bars represent standard error of the mean. Scale bar = 1 mm (A and B). Please click here to view a larger version of this figure.
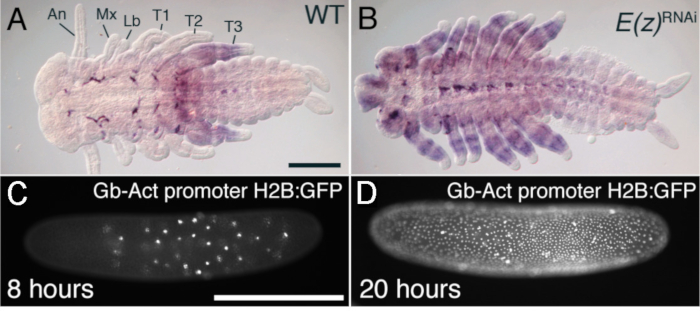
Figure 4: Assessing injection results. (A-B) Injection of Enhancer of Zeste (E(z)) dsRNA alters the normal expression of Ubx mRNA (wild type shown in (A) and leads to the transformation of antennae (AN) and mouthparts (Mx, Lb) into leg-like appendages (B). (C-D) Injection of early eggs with the transposable element piggyBac and histone2B-eGFP produces transgenic embryos expressing GFP in all nuclei. Location of nuclei can be observed 8 h after egg laying (C) and 20 h after egg laying (D). Abbreviations: An = Antenna; Mx = maxilla; Lb = labium; T1-3 = thoracic legs 1 to 3; RNAi = RNA interference; Gb = G. bimaculatus; Act = actin; H2B = Histone H2B; GFP = green fluorescent protein. Scale bars = 200 µm (A and B); 500 µm (E and F). These experimental results were originally described elsewhere7,20. Please click here to view a larger version of this figure.
Discussion
The two main challenges with this technique are the related issues of optimal needle size and survivability. Though smaller needles improve survivability, needles with narrower lumens have a greater degree of capillary forces at work, which makes it more likely that yolk will move into the needle causing it to clog. In the best case, blockages can be cleared simply by injecting another egg or by clearing the needle as described above. One can also attempt to increase the balance pressure on the microinjector until the yolk is pushed out of the needle. The other major challenge with this technique is survivability. In our hands, survival rates for eggs injected with experimental solutions are typically between 40 and 80%. Survival rates can be as low as 10% for those beginning to learn this technique but will improve with practice. Negative controls, as described above, are important for researchers to accurately assess changes in survival rate with practice, given that the introduction of various materials can lower survival rates (Figure 3C).
The timing of injections is an important consideration for each experimental approach as well. Older eggs are more difficult to inject than younger ones, and in general, survival rates are higher when injecting younger rather than older eggs. The introduction of the needle and injected material at later stages can lead to unintended anatomical damage. With experience, however, injection of two- to three-day old eggs is possible. Targeting the dorsal side of the egg at these stages helps to avoid damaging the embryo, which first forms on the ventral side. Targeting the posterior end may increase reagent access to the first nuclear divisions and germ band formation, which occur near this end of the egg19,21. If one is attempting to manipulate the genome, with the aim of achieving either widespread somatic or germline transmission of the genome modification, injections should be performed before cellularization, which starts approximately 14 h after egg laying19. If injecting dsRNA to knockdown mRNA levels, the time window for injections should be determined based on how early in development one wishes to knock down a gene of interest. While the effects of RNAi likely last for some time, dsRNA needs to be introduced in advance of the time at which one plans to assess any changes in phenotype. Keeping track of the time elapsed during injection and the progression of embryonic development may be important to ensure that the proper stage eggs are being injected. Regardless of what material is injected, developmental delays following injection are common, although the degree of the delay may vary depending on the injectant.
The protocol presented here relies on a number of relatively expensive pieces of equipment. It should be possible, however, to inject eggs successfully without the use of some or even all of these items. For example, there are a variety of microinjectors on the market, and some may be less expensive than the one suggested in the Table of Materials. It is also possible to simply use a syringe to inject an appropriate amount of material, though this would take some practice. Instead of an expensive micromanipulator, one could use a variety of materials to hold the needle steady and allow a slow, controlled advance, and a simple plasticine design has been used by others22. It may also be possible to avoid the use of a beveller, though in our experience proper beveling can make an enormous difference to the success of injections. It may be possible to sharpen a pulled needle by dragging the tip of the needle by hand across the finest sand paper, assessing this process under a microscope. Regardless, sharpened needles will make injections easier and improve survivability.
This injection technique is flexible and can be used for a variety of different manipulations. Here, we have shown results for dsRNA and piggyBac-dependent genomic modification experiments, but it is also possible to use CRISPR/Cas or other approaches with this technique. In the experiments included here, resulting phenotypes were obvious and easy to assess. Some experiments may require the use of immunohistochemistry or other visualization tools in order to see the effects of experimental manipulation. One alternative to injections is the use of electroporation, which can be used to introduce DNA or other macromolecules into cells and tissues in vivo. Electroporation can be used in older cricket eggs where embryos have already formed in order to target specific regions8, but injection is more useful when the experiment requires that the entire developing embryo is targeted. This injection technique should also be easily adaptable for use in eggs from a variety of different hemimetabolous and holometabolous insects, ideally improving the ability to undertake meaningful comparative studies.
Declarações
The authors have nothing to disclose.
Acknowledgements
Research reported in this project was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM10342 to HH3, and by NSF award number IOS-1257217 to CGE.
Materials
| Fluorescent dissecting microscope | Leica | M165 FC | Stereomicroscope with fluorescence | |
| External light source for fluorescence | Leica | EL 6000 | ||
| Microinjector | Narishige | IM-300 | -Accessories may include Injection Needles Holder, Input Hose (with a hose connector), AC Power Cord, Foot Switch, Silicone Rubber Gasket- | |
| mCherry filter cube | Leica | M205FA/M165FC | Filter cube for mCherry or similar red dye will work | |
| Micromanipulator | World Precision Instruments, Inc. | M3301R | Used with Magnetic Stand (Narishige, Type GJ-8) | |
| Magnetic stand | Narishige | MMO-202ND | ||
| Pipette Holder (Needle holder) | Narishige | HD-21 | ||
| Tubing to connect air source to microinjector | ||||
| Egg well stamp | 3D printed | custom | 3D printed on a Lulzbot Taz 5 using Poly Lactic Acid thermoplastic | |
| Microwave | various | |||
| Incubator or temperature controlled room | various | Temperatures of 23.5-26°C are needed. | ||
| cricket food | various | cat food or fish flakes are appropriate food. | ||
| cricket wter | vairous | Water can be held in vials and presented to crickets through cotton balls | ||
| cricket shelter | arious | Shelter materials can include crumpled paper towels or egg cartons | ||
| Glass capillary tubes | World Precision Instruments, Inc. | Item no. 1B100F-4 | Kwik-Fil™ Borosilicate Glass Capillaries, 100mm length, 0.58 mm ID, 1.0 mm OD, with filament | |
| Micropipette puller | Flaming/Brown | Model P-97 | Distributed by Sutter Instrument Co. | |
| Beveller/Micro grinder | Narishige | Model EG-45/EG-400 | EG-400 includes a microscope head | |
| Petri dishes | CellTreat | Product code 229693 | 90mm diameter | |
| Play Sand | Sandtastik Products Ltd. | B003U6QLVS | White play sand | |
| Agarose | American Bioanalytical | AB000972 | Agarose GPG/LE ultrapure | |
| Egg Strainer: Extra Fine Twill Mesh Stainless Steel Conical Strainers | US Kitchen Supply | Model SS-C123 | Pore size should be between 0.5 – 1.0 mm | |
| Penicillin Streptomycin | Gibco by Life Technologies | Ref 15070-063 | Pen Strep | |
| Plastic tweezers | Sipel Electronic SA | P3C-STD | Black Static Dissipative, 118mm | |
| syringe filters, 25mm diameter, 0.45 µm | Nalgene | 725-2545 | Use with 1 ml syringe | |
| 1 mL syringe, with Tuberculin Slip Tip | Becton Dickinson | 309602 | Use with syring filter to filter Injection Buffer , Luer-Lok tip syringes would also work | |
| Air tank (optional) | Midwest Products | Air Works® | Portable air tank | |
| Rhodamine dye | Thermofisher | D-1817 | dextran, tetramethylrhodamine 10,000MW, | |
| 20 mL loading tips | Eppendorf | Order no. 5242 956.003 | epT.I.P.S. 20uL Microloader | |
| Compound microscope | Zeiss | Axioskope 2 plus | ||
| 20X objective | Ziess | Plan-Apochromat 20x/0.75 M27 | ||
| camera | Leica | DMC 5400 | ||
| Leica Application Suite software | Leica | LAS | Version 4.6.2 used here | |
Referências
- Abzhanov, A., et al. Are we there yet? Tracking the development of new model systems. Trends in Genetics. 24 (7), 353-360 (2008).
- Krebs, H. A. The August Krogh principle: “For many problems there is an animal on which it can be most conveniently studied. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 194 (1), 221-226 (1975).
- Krogh, A. The Progress of Physiology. American Journal of Physiology. 90 (2), 243-251 (1929).
- Huber, F., Moore, T. E., Loher, W. . Cricket neurobiology and behavior. , (1989).
- Horch, H. W., Mito, T., Popadic, A., Ohuchi, H., Noji, S. . The Cricket as a Model Organism: Development, Regeneration, and Behavior. , (2017).
- Misof, B., et al. Phylogenomics resolves the timing and pattern of insect evolution. Science. 346 (6210), 763-767 (2014).
- Nakamura, T., et al. Imaging of transgenic cricket embryos reveals cell movements consistent with a syncytial patterning mechanism. Current Biology. 20 (18), 1641-1647 (2010).
- Shinmyo, Y., et al. piggyBac-mediated somatic transformation of the two-spotted cricket, Gryllus bimaculatus. Development, growth & differentiation. 46 (4), 343-349 (2004).
- Watanabe, T., et al. Non-transgenic genome modifications in a hemimetabolous insect using zinc-finger and TAL effector nucleases. Nature communications. 3, 1017 (2012).
- Wang, H., La Russa, M., Qi, L. S. CRISPR/Cas9 in Genome Editing and Beyond. Annual Review of Biochemistry. 85 (1), 227-264 (2016).
- Awata, H., Watanabe, T., Hamanaka, Y., Mito, T., Noji, S., Mizunami, M. Knockout crickets for the study of learning and memory: Dopamine receptor Dop1 mediates aversive but not appetitive reinforcement in crickets. Scientific Reports. 5, 15885 (2015).
- Horch, H. W., Liu, J. J., Mito, T., Popadic, A., Watanabe, T. Protocols in the Cricket. The Cricket as a Model Organism: Development, Regeneration, and Behavior. , 327-370 (2017).
- Watanabe, T., Noji, S., Mito, T. Genome Editing in the Cricket, Gryllus bimaculatus. Genome Editing in Animals. , 219-233 (2017).
- Kainz, F., Ewen-Campen, B., Akam, M., Extavour, C. G. Notch/Delta signalling is not required for segment generation in the basally branching insect Gryllus bimaculatus. Development. 138 (22), 5015-5026 (2011).
- Miyawaki, K., et al. Involvement of Wingless/Armadillo signaling in the posterior sequential segmentation in the cricket, Gryllus bimaculatus (Orthoptera), as revealed by RNAi analysis. Mechanisms of Development. 121 (2), 119-130 (2004).
- Donoughe, S., Kim, C., Extavour, C. G. High-throughput live-imaging of embryos in microwell arrays using a modular specimen mounting system. Biology Open. 7 (7), bio031260 (2018).
- Donoughe, S., Nakamura, T., Ewen-Campen, B., Green, D. A., Henderson, L., Extavour, C. G. BMP signaling is required for the generation of primordial germ cells in an insect. Proceeding of the National Academy of Science USA. 111 (11), 4133-4138 (2014).
- Larson, E., Andres, J., Harrison, R. Influence of the male ejaculate on post-mating prezygotic barriers in field crickets. PLOS ONE. 7 (10), e46202 (2012).
- Donoughe, S., Extavour, C. G. Embryonic development of the cricket Gryllus bimaculatus. Biologia do Desenvolvimento. , (2016).
- Matsuoka, Y., et al. Short germ insects utilize both the ancestral and derived mode of Polycomb group-mediated epigenetic silencing of Hox genes. Biology Open. 4 (6), 702-709 (2015).
- Rosenberg, M., Lynch, J., Desplan, C. Heads and tails: Evolution of antero-posterior patterning in insects. Biochimica et biophysica acta. 1789 (4), 333-342 (2009).
- Bacon, J., Strausfeld, N. Nonrandom resolution of neuron arrangements. Neuroanatomical Techniques: Insect Nervous System. , 357-372 (1980).

