环境细颗粒物小鼠组织中三种 DNA 损伤的质谱定量及水平评价
Summary
我们在这里描述的方法敏感和准确定量的病变 8-氧-7-二氢-2 ‘-脱氧鸟苷 (8-氧 ‘,1, n6-乙基 2 ‘-脱氧腺苷 (1,n6-do)和 1,n2-在 DNA 中的乙二-2 ‘-脱氧鸟苷 (1,n2-dguo)。应用该方法对暴露的阿可姆小鼠组织 (肺、肝、肾) 环境细颗粒物 (PM2.5) 的影响进行了评价。
Abstract
DNA 加合物和氧化 DNA 碱基是 DNA 病变的例子, 是对亲电物质的毒性评估、生物转化后产生活性电极或诱发氧化应激的有用生物标志物。在氧化核酸酶中, 研究最多的是 8-氧-7-8-二氢鸟嘌呤 (8-Xocogua) 或 8-oxo-7-8-二氢-2 ‘-脱氧鸟苷 (8-氧果), 这是氧化诱导的 DNA 碱基损伤的生物标志物。脂质过氧化过程产生的醛和环氧醛是能够形成诱变外环 DNA 加合物的亲电分子, 如乙二烯加合物 1,n2-乙二-2 ‘-脱氧鸟苷 (1,n2-1,n6-乙二-2 ‘-脱氧腺苷 (1,n 6-dado), 已被认为是炎症病理生理学中潜在的生物标志物。选择和敏感的方法, 他们在 DNA 中量化是必要的制定预防战略, 以减缓细胞突变率和慢性病的发展 (例如, 癌症, 神经退行性疾病)。在可用于检测它们的敏感方法 (与电化学或串联质谱检测器耦合的高效液相色谱 (彗星分析、免疫检测、 32p 后标记) 中, 最具选择性的是基于这些方法。在高效液相色谱联接到串联质谱 (HPLC-ESI-MSMS)。在分析复杂的生物样本时, 选择性是一个重要优势, HPLC-ESI-MMSY 演变为 DNA、尿液、血浆和唾液等生物基质中修饰核苷的黄金标准。使用同位素标记的内部标准增加了在 DNA 水解和分析物富集步骤中更正分子损失的优势, 以及样品之间分析物电离的差异。当存在多个峰时, 它还有助于识别正确的色谱峰。
我们在这里提出了验证敏感, 准确的 HPLC-ESIC-MMSMS 方法, 成功地应用于定量的 8-oxodGuo, 1,n-dAdo和1,n-dAdo 在肺, 肝脏和肾脏 do在肺, 肝脏和肾脏 do环境 PM2.5暴露的影响的评估。
Introduction
一些活性氧物种 (ROS) 能够氧化 DNA 碱基的碳双键和脱氧核糖中的一些碳键, 产生氧化碱基和 DNA 链断裂1。作为一种富含氮和氧原子的负电荷分子, DNA 也是与亲核位点 (氮和氧) 共价反应的亲电基团的靶标, 其产物被称为 DNA 加合物2。因此, DNA 加合物和氧化 DNA 碱基是 DNA 病变的例子, 这些病变是对亲电物质的毒性评估、生物转化时产生活性电泳或诱导氧化应激1的有用生物标志物, 2。虽然修改后的 DNA 碱基可以通过碱基或核苷酸切除修复 (BER 或 NER) 从 DNA 中去除, 但诱导 DNA 病变的产生和去除之间的不平衡有利于前者导致他们的 DNA 加班水平净增加 3 。结果是 dna 突变率的增加, 基因表达的减少, 蛋白质活性降低2,4,5,6, 7,与疾病的发展。DNA 突变可能会影响多种细胞功能, 如细胞信号转导、细胞周期、基因组完整性、端粒稳定性、表观基因组、染色质结构、RNA 拼接、蛋白质稳态、代谢、凋亡和细胞分化 8 ,9。减缓细胞突变率和慢性病发展 (如癌症、神经退行性疾病) 的策略通过突变来源的知识, 其中包括 DNA 病变及其原因。
由于接触污染物、持续炎症、疾病病理生理学 (如糖尿病) 等原因, 内生异常过度, 是生物分子损伤的重要原因, 包括 DNA 和脂质损伤1。例如, 由过渡金属离子 (Fe2 +, cu+) 还原的 h2o2 形成的高反应羟基自由基 (oh) 氧化扩散控制下的 DNA 碱基、dna 糖层和多不饱和脂肪酸率10。在已经有特征的80种氧化核酸酶3中, 研究最多的是 8-oxo-7, 8-二氢鸟嘌呤 (8-oxoGua) 或 8-oxo-7-二氢-2 ‘-脱氧鸟苷 (8-奥科德戈戈,图 1), 这种病变能够诱导 gt 转化。哺乳动物细胞10,11。它是由鸟嘌呤的单电子氧化或 dna 1 中的羟基自由基或鸟嘌呤的单氧攻击而形成的。多不饱和脂肪酸是高活性氧化剂的其他重要靶点, 如·oh , 它启动了脂质过氧化1,12的过程。它产生脂肪酸过氧化氢, 可能分解为亲电醛和环氧醛, 如丙二醛, 4-羟基-2-非烯, 2, 4-十二烷基, 4, 5-py-(2e)-十二烯, 六烯, 阿克罗林, 氯酮醛, 这些能够形成诱变的外环 dna 加合物, 如丙二醛、丙醇或乙二醇加合物1,12,13。乙二醇加合物 1,n2-乙二 ‘-脱氧鸟苷 (1,n2-dguo,图 1) 和 1,n6-乙二-2 ‘-脱氧腺苷 (1,n6-dado,图1) 已被认为是炎症病理生理学中潜在的生物标志物 14,15。
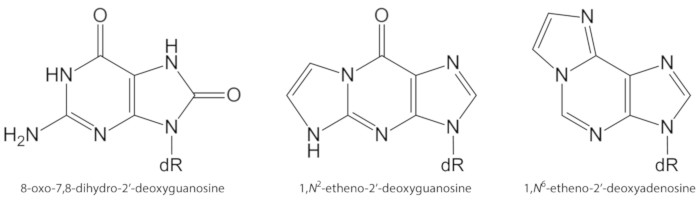
图 1.本研究量化的 DNA 病变的化学结构.dR = 2 脱氧核糖。奥利维拉等人对这一数字作了修改。请点击这里查看此图的较大版本.
20世纪80年代初进行的研究使其能够通过高效液相色谱结合电化学检测 (HPLC-ECD) 对 8-氧郭进行灵敏检测。在氧化条件下, hplc-ecd 对 8-氧果进行定量, 使人们认识到, 8-氧果是 dna1,16中氧化诱导碱基损伤的生物标志物。虽然在低 fmol 范围17中, Hplc-ecd 测量具有鲁棒性, 并允许对 8-氧郭进行量化, 但它依赖于分析物保留时间的准确性进行分析物的识别, 并依靠色谱分辨率来避免干扰。其他样品成分。由于电化学检测需要在流动阶段使用盐 (例如, 磷酸钾、醋酸钠), 因此维持适当的分析条件需要常规的柱和设备清洗时间。
或者, 使用细菌 DNA 修复酶甲胺吡啶 dna 糖基酶 (FPG) 和人类8-oxoguanine 糖基酶 1 (Gggg1), 从 DNA 中检测和去除 8-Xooga, 作为诱导 DNA 碱的一种方法网站。碱基不稳定位点转化为 DNA 链断裂, 并允许非常敏感的间接定量 8-奥克瓜通过碱性单细胞凝胶电泳 (“彗星检测”)。高灵敏度和完成的分析, 而不需要细胞 DNA 提取是这种类型的检测的主要优点。它给出了 DNA 中 8-氧瓜的最低稳态水平, 通常比基于高效液相色谱的生物分析方法获得的水平低7-10 倍。然而, 它是一个间接测量的8-oxoGua 和一些缺点是缺乏特异性或未知的效率, 使用的修复酶 1,16,18。
免疫检测是用于检测 8-oxoGua 1 和外环 DNA 加合物的其他方法, 如 1,n6-Dado 和 1,n2-dowo12。尽管灵敏度很高, 但使用抗体检测 dna 病变的一个缺点是, 由于与生物样本的其他成分 (包括正常 dna 碱基1,12)的交叉反应, 缺乏特异性。外环 DNA 加合物, 包括 1,n-dAdo 和 1,n2-dguo, 也可以检测和量化高度敏感的32p 后标记检测12。32p 后贴标的高灵敏度允许使用极少量的 dna (例如, 10 微克) 来检测每10个正常碱基中大约1个加合物 19.然而, 放射性化学品的使用、缺乏化学特异性和精度低是一些缺点 19、20。
上述方法的一个共同局限性是检测所需分子的选择性或特异性较低。在这种情况下, HPLC 与电喷雾电离串联质谱 (HPLC-ESI-MSMS 和 HPLC-MS3) 相结合, 发展成为在 dna、尿液、血浆和唾液等生物基质中定量修饰核苷的黄金标准1,19,20. hplc-esi-mmsms 方法的优点是灵敏度 (通常在低 fmol 范围内) 和高特异性提供的 i) 色谱分离, ii) 在质量内分子分裂的特征和已知模式分光计碰撞室, 以及 iii) 在多反应监测模式1,19中准确测量所选质量电荷比 (m/z)。使用同位素标记的内部标准增加了在 DNA 水解和分析物富集步骤中更正分子损失的优势, 以及样品之间分析物电离的差异。当一个峰出现 1、12、19、20时, 它还有助于识别正确的色谱峰。
在从不同生物样本 12、15、20中提取的 dna 中, 采用了几种基于 hplc-esi-mmsms 的方法对其进行了定量. , 21,22,23,24,25, 26,27, 28,29.细颗粒 (PM2.5) 携带有机和无机化学物质, 如多环芳烃 (pahs)、硝基多环芳烃、醛、酮、羧酸、奎诺、金属和水溶性离子, 这些化学物质可能会引起炎症和氧化应激, 有利于生物分子损伤发生的条件和疾病30,31,32,33。我们在这里验证了 HPLC-ESI-MMSMS 方法, 成功地应用于定量的 8-oxodGuo, 1,n6-do和 1,n2-dguo 在肺, 肝脏和肾脏 do对阿美鼠的评估环境 PM2.5曝光的影响34。
Protocol
Representative Results
Discussion
采用高效液相色谱法进行的8-oxodguo 分析中发现的一个主要问题是, 在 dna 提取、dna 水解和 dna 水解物浓度 22,38的研究过程中, 可能会诱导其形成。为了最大限度地减少8-Oxodoguo 人工物形成的问题, 建议在所有 DNA 提取、储存和水解溶液中添加脱氧胺, 使用碘化钠碎屑法, 并避免 DNA 提取中的苯酚, 如并且脱氧核糖核酸的用途共计接近100μg 在水解做法, 以最大?…
Declarações
The authors have nothing to disclose.
Acknowledgements
Fapesp (和平与安全基金会), cnpq (prc. 454214/2014-6 和 42918m/2016-6)、capes、prpusp (próreitoria de pesquisa da o d sculo)、inct inaira (mct/cnpq/fndc-capese/capese/FEMIG/FAPERJ/FAPESP;Proc. 573813/2008-6), INCT Redoxoma (Fapsp/cnpqq/capes;Proc. 573530/2008-4), NAP 氧化还原瘤 (PRPUSP;Proc. 2011.1.9352.1.8) 和 CENID 氧化还原瘤 (FAPESP;方案 2012-0797-8)。T. f. Oliveira 和 A. A. F. Oliveira 获得了 FAPESP (Proc. 2012163-8、201-r8891比0、2012/08617-4) 和 CAPES (卫六-德苏格) 和 CAPES (卫六) 的奖学金。M. H. g. Medeiros、P. di Mascio、P. h. n. Saldiva 和 a. p. m. Loureiro 接受了 CNPq 的研究金。
这项工作中的一些数字和表格最初发表在奥利维拉 a. a. f. 等人身上, 对来自巴西圣保罗市的接触浓缩环境微粒物质的小鼠的遗传毒性和表观毒性作用 (PM2.5)。粒子和纤维毒理学.15、40 (2018年)。
Materials
| [15N5]-2’-deoxyadenosine | Cambridge Isotope Laboratories | NLM-3895-25 | |
| [15N5]-2’-deoxyguanosine | Cambridge Isotope Laboratories | NLM-3899-CA-10 | |
| acetonitrile | Carlo Erba Reagents | 412413000 | |
| alkaline phosphatase from bovine intestinal mucosa | Sigma | P5521 | |
| ammonium acetate | Merck | 101116 | |
| calf thymus DNA | Sigma | D1501 | |
| cell lysis solution | QIAGEN | 158908 | |
| chloroform | Carlo Erba Reagents | 412653 | |
| deferoxamine | Sigma | D9533 | |
| deoxyribonuclease I (DNase I) | Bio Basic Inc | DD0649 | |
| ethanol | Carlo Erba Reagents | 414542 | |
| formic acid | Sigma-Aldrich | F0507 | |
| HPLC-ESI-MS/MS system | HPLC: Agilent 1200 series ESI-MS/MS: Applied Biosystems/MDS Sciex Instruments | HPLC: binary pump (G1312B), isocratic pump (G1310A), column oven with a column switching valve (G1316B), diode array detector (G1315C), auto sampler (G1367C). ESI-MS/MS: Linear Quadrupole Ion Trap mass spectrometer, Model 4000 QTRAP. | |
| HPLC/DAD system | Shimadzu | Two pumps (LC-20AT), photo diode array detector (DAD-20AV), auto-injector (Proeminence SIL-20AC), column oven (CTO-10AS/VP) | |
| HPLC column (50 x 2.0 mm i.d., 2.5 µm, C18) | Phenomenex | 00B-4446-B0 | |
| HPLC column (150 x 2.0 mm i.d., 3.0 µm, C18) | Phenomenex | 00F-4251-B0 | |
| HPLC column (250 x 4.6 mm i.d., 5.0 µm, C18) | Phenomenex | 00G-4252-E0 | |
| HPLC C18 security guard cartridge (4.0 x 3.0 mm i.d.) | Phenomenex | AJO-4287 | |
| isoamyl alcohol | Sigma-Aldrich | M32658 | |
| isopropyl alcohol (isopropanol) | Carlo Erba Reagents | A412790010 | |
| ketamine | Ceva | Commercial name: Dopalen | |
| magnesium chloride | Carlo Erba Reagents | 349377 | |
| magnesium chloride | Sigma | M2393 | |
| methanol | Carlo Erba Reagents | L022909K7 | |
| phosphodiesterase I from Crotalus atrox | Sigma | P4506 | |
| protein precipitation solution | QIAGEN | 158912 | |
| proteinase K | Sigma-Aldrich | P2308 | |
| ribonuclease A | Sigma | R5000 | |
| sodium chloride | Sigma-Aldrich | S9625 | |
| SPE-C18 (Strata-X) | Phenomenex | 8B-S100-TAK | |
| tris(hydroxymethyl)-aminomethane | Carlo Erba Reagents | 489983 | |
| xylazine | Syntec do Brasil | Commercial name: Xilazin |
Referências
- Cadet, J., Davies, K. J. A., Medeiros, M. H. G., Di Mascio, P., Wagner, J. R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radical Biology and Medicine. 107, 13-34 (2017).
- Barnes, J. L., Zubair, M., John, K., Poirier, M. C., Martin, F. L. Carcinogens and DNA damage. Biochemical Society Transactions. 46, 1213-1224 (2018).
- Cadet, J., Davies, K. J. A. Oxidative DNA damage & repair: An introduction. Free Radical Biology and Medicine. 107, 2-12 (2017).
- Cao, H., Jiang, Y., Wang, Y. Stereospecific synthesis and characterization of oligodeoxyribonucleotides containing an N2-(1-carboxyethyl)-2′-deoxyguanosine. Journal of the American Chemical Society. 129, 12123-12130 (2007).
- Breyer, V., et al. Analysis and biological relevance of advanced glycation end-products of DNA in eukaryotic cells. The FEBS Journal. 275, 914-925 (2008).
- Tamae, D., Lim, P., Wuenschell, G. E., Termini, J. Mutagenesis and repair induced by the DNA advanced glycation end product N2-1-(carboxyethyl)-2′-deoxyguanosine in human cells. Bioquímica. 50, 2321-2329 (2011).
- Hecht, S. S. Lung carcinogenesis by tobacco smoke. International Journal of Cancer. 131, 2724-2732 (2012).
- Garraway, L. A., Lander, E. S. Lessons from the cancer genome. Cell. 153, 17-37 (2013).
- Ong, T. P., Loureiro, A. P. M. Nutritional interventions in age-related genetic and epigenetic instability and cancer. Anti-ageing nutrients: Evidence-based prevention of age-associated diseases. , (2015).
- Evans, M. D., Dizdaroglu, M., Cooke, M. S. Oxidative DNA damage and disease: induction, repair and significance. Mutation Research. 567, 1-61 (2004).
- Moriya, M. Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-oxoguanine in DNA induces targeted GC → TA transversions in simian kidney cells. Proceedings of the National Academy of Sciences of the United States of America. 90, 1122-1126 (1993).
- Medeiros, M. H. G. Exocyclic DNA adducts as biomarkers of lipid oxidation and predictors of disease. Challenges in developing sensitive and specific methods for clinical studies. Chemical Research in Toxicology. 22, 419-425 (2009).
- Guéraud, F. 4-Hydroxynonenal metabolites and adducts in pre-carcinogenic conditions and cancer. Free Radical Biology and Medicine. 111, 196-208 (2017).
- Nair, U., Bartsch, H., Nair, J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: A review of published adduct types and levels in humans. Free Radical Biology and Medicine. 43, 1109-1120 (2007).
- Pang, B., et al. Lipid peroxidation dominates the chemistry of DNA adduct formation in a mouse model of inflammation. Carcinogenesis. 28, 1807-1813 (2007).
- Møller, P., et al. Harmonising measurements of 8-oxo-7,8-dihydro-2′-deoxyguanosine in cellular DNA and urine. Free Radical Research. 46, 541-553 (2012).
- Hofer, T., Moller, L. Optimization of the workup procedure for the analysis of 8-oxo-7,8-dihydro-2′-deoxyguanosine with electrochemicaldetection. Chemical Research in Toxicology. 15, 426-432 (2002).
- Collins, A., El Yamani, N., Dusinska, M. Sensitive detection of DNA oxidation damage induced by nanomaterials. Free Radical Biology and Medicine. , 69-76 (2017).
- Zubel, T., Buerkle, A., Mangerich, A. Mass spectrometric analysis of sulfur mustard-induced biomolecular adducts: Are DNA adducts suitable biomarkers of exposure?. Toxicology Letters. 293, 21-30 (2018).
- Tretyakova, N., Goggin, M., Sangaraju, D., Janis, G. Quantitation of DNA adducts by stable isotope dilution mass spectrometry. Chemical Research in Toxicology. 25, 2007-2035 (2012).
- Churchwell, M. I., Beland, F. A., Doerge, D. R. Quantification of multiple DNA adducts formed through oxidative stress using liquid chromatography and electrospray tandem mass spectrometry. Chemical Research in Toxicology. 15, 1295-1301 (2002).
- Chao, M. R., Yen, C. C., Hu, C. W. Prevention of artifactual oxidation in determination of cellular 8-oxo-7,8-dihydro-2′-deoxyguanosine by isotope-dilution LC-MS/MS with automated solid-phase extraction. Free Radical Biology and Medicine. 44, 464-473 (2008).
- Danielsen, P. H., et al. Oxidative stress, inflammation, and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicological Sciences. 118, 574-585 (2010).
- Danielsen, P. H., et al. Oxidative stress, DNA damage, and inflammation induced by ambient air and wood smoke particulate matter in human A549 and THP-1 cell lines. Chemical Research in Toxicology. 24, 168-184 (2011).
- Garcia, C. C. M., et al. [13C2]-Acetaldehyde promotes unequivocal formation of 1,N2-propano-2′-deoxyguanosine in human cells. Journal of the American Chemical Society. 133, 9140-9143 (2011).
- Angeli, J. P. F., et al. Lipid hydroperoxide-induced and hemoglobin-enhanced oxidative damage to colon cancer cells. Free Radical Biology and Medicine. 51, 503-515 (2011).
- Yu, Y., et al. Comprehensive assessment of oxidatively induced modifications of DNA in a rat model of human Wilson’s disease. Molecular and Cellular Proteomics. 15, 810-817 (2016).
- Torres-Cuevas, I., Aupi, M., Asensi, M. A., Vento, M., Ortega, &. #. 1. 9. 3. ;., Escobar, J. 7,8-Hydroxy-2′-deoxyguanosine/2′-deoxiguanosine ratio determined in hydrolysates of brain DNA by ultrachromatrography coupled to tandem mass spectrometry. Talanta. 170, 97-102 (2017).
- Wu, D., et al. Detection of 8-hydroxydeoxyguanosine (8-OHdG) as a biomarker of oxidative damage in peripheral leukocyte DNA by UHPLC-MS/MS. Journal of Chromatography B. 1064, 1-6 (2017).
- IARC. . Monographs on the Evaluation of Carcinogenic Risks to Humans: Outdoor Air Pollution. 109, (2016).
- De Martinis, B. S., Kado, N. Y., Carvalho, L. R. F., Okamoto, R. A., Gundel, L. A. Genotoxicity of fractionated organic material in airborne particles from São. Mutation Research. 446, 83-94 (1999).
- Karlsson, H. L., Nygren, J., Möller, L. Genotoxicity of airborne particulate matter: The role of cell-particle interaction and of substances with adduct-forming and oxidizing capacity. Mutation Research. 565, 1-10 (2004).
- Bell, M. L., Dominici, F., Ebisu, K., Zeger, S. L., Samet, J. M. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environmental Health Perspectives. 115, 989-995 (2007).
- Oliveira, A. A. F., et al. Genotoxic and epigenotoxic effects in mice exposed to concentrated ambient fine particulate matter (PM2.5) from São Paulo city, Brazil. Particle and Fibre Toxicology. 15, 40 (2018).
- Loureiro, A. P. M., Zhang, W., Kassie, F., Zhang, S., Villalta, P. W., Wang, M., Hecht, S. S. Mass spectrometric analysis of a cyclic 7,8-butanoguanine adduct of N-nitrosopyrrolidine: comparison to other N-nitrosopyrrolidine adducts in rat hepatic DNA. Chemical Research in Toxicology. 22, 1728-1735 (2009).
- Loureiro, A. P. M., Marques, S. A., Garcia, C. C. M., Di Mascio, P., Medeiros, M. H. G. Development of an on-line liquid chromatography-electrospray tandem mass spectrometry assay to quantitatively determine 1,N2-etheno-2′-deoxyguanosine in DNA. Chemical Research in Toxicology. 15, 1302-1308 (2002).
- Mangal, D., et al. Analysis of 7,8-dihydro-8-oxo-2′-deoxyguanosine in cellular DNA during oxidative stress. Chemical Research in Toxicology. 22, 788-797 (2009).
- ESCODD (European Standards Committee on Oxidative DNA Damage). Comparative analysis of baseline 8-oxo-7,8-dihydroguanine in mammalian cell DNA, by different methods in different laboratories: an approach to consensus. Carcinogenesis. 23, 2129-2133 (2002).
- Helbock, H. J., et al. DNA oxidation matters: The HPLC-electrochemical detection assay of 8-oxo-deoxyguanosine and 8-oxo-guanine. Proceedings of the National Academy of Sciences of the United States of America. 95, 288-293 (1998).
- Risom, L., et al. Oxidative DNA damage and defence gene expression in the mouse lung after short-term exposure to diesel exhaust particles by inhalation. Carcinogenesis. 24, 1847-1852 (2003).
- Risom, L., et al. Repeated inhalations of diesel exhaust particles and oxidatively damaged DNA in young oxoguanine DNA glycosylase (OGG1) deficient mice. Free Radical Research. 41, 172-181 (2007).
- Tsurudome, Y., et al. Changes in levels of 8-hydroxyguanine in DNA, its repair and OGG1 mRNA in rat lungs after intratracheal administration of diesel exhaust particles. Carcinogenesis. 20, 1573-1576 (1999).
- Marie-Desvergne, C., Maître, A., Bouchard, M., Ravanat, J. L., Viau, C. Evaluation of DNA adducts, DNA and RNA oxidative lesions, and 3-hydroxybenzo(a)pyrene as biomarkers of DNA damage in lung following intravenous injection of the parent compound in rats. Chemical Research in Toxicology. 23, 1207-1214 (2010).
- Iwai, K., et al. Early oxidative DNA damages and late development of lung cancer in diesel exhaust-exposed rats. Environmental Research. 84, 255-264 (2000).
- Ichinose, T., et al. Lung carcinogenesis and formation of 8-hydroxy-deoxyguanosine in mice by diesel exhaust particles. Carcinogenesis. 18, 185-192 (1997).
- Schmerold, I., Niedermu, H. Levels of 8-hydroxy-2′-deoxyguanosine in cellular DNA from 12 tissues of young and old Sprague Dawley rats. Experimental Gerontology. 36, 1375-1386 (2001).
- Garcia, C. C. M., Freitas, F. P., Di Mascio, P., Medeiros, M. H. G. Ultrasensitive simultaneous quantification of 1,N2-etheno-2′-deoxyguanosine and 1,N2-propano-2′-deoxyguanosine in DNA by an online liquid chromatography-electrospray tandem mass spectrometry assay. Chemical Research in Toxicology. 23, 1245-1255 (2010).
- Godshalk, R., et al. Comparison of multiple DNA adduct types in tumor adjacent human lung tissue: effect of cigarette smoking. Carcinogenesis. 23, 2081-2086 (2002).
- Dechakhamphu, S., et al. Lipid peroxidation and etheno DNA adducts in white blood cells of liver fluke-infected patients: protection by plasma alpha-tocopherol and praziquantel. Cancer Epidemiology Biomarkers and Prevention. 19, 310-318 (2010).
- Arab, K., et al. Typical signature of DNA damage in white blood cells: a pilot study on etheno adducts in Danish mother-newborn child pairs. Carcinogenesis. 30, 282-285 (2009).
- Nair, J., et al. High dietary omega-6 polyunsaturated fatty acids drastically increase the formation of etheno-DNA base adducts in white blood cells of female subjects. Cancer Epidemiology Biomarkers and Prevention. 6, 597-601 (1997).

