Discrimintion and Mapping of the Primary and Processed Transcripts in Maize Mitochondrion Using a Circular RT-PCR-based Strategy
Summary
We present a circular RT-PCR-based strategy by combining circular RT-PCR, quantitative RT-PCR, RNA 5' polyphosphatase-treatment, and Northern blot. This protocol includes a normalization step to minimize the influence of unstable 5' triphosphate, and it is suitable for discriminating and mapping the primary and processed transcripts stably accumulated in maize mitochondrion.
Abstract
In plant mitochondria, some steady-state transcripts have 5' triphosphate derived from transcription initiation (primary transcripts), while the others contain 5' monophosphate generated post-transcriptionally (processed transcripts). To discriminate between the two types of transcripts, several strategies have been developed, and most of them depend on presence/absence of 5' triphosphate. However, the triphosphate at primary 5' termini is unstable, and it hinders a clear discrimination of the two types of transcripts. To systematically differentiate and map the primary and processed transcripts stably accumulated in maize mitochondrion, we have developed a circular RT-PCR (cRT-PCR)-based strategy by combining cRT-PCR, RNA 5' polyphoshpatase treatment, quantitative RT-PCR (RT-qPCR), and Northern blot. As an improvement, this strategy includes an RNA normalization step to minimize the influence of unstable 5' triphosphate.
In this protocol, the enriched mitochondrial RNA is pre-treated by RNA 5' polyphosphatase, which converts 5' triphsophate to monophosphate. After circularization and reverse transcription, the two cDNAs derived from 5' polyphosphatase-treated and non-treated RNAs are normalized by maize 26S mature rRNA, which has a processed 5' end and is insensitive to 5' polyphosphatase. After normalization, the primary and processed transcripts are discriminated by comparing cRT-PCR and RT-qPCR products obtained from the treated and non-treated RNAs. The transcript termini are determined by cloning and sequencing of the cRT-PCR products, and then verified by Northern blot.
By using this strategy, most steady-state transcripts in maize mitochondrion have been determined. Due to the complicated transcript pattern of some mitochondrial genes, a few steady-state transcripts were not differentiated and/or mapped, though they were detected in a Northern blot. We are not sure whether this strategy is suitable to discriminate and map the steady-state transcripts in other plant mitochondria or in plastids.
Introduction
In plant mitochondria, many mature and precursor RNAs are accumulated as multiple isoforms, and the steady-state transcripts can be divided into two groups based on the difference at their 5' ends1,2,3,4. The primary transcripts have 5' triphosphate ends, which are derived from transcription initiation. By contrast, the processed transcripts have 5' monophosphate generated by post-transcriptional processing. Discrimination and mapping of the two types of transcripts are important to unravel the molecular mechanisms underlying transcription and transcript end maturation.
To distinguish between the primary and processed transcripts in plant mitochondrion, four major strategies have been developed. The first strategy is to pre-treat the mitochondrial RNAs with tobacco acid pyrophosphatase (TAP), which converts 5' triphosphate to monophosphate and enables primary transcripts to be circularized by RNA ligase. The transcript abundances of TAP-treated and non-treated RNA samples are then compared by rapid amplification of cDNA ends (RACE) or circular RT-PCR (cRT-PCR)2,3,4. In the second strategy, processed transcripts are firstly depleted from mitochondrial RNAs using terminator 5'-phosphate-dependant exonuclease (TEX), and the primary transcripts left are then mapped by primer extension analysis5,6. The third strategy is to pre-cap the primary transcripts using guanylyl transferase, and then the position of triphosphated 5' termini is determined by primer extension together with ribonuclease or S1 nuclease protection analysis7,8,9. Different from those depending on the presence/absence of 5' triphosphate, the fourth strategy combines in vitro transcription, site-directed mutagenesis, and primer extension analysis to characterize the putative promoters and determine the transcription initiation sites8,10,11. By using these strategies, many primary and processed transcripts have been determined in plant mitochondria.
However, several studies have reported that the 5' triphosphate of primary transcripts were unstable, and they were easily converted to monophosphate for unknown reason2,4,12,13. This problem hinders a clear discrimination of the two types of transcripts by using techniques depending on the presence/absence of 5' triphosphate, and previous efforts to systematically discriminate between the primary and processed transcripts in plant mitochondria failed2,12.
In this protocol, we combine cRT-PCR, RNA 5' polyphosphatase treatment, RT-qPCR, and Northern blot to systematically distinguish the primary and processed transcripts stably accumulated in maize (Zea mays) mitochondrion (Figure 1). cRT-PCR allows simultaneous mapping of 5' and 3' extremities of a RNA molecule, and it is usually adapted to map transcript termini in plants2,12,14,15. RNA 5' polyphosphatase could remove two phosphates from the triphosphated 5' termini, which makes the primary transcripts available for self-ligation by RNA ligase. Previous studies showed that mature 26S rRNA in maize had processed 5' terminus, and it was insensitive to RNA 5' polyphosphatase1,16. To minimize the influence of unstable triphosphate at primary 5' termini, the 5' polyphosphatase-treated and non-treated RNAs are normalized by mature 26S rRNA, and the primary and processed transcripts are then differentiated by comparing the cRT-PCR products obtained from the two RNA samples. The cRT-PCR mapping and discrimination results are verified by Northern blot and RT-qPCR, respectively. Finally, alternative primers are used to amplify those transcripts detected in Northern blot but not by cRT-PCR. By using this cRT-PCR-based strategy, most steady-state transcripts in maize mitochondrion have been differentiated and mapped1.
Protocol
1. Primer Design
- Design gene-specific primers for reverse transcription (RT) using PCR primer design software (Table of Materials) based on the general rules of primer design17.
NOTE: RT primers are highly specific to the target transcripts, and they are generally anchored on the 5’ part of coding sequences (mature mRNAs and precursor RNAs), or ~500–600 nt downstream of the anticipated 5’ end (18S and 26S rRNAs). - Design pairs of divergent primers to amplify the circularized transcripts by cRT-PCR.
NOTE: The paired divergent primers flank the 5’-3’ junction of the circularized transcripts, and their positions vary among the target transcripts analyzed (Figure S1). Some transcripts have long UTRs; for example, nad2-1 5’ UTR and rps4-1 3’ UTR are 1,985 and 1,826/1,834 nt, respectively1. If both primers are anchored on coding region, it will be hard to amplify the target transcripts. By contrast, some UTRs are short; for example, the 3’ UTRs of nad2-1 and nad4-1 are 34/35 and 29–31 nt, respectively1. If the paired primers are located far away from the coding sequences, the PCR will fail. Generally, two pairs of divergent primers are designed for each target transcripts, while multiple pairs may be necessary to map those whose transcript patterns are complicated and/or UTRs are very long. - Design pairs of convergent primers to prepare RNA probes for Northern blot.
NOTE: The paired primers are located on coding region of the target gene, and the size of the PCR product should be in the range of 100 to 1,000 bp. Each forward primer should contain a restriction enzyme site to minimize vector sequences in the resulting probes.
2. Preparation of Crude Mitochondrion from Maize Developing Kernels
- Sterilize pestles, mortars, glass funnels, tubes, and tips by autoclave, and dry them in oven.
- Perform all procedures at 4 °C or on ice, and pre-cool all solutions.
- Prepare 100 mL of extraction buffer (EB), composed of 0.3 M sucrose, 5 mM tetrasodium pyrophosphate, 10 mM KH2PO4, 2 mM EDTA, 1% [w/v] polyvinylpyrrolidone 40, 1% [w/v] bovine serum albumin, 5 mM L-cysteine, and 20 mM ascorbic acid. Adjust to pH 7.3 with KOH and sterilize by filtration.
- Prepare 100 mL of wash buffer (WB) consisting of 0.3 M sucrose, 1 mM EGTA, and 10 mM MOPS (3-(N-morpholino)propanesulfonic acid). Adjust to pH 7.2 with NaOH and sterilize by filtration.
NOTE: It is suggested to prepare EB and WB using DEPC-treated deionized H2O. - Collect 20 g developing kernels at 11–20 days after pollination (DAP) to a 50-mL tube placed on ice, and then transfer the kernels to pre-cooled mortars.
NOTE: Use a ratio of 100 mL of EB to 20 g of maize kernels. - Add 10–20 mL of ice-cold EB to each mortar, and grind the kernels completely.
- Add more EB, and filter the ground tissues through two layers of filter cloth (Table of Materials).
- Centrifuge the filtrate at 8,000 x g for 10 min, and discard the pellet.
- Transfer the supernatant to a new tube, and centrifuge it at 20,000 x g for 10 min.
- Pour off the supernatant, and resuspend the pellet in 6 mL WB.
- Aliquot the suspension to five 1.5 mL RNase-free tubes, and centrifuge them at 14,000 x g for 5 min.
- Discard the supernatant, freeze the mitochondrial pellet in liquid nitrogen, and store at -80 °C.
3. Extraction of Mitochondrial RNA
- Extract mitochondrial RNA with a commercial reagent (Table of Materials) according to the manufacture’s instructions.
CAUTION: This reagent contains phenol and guanidine isothiocyanate. Work with it in a fume hood, and wear lab coat and gloves. - Dissolve the isolated mitochondrial RNA in DEPC-treated deionized H2O, and estimate RNA concentration and purity with a spectrophotometer (Table of Materials).
NOTE: Generally, ~250 μg mitochondrial RNA is obtained from 20 g of 15-DAP maize kernels. - Prepare one agarose gel composed of 1x TAE buffer (40 mM Tris, 20 mM acetic acid, 1mM EDTA), 1.5% agarose (Table of Materials), and 1x nuclear staining dye (Table of Materials).
- Add an appropriate volume of 10x loading buffer (0.5% bromophenol blue, 0.5% xylene cyanol FF, and 50% glycerol), and load mitochondrial RNA/Loading buffer mixture on the 1.5% agarose gel.
- Run the gel in 1x TAE buffer at 5–6 V/cm for 20–25 min, and evaluate mitochondrial RNA integrity by imaging the gel with a gel documentation system (Table of Materials).
NOTE: The presence of two distinct bands (~3,510 and ~1,970 nt for maize mitochondrial 26S and 18S rRNAs, respectively) is an acceptable standard for intact mitochondrial RNA. To exclude possible degradation, RNA integrity should be investigated during the multiple steps of circularized RNA preparation (Figure 2).
4. RNA 5’ Polyphosphatase Treatment
- Set up RNA 5’ polyphosphatase (Table and Materials) treatment (Table 1), and incubate at 37 °C for 30–60 min.
- Recover the 5’ polyphosphatase-treated RNA with an RNA purification kit (Table of Materials) according to the manufacturer’s instructions.
- Repeat steps 3.3 to 3.5.
5. RNA Circularization
- Prepare two circularization reactions using the same amounts of 5’ polyphosphatase-treated and non-treated mitochondrial RNAs (Table 2), and incubate both reactions at 16 °C for 12–16 h.
- Recover the two sets of self-ligated RNAs using the same kit as in step 4.2.
NOTE: It should be noted that only a fraction of mitochondrial RNA will be self-ligated, and the recovered RNA will be a mixture of linear and circularized transcripts. - Repeat steps 3.3 to 3.5.
6. Reverse Transcription
- Synthesize two sets of cDNAs from the same amounts of circularized 5’ polyphosphatase-treated and non-treated RNAs (200 ng).
- Prepare a primer mixture by adding an equal ratio of 26S-CRT and up to 7 other RT primers.
NOTE: The final concentration of the primer mixture should be 1 μM. - Prepare two pre-mixtures by combining the reagents listed in Table 3, incubate at 65 °C for 5 min, and then chill on ice for 2 min.
- Assemble two RT reaction systems (Table 4), and incubate them at 42 °C for 50 min.
- Heat both RT reactions at 70 °C for 5 min, and then chill them on ice.
7. Normalization
- Prepare two PCR reactions by adding the same volume of template cDNAs derived from 5’ polyphosphatase-treated or non-treated RNAs, divergent primers flanking the 5’-3’ junction of circularized 26S rRNA (i.e. 26S-CF1 and -CR1), etc. (Table 5).
- Run the two reactions in a thermal cycler (Table of Materials) under conditions described in Table 6.
- Prepare one agarose gel composed of 1x TAE buffer, 1.0% agarose, and 1x nuclear staining dye.
- Add 2 μL 10x loading buffer (0.5% bromophenol blue, 0.5% xylene cyanol FF, and 50% glycerol) to each of the two PCR products, and load them on the gel.
- Run the gel in 1x TAE buffer at 5–6 V/cm for 30–40 min, and image it using the same gel documentation system as step 3.5.
- Compare the abundance of the two PCR products using computer software (Table of Materials, Figure 4A), and optimize the normalization by changing the amounts of template cDNAs if necessary.
8. PCR Amplification
- Prepare pairs of PCR reactions by adding appropriate volume of the normalized cDNAs and a pair of divergent primers flanking 5’-3’ junction of the target transcripts (Table 7).
NOTE: The amount of template cDNAs used for PCR amplification of the target transcripts are determined by the 26S rRNA normalization results. - Perform the PCR reactions according to the program described in Table 8.
- Repeat steps 7.3 to 7.5.
- Change to nested divergent primers, and verify the first round PCR results by repeating steps 8.1 and 8.2.
- Repeat steps 7.3 to 7.5.
- Recover the prominent bands that could be repeated in two rounds of PCR amplification using a gel DNA recovery kit (Table of Materials).
9. Determination of Transcript Termini
- Clone the gel-purified PCR products into a blunt-end vector (Table of Materials) using standard techniques.
- Perform colony PCR to select positive clones containing the target inserts, and sequence them commercially.
NOTE: Positive clones containing inserts with variable size are usually detected from a single recovered band because many steady-state transcripts in plant mitochondrial have heterogeneous 5’ and/or 3’ ends1,12. - Align the sequencing data with maize mitochondrial genome using basic local alignment search tool (BLAST) of national center for biotechnology information (NCBI). Choose organism “maize (taxid:4577)”, and search database “Nucleotide collection (nr/nt)”.
- Find the 5’-3’ junction of the circularized transcript, and determine the positions of 5’ and 3’ transcript termini.
- Calculate the size of target transcripts.
10. Verification of the cRT-PCR Mapping Results by RNA Gel Blot Hybridization
NOTE: RNA gel blot hybridization is performed by using a commercial kit (Table of Materials), which contains the reagents for transcription-labeling of RNA with digoxigenin (DIG) and T7 RNA polymerase, hybridization, and immunological detection. Please refer to the protocols provided in this kit for more details. Make sure that only RNase free equipment is used for the whole procedure.
- Amplify the DNA fragment used to prepare RNA probe, and clone it to the same vector as in step 9.1, which contains a T7 promoter 17 bp upstream of the insertion site.
- Linearize the construct using proper restriction enzyme, recover the linearized plasmid by a DNA purification kit (Table of Materials), and dissolve it in DEPC-treated deionized H2O.
- Label RNA probes with DIG-11-UTP by a commercial kit (Table of Materials) according to the manufacturer’s instructions.
- Prepare 500 mL of 10x MOPS buffer (0.2 M MOPS, 50 mM sodium acetate, and 10 mM EDTA) using DEPC-treated deionized H2O. Adjust to pH 7.0 by NaOH, and sterilize by filtration.
- Add 2–3 volumes of loading buffer (50% formamide, 6.2% formaldehyde, 1x MOPS, 10% glycerol, and 0.1% bromophenol blue) to the mitochondrial RNA prepared in steps 3.1–3.3.
- Denature the RNA sample/Loading buffer mixture at 65 °C for 10 min, and then chill on ice for 1 min.
- Prepare a denatured agarose gel (2% formaldehyde, 1.2% agarose, and 1x MOPS), and load the RNA sample/Loading buffer mixture to the gel (1–2 μg mitochondrial RNA per well).
- Run the gel in 1x MOPS at 3–4 V/cm for ~4 h.
- Prepare 2 L of 20x SSC (3 M NaCl, 0.3 M sodium citrate, pH 7.0). Treat it with 0.1% DEPC overnight, and sterilize by autoclave.
- Rinse the gel twice in 20x SSC (15 min each time), and transfer gel RNA to a nylon membrane (Table of Materials) by capillary transfer with 20x SSC for 10–16 h.
- Fix the RNA to membrane by baking at 120 °C for 30 min.
- Perform RNA hybridization and immunological detection with a commercial kit (Table of Materials) according to the manufacturer’s instruction.
11. Discrimination of Primary and Processed 5’ Ends
- Discriminate the primary and processed 5’ ends by comparing the cRT-PCR products obtained from the normalized 5’ polyphosphatase-treated and non-treated RNAs.
NOTE: To primary transcripts, the abundance of PCR products from 5’ polyphosphatase-treated RNA is much higher than that from non-treated counterpart (Figure 1, ‘Gene A,’ and Figure 4A). However, to processed transcripts, a comparable level of PCR products would be amplified from the two sets of mitochondrial RNAs (Figure 1, ‘Gene B’). - Design RT-qPCR primers based on the cRT-PCR mapping results.
- Verify the cRT-PCR discrimination results by RT-qPCR.
NOTE: The two cDNA samples derived from 5’ polyphosphatase-treated and non-treated RNAs are normalized by the RT-qPCR products of 26S mature rRNA.
Representative Results
Estimation of mitochondrial RNA circularization efficiency
In a previous study, both total and mitochondrial RNAs were used for cRT-PCR mapping of mitochondrial transcript termini in Arabidopsis (Arabidopsis thaliana), and the two types of RNAs gave similar mapping results12. Initially, we also used total RNAs for cRT-PCR mapping of mitochondrial transcript termini in maize. After many trials, we found the target transcripts were hard to detect. As an improvement, we enriched the mitochondrial RNA from maize developing kernels, and it made the amplification of the circularized target transcripts in one round of PCR possible.
To explain the easy amplification of target transcripts after the enrichment, we estimated RNA circularization efficiency by performing RT-PCRs on representative mitochondrial gene nad5 (Figure 3 and Figure S2). The maize nad5 gene contains two trans– and two cis-splicing introns. After self-ligation of total and mitochondrial RNAs, first strand cDNAs were independently synthesized using nad5-RT1 and -RT2 primers. The two primers could reverse transcribe both linear and circularized nad5 mRNAs (Figure S2). Four pairs of convergent primers are used for PCR amplification: sqF1&sqR1 and sqF2&sqR2 for semi-quantitative RT-PCR (RT-sqPCR) and qF1&qR1 and qF2&qR2 for RT-qPCR. For cDNAs transcribed by nad5-RT1, all four pairs of primers detected both circularized and linear nad5 mature mRNAs; for the nad5-RT2 reverse transcription reaction, sqF2&sqR2 and qF2&qR2 surveyed both forms of mRNA, while sqF1&sqR1 and qF1&qR1 PCR products were derived from circularized nad5 only. Based on this analysis, we used the sqF2&sqR2 (for RT-sqPCR) and qF2&qR2 (for RT-qPCR) PCR products to normalize the nad5-RT1 and -RT2 reverse transcription reactions, and estimated the ratio of circularized nad5 by comparing the sqF1&sqR1 (for RT-sqPCR) or qF1&qR1 (for RT-qPCR) PCR products. Prior to self-ligation, RNAs were pre-treated by RNA 5' polyphosphatase to make all transcripts available for circularization by RNA ligase. The RT-sqPCR analysis showed a very small fraction of nad5 mRNA was circularized when total RNA was used, but the ratio was dramatically increased when the enriched mitochondrial RNA was used (Figure 3B). RT-qPCR results showed that the ratio of self-ligated nad5 mRNA increased from 3.7% to 32% after the enrichment (Figure 3C). Analysis of nad1 gave similar results, and the circularization efficiency of nad1 mRNA increased from 0.7% to 30% (Figure 3D–F).
Per these analyses, about 30% maize mitochondrial RNAs were self-ligated after the improvement, and the increased circularization efficiency explains the easy detection of the target transcripts by cRT-PCR.
Determination of maize cox2 mRNA termini using the cRT-PCR-based strategy
We used the cox2 gene as an example to introduce the mapping and discrimination of maize mitochondrial transcripts by using the cRT-PCR-based strategy (Figure 4).
In the beginning of mapping cox2 mRNA, three outward-facing primers CF1, CF2, and CR1 were designed on the gene coding region (Figure 4D). CF1 and CF2 were nested primers, and CF2 was 69 bp downstream of CF1. Using the template cDNAs synthesized in steps 6.1–6.5 and normalized at steps 7.1–7.6, the CF1&CR1 primer pair amplified two prominent bands, and the amplification results were repeated by nested primers CF2&CR1 (Figure 4A). Moreover, the two bands were strongly amplified from the 5' polyphsophatase-treated RNA, while they were hard to detect in the non-treated counterpart, suggesting that they are sensitive to RNA 5' polyphsophatase and have primary 5' termini.
The two candidate bands were named cox2-1 and -2, and they were recovered independently from the agarose gel and cloned into vectors. Colony PCR results showed that the positive clones contain inserts with variable size, implying heterogeneous 5' and/or 3' termini of the transcripts (Figure 5). The positive clones were sequenced commercially, and the sequencing data were aligned with maize mitochondrial genome as described in step 9.3.
The sequencing and alignment results showed that the two transcripts were identical at 3' ends, but different in the length of 5' UTRs (Figure 4D). The 5' ends of cox2-1 and -2 were enriched at 992-1,030 and 1,276-1,283 nt upstream of AUG, respectively, while their 3' end was 39 nt downstream of the stop codon. Deduced from the cRT-PCR results, the calculated sizes of cox2-1 and -2 were 1,804–1,832 and 2,088–2,095 nt, respectively.
To verify the cRT-PCR mapping results, RNA gel blot hybridization was performed by using the probe derived from cox2 gene coding region, and two major bands with similar size as cox2-1 and -2 were detected (Figure 4C). Additionally, two larger bands about 2,500 and 2,800 nt were detected in the Northern blot, but they were not amplified by cRT-PCR. The two larger bands were named as cox2-3 and -4, respectively. To map them, another two outward-facing primers (i.e. CR2 and CF3), anchored on the 5' and 3' UTRs of cox2-2, were designed, and their positions were close to the expected transcript ends of cox2-3 and -4. Using the primer pair CF3&CR2, two major bands were strongly amplified from cDNAs derived from 5' polyphsophatase-treated RNA but not from the non-treated counterpart (Figure 4A). The sequencing results showed that they had identical 5' termini while variable 3' ends. The calculated size of the two transcripts were 2,512/2,513 and 2,833–2,835 nt, respectively, and they were close in size with the two larger bands detected in the Northern blot. Moreover, the cRT-PCR results suggested that cox2-3 and -4 have primary 5' termini.
To confirm the discrimination results, five outward-facing primers were designed according to the cRT-PCR mapping results, i.e. qCF1, qCF2, qCR1, qCR2, and qCR3, and the primer pairs qCF1&qCR1, qCF1&qCR2, qCF1&qCR3, and qCF2&qCR3 were used for RT-qPCR analysis of cox2-1, -2, -3, and -4, respectively. The relative abundance of all four RT-qPCR products in 5' polyphsophatase-treated sample were much higher that those in the non-treated counterpart, which confirms the cRT-PCR discrimination results.
In summary, the transcript pattern of maize cox2 gene is complicated, and the cRT-PCR-based strategy effectively discriminates and maps the multiple isoforms of cox2 mRNA.
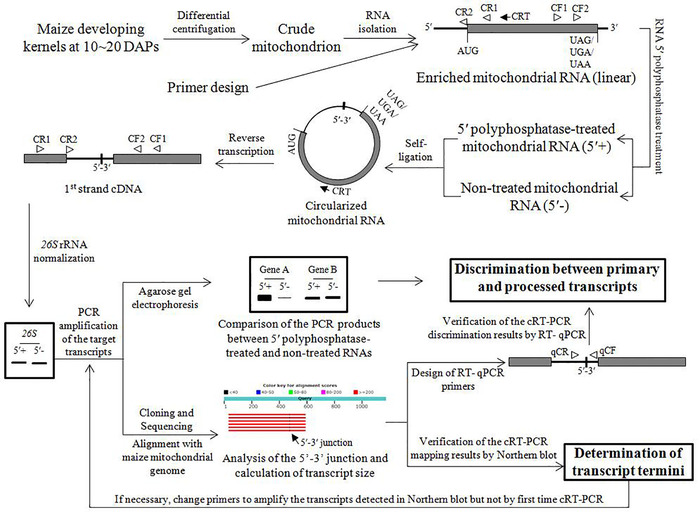
Figure 1: Overview of the cRT-PCR-based strategy. Illustration of the major steps of the cRT-PCR-based strategy. 5'+ and 5'- = the two RNA samples treated and non-treated by RNA 5' polyphosphatase, respectively. The two cDNA samples derived from 5' polyphosphatase-treated and non-treated RNAs are normalized by 26S mature rRNA in maize, and the primary and processed transcripts are discriminated by comparing the cRT-PCR products. For gene A, the corresponding RNA has a primary 5' end because the PCR product abundance from 5' polyphosphatase-treated RNA is much higher than that from the non-treated counterpart; for gene B, the PCR products from the two sets of mitochondrial RNAs are amplified at a comparable level, implying a processed 5' terminus of the corresponding RNA. The coding region and UTRs are represented by gray box and bold lines, respectively. CRT = primer for reverse transcription; CF1, CF2, CR1, and CR2 = PCR primers flanking the 5'-3' junction of the circularized transcript; qCF and qCR = divergent primers for RT-qPCR. Please click here to view a larger version of this figure.
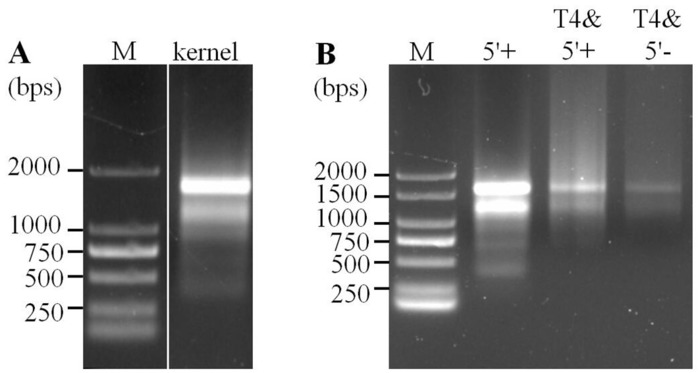
Figure 2: Evaluation of mitochondrial RNA integrity by agarose gel electrophoresis. (A) Mitochondrial RNAs prepared from 15-DAP maize kernels. M = DNA molecular marker. (B) Kernel mitochondrial RNA after the treatment by 5' polyphosphatase and/or circularization by T4 RNA ligase. 5'+ = mitochondrial RNA after the treatment by 5' polyphosphatase; T4&5'+ = mitochondrial RNA after the treatment by 5' polyphosphatase and circularization by T4 RNA ligase; T4&5'- = mitochondrial RNA after circularization only. Please click here to view a larger version of this figure.
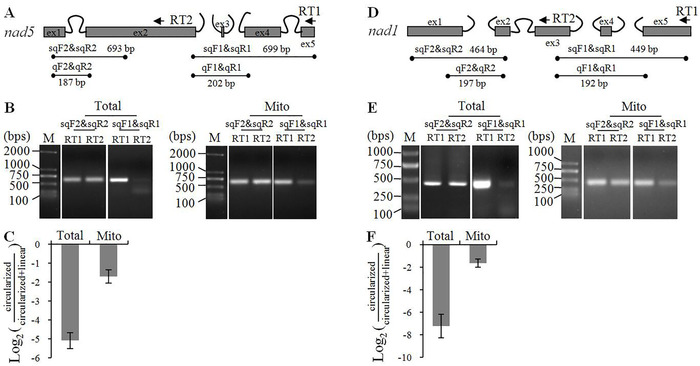
Figure 3: Circularization efficiency of nad5 and nad1 mRNAs estimated by RT-PCRs. (A) and (D) Schematic diagram of nad5 and nad1 genes, respectively. Ex = exon. Exons and introns are shown as gray boxes and curved lines, respectively. The position of reverse transcription primers RT1 and RT2 are indicated by arrows. Black dots indicate the position of PCR primers, and the predicted size of the PCR products is shown. sqF1, sqF2, sqR1, and sqR2 are primers for RT-sqPCR; qF1, qF2, qR1, and qR2 are primers for RT-qPCR. (B) and (E) RT-sqPCR analysis of the circularization efficiency of nad5 and nad1 mRNAs, respectively. M = DNA molecular marker. (C) and (F) RT-qPCR analysis of the circularization efficiency of nad5 and nad1 mRNAs, respectively. The two cDNA samples reverse transcribed by RT1 and RT2 primers are normalized by sqF2&sqR2 (for RT-sqPCR) or qF2&qR2 (for RT-qPCR) PCR products. The circularization efficiency of nad5 and nad1 mRNAs is estimated by comparing the PCR products of sqF1&sqR1 (for RT-sqPCR) or qF1&qR1 (for RT-qPCR). circularized/(circularized+linear) = qF1&qR1 over qF2&qR2 in RT2 reaction/ qF1&qR1 over qF2&qR2 in RT1 reaction. Values are means and SDs of three biological replicates. To exclude the potential influence by 5′ triphosphate, the RNAs were treated by 5′ polyphosphatase prior to self-ligation. Total and Mito = total and mitochondrial RNAs, respectively. RT1 and RT2 = cDNAs reverse transcribed by RT1 and RT2 primers, respectively Please click here to view a larger version of this figure.
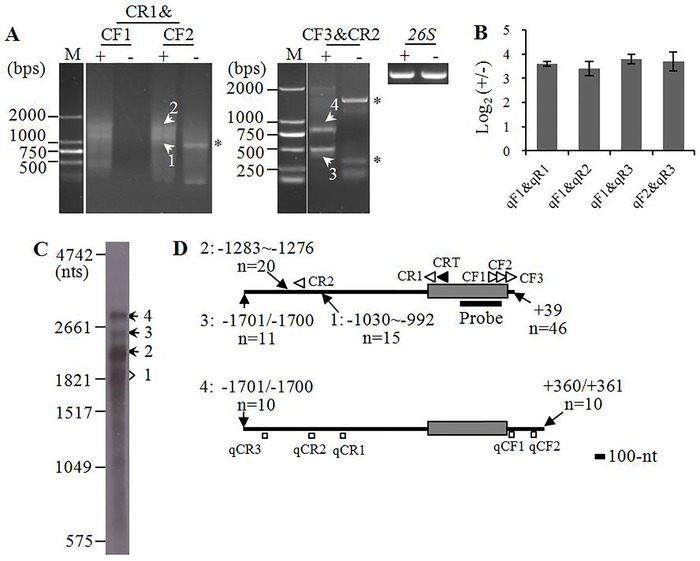
Figure 4: Mapping of cox2 mature mRNA termini by using the cRT-PCR-cased strategy. (A) Gel separation of cox2 cRT-PCR products. + and – = cDNAs derived from 5′ polyphosphatase-treated and non-treated mitochondrial RNAs, respectively. The two cDNA samples were normalized by amplification of 26S cDNA (26S). Five primers are used for cRT-PCR analysis of circularized cox2 mRNA: CF1, CF2, and CF3 are nested primers, and CF2 and CF3 are 69 and 138 bp downstream of CF1, respectively; CR1 and CR2 are nested primers, and CR2 is 1,288 bp upstream of CR1. The bands indicated by '1' and '2' were amplified by both CF1&CR1 and CF2&CR1, while the '3' and '4' bands were amplified by CF3&CR2. All four of the bands were sequenced by cloning. The bands marked by asterisks could not be repeated, and they were excluded from the results. M = DNA molecular marker. (B) RT-qPCR analysis of the relative abundance of circularized cox2 mRNA after 5' polyphosphatase treatment. The two cDNA samples were synthesized by a primer mixture containing 26S-CRT, cox2-CRT, nad5-CRT, nad6-CRT, nad7-CRT, nad9-CRT, cob-CRT, and cox1-CRT at equal ratio, and the cDNA derived from 26S mature rRNA was used for normalization. +/- = cox2 over 26S in treated sample/cox2 over 26S in non-treated sample. Values represent means and SD of three biological replicates. The primers used for RT-qPCR are indicated. (C) Northern blot analysis of cox2 transcripts. 2 µg mitochondrial RNA was loaded. The bands corresponding to different isoforms of cox2 mature mRNA are marked. (D) Transcript termini of cox2 mRNA deduced from cRT-PCR results. UTRs and open reading frames are shown as bold lines and gray boxes, respectively. The positions of 5' and 3' termini relative to AUG (+1) and UAA (-1), and numbers of single clones sequenced at those positions are shown. The positions of reverse transcription and PCR amplification primers are shown as closed and open arrow heads, respectively. Outward-facing primers used for RT-qPCR are shown as open squares, and the position of cox2 probe is as indicated. Please click here to view a larger version of this figure.
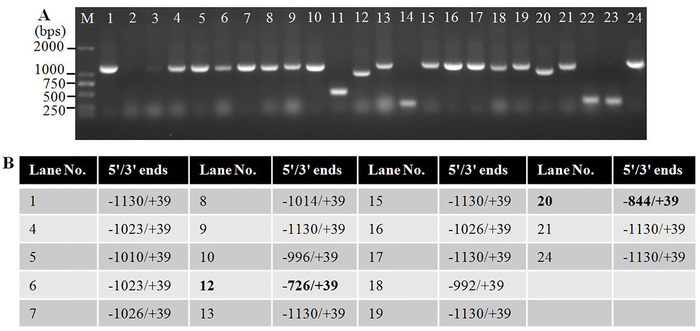
Figure 5: Selection of positive clones containing cox2-4 inserts by colony PCR. (A) Colony PCR to screen single clones containing cox2-4 inserts. The size of cox2-4 insert is about 1,165 bp, and that of the vector sequences is 100 bp. The calculated size of colony PCR products is about 1,265 bp, and those clones containing small size inserts were not sequenced, i.e., numbers 11, 14, 22, and 23. (B) Sequencing results of the positive clones screened in (A). 5'/3' ends = the 5' and 3' ends relative to AUG (+1) and UAA (-1), respectively. The sequencing results of numbers 12 and 20 were excluded because they were much smaller than the others. Please click here to view a larger version of this figure.
| Component | Volume for 50 μL reaction (μL) | Final concentration |
| 10x RNA 5' polyphosphatase buffer | 5 | 1x |
| RNase inhibitor (40 U/μL) | 0.75 | 0.6 U/μL |
| Mitochondrial RNA | x | 0.2 μg/μL |
| RNA 5' polyphosphatase (20 U/μL) | 2.5 | 1 U/μL |
| DEPC-treated deionized H2O | to 50 μL | – |
Table 1: Reaction components of RNA 5' polyphosphatase treatment.
| Component | Volume for 20 μL reaction (μL) | Final concentration |
| 10x T4 RNA ligase I buffer | 2 | 1x |
| dATP (1 mM) | 1 | 50 μM |
| PEG8000 (50%) | 6 | 15% |
| RNA inhibitor (40 U/μL) | 0.5 | 1 U/μL |
| 5' polyphosphatase-treated or non-treated mitochondrial RNA | x | 0.1~0.2 μg/μL |
| T4 RNA ligase I (30 U/μL) | 0.4 | 0.6 U/μL |
| DEPC-treated deionized H2O | to 20 μL | – |
Table 2: Reaction components of mitochondrial RNA circularization.
| Component | Volume for 10 μL pre-mixture (μL) |
| Primer mixture (1 μM total)a | 2 |
| dNTP mixture (10 mM each) | 1 |
| Circularized 5' polyphosphatase-treated or non-treated mitochondrial RNAb | x |
| DEPC-treated deionized H2O | to 10 μL |
Table 3: Pre-mixture for reverse transcription reaction. aThe primer mixture contains equal ratio of 26S-CRT and up to 7 other RT primers, and the final concentration is 1 µM. bTwo pre-mixtures are prepared side-by-side, and they contain the same amount (200 ng) of circularized 5' polyphosphatase-treated or non-treated mitochondrial RNA.
| Component | Volume for 20 μL reaction system (μL) | Final concentration |
| Template RNA/primer/dNTP* | 10 | – |
| 5x RTase buffer | 4 | 1x |
| RNase inhibitor (40 U/μL) | 0.5 | 1 U/μL |
| RTase (200 U/μL) | 1 | 10 U/μL |
| DEPC-treated deionized H2O | to 20 μL | – |
Table 4: Reaction components of reverse transcription. *The template RNA/primer/dNTP pre-mixtures prepared at step 6.3.
| Component | Volume for 20 μL reaction system (μL) | Final concentration |
| Deionized H2O | 7 | – |
| 2x reaction buffer | 10 | 1x |
| dNTP mixture (10 mM each) | 0.4 | 0.2 mM each |
| 26S-CF1 (10 μM) | 0.8 | 0.4 mM |
| 26S-CR1 (10 μM) | 0.8 | 0.4 mM |
| Templates cDNA derived from circularized 5' polyphosphatase-treated or non-treated RNAs * | 0.6 | – |
| DNA polymerase (1 U/μL) | 0.4 | 0.02 U/μL |
Table 5: Reaction components for PCR amplification of 26S cDNA. *Initially, equal volume of template cDNAs (0.6 µL) are used for normalization. If necessary, change the amounts of template cDNAs to ensure the same abundance of 26S PCR products between the two PCR reactions.
| Step | Temperature | Time | Cycle number |
| Initial denaturation | 95 °C | 3 min | 1 cycle |
| Denaturation | 95 °C | 15 sec | 22–25 cycles |
| Primer annealing | 56 °C | 15 sec | |
| Extension | 72 °C | 30 sec | |
| Final extension | 72 °C | 5 min | 1 cycle |
| Hold | 4 °C | ∞ | – |
Table 6: PCR conditions to amplify 26S cDNA for normalization.
| Component | Volume for 20-μL reaction system (μL) | Final concentration |
| Deionized H2O | to 20 μL | – |
| 2x reaction buffer | 10 | 1x |
| dNTP mixture (10 mM each) | 0.4 | 0.2 mM each |
| Divergent primer-forward (10 μM) | 0.8 | 0.4 mM |
| Divergent primer-reverse (10 μM) | 0.8 | 0.4 mM |
| Template cDNA derived from the circularized 5' polyphosphatase-treated or non-treated RNAs * | x | – |
| DNA polymerase (1 U/μL) | 0.4 | 0.02 U/μL |
Table 7: Reaction components for PCR amplification of the circularized target transcripts. *The volume of template cDNAs used in these reactions are determined by the 26S rRNA normalization results.
| Step | Temperature | Time | Cycle number |
| Initial denaturation | 95 °C | 3 min | 1 cycle |
| Denaturation | 95 °C | 15 sec | 22-40 cycles c |
| Primer annealing a | 50-65 °C | 15 sec | |
| Extenstion b | 72 °C | 0.5-1 min | |
| Finatl extension | 72 °C | 5 min | 1 cycle |
| Hold | 4 °C | ∞ | – |
Table 8: PCR conditions to amplify the target transcripts. aExact annealing temperate depends on the melting temperature of the PCR primers. bElongation time depends on the length of target to be amplified. Recommended time is 1 min per 1 kb of the PCR fragment. cIn general, 30~35 cycles are enough to produce an adequate amount of PCR product. For low abundant transcripts, increase the number of cycles up to 40 cycles.
Figure S1: Position of primers for representative maize mitochondrial transcripts. CRT = reverse transcription primer; CF1, CF2, CR1, and CR2 = divergent primers for PCR amplification; qCF and qCR = divergent primers for RT-qPCR. The transcript termini of maize nad2-1, rps4-1, and nad4-1 have been determined previously (Zhang et al., 20191). Positions of 5'- and 3'-ends relative to AUG (+1) and the last nucleotide of stop codon (-1) are shown. The coding regions and UTRs are indicated as gray boxes and bold lines, respectively. Please click here to download this file.
Figure S2: Principle to estimate circularization efficiency of nad5 mRNA in maize. In a self-ligation reaction, only a fraction of mitochondrial RNAs is circularized. To calculate the ratio of circularized nad5 mRNA, two gene-specific primers are used to synthesize the first strand cDNAs, i.e., nad5-RT1 and -RT2. In the nad5-RT2 reverse transcription (RT) reaction, the PCR products amplified by sqF2&sqR2 (for RT-sqPCR) and qF2&qR2 (for RT-qPCR) are derived from both linear and circularized nad5, while the sqF1&sqR1 (for RT-sqPCR) and qF1&qR1 (for RT-qPCR) PCR products are derived from circularized nad5 only; in nad5-RT1 reaction, all four pairs of PCR primers could amplify both forms of nad5. To calculate the ratio of circularized nad5 mRNA, the two reactions are normalized by sqF2&sqR2 or qF2&qR2 PCR products. By comparing the abundance of sqF1&sqR1 or qF1&qR1 PCR products between the two RT reactions, the circularization efficiency of nad5 mRNA is roughly estimated. ex = exon. Exons and introns are shown as gray boxes and curved lines, respectively. The positions of nad5-RT1 and -RT2 primers are indicated by arrows. Black dots indicate the positions of PCR primers, and the predicted size of the PCR products is shown. Please click here to download this file.
Table S1: Primer information. Please click here to download this file.
Discussion
In a previous study, total and mitochondrial RNAs from cell suspension culture of Arabidopsis were used to map mitochondrial transcript termini by cRT-PCR, and similar results were obtained12. However, only enriched mitochondrial RNA was used to map mitochondrial transcript termini in many other studies1,2,3,9. We found that the enrichment of mitochondrial RNA is an important step for cRT-PCR mapping of mitochondrial transcript termini in maize. After this enrichment, the ratio of circularized mitochondrial RNA is dramatically increased from 0.3%–3.7% to ~30% (Figure 3 and Figure S2), which makes the amplification of circularized target transcripts possible in one round of PCR.
The circularization efficiency of mitochondrial RNA was estimated by RT-PCR analysis on maize nad1 and nad5, both of which are divided into independent precursors by cis-splicing introns. Because the potential influence from the presence of precursor RNAs as well as the variation of RT efficiency could not be ruled out, this is only a rough estimation of the real mitochondrial RNA circularization efficiency.
We altered the self-ligation conditions by altering the concentration of mitochondrial RNA and PEG8000, as well as by elongating the incubation time, but these changes did not seem to affect mitochondrial RNA circularization efficiency. Although we are not sure whether further Percoll gradient purification could increase the circularization efficiency, the quality of crude mitochondrion prepared in section 2 is good enough for cRT-PCR mapping of mitochondrial transcript termini in maize. Since multiple rounds of PCRs may cause false positive results, the amplification of target transcripts in one round PCR makes the mapping results more reliable.
The 5' triphosphate of primary transcripts is unstable, and it could be converted to 5' monophosphate for unknown reasons. This problem hinders a clear differentiation between the primary and processed transcripts by using approaches depending on the presence/absence of 5' triphosphate1,2,4. The mature form of maize 26S rRNA has a monophosphated 5' end, and it is insensitive to RNA 5' polyphosphatase. To minimize the influence of the unstable 5' triphospahte, mature 26S rRNA is used to normalize the two RNA samples, treated and untreated by 5' polyphosphatase, and it is shown to be an important step to differentiate the two types of transcripts in maize mitochondrion. After normalization, the primary and processed transcripts could be discriminated by comparing cRT-PCR and RT-qPCR results obtained from 5' polyphosphatase-treated and non-treated RNA samples. The primary transcripts are sensitive to 5' polyphosphatase, and they are strongly amplified from the 5' polyphosphatase-treated sample but not (or at a very low level due to the unstable 5' triphosphate) from the non-treated counterpart. By contrast, the processed transcripts are detected at comparable levels between the two samples.
In plants, the transcript patterns of many mitochondrial genes are rather complicated1,2. For example, the maize nad6 and atp6 genes are expressed as both monocistrons and dicistrons, and nad6, atp6, and atp6–na6 mature mRNAs have two, three, and two isoforms, respectively. Moreover, the transcript population of one mitochondrial gene is actually a mixture of precursor, mature, and degradation RNAs. PCRs are prone to amplify small size molecules, and it is hard to detect all transcript isoforms using one pair of primers. Therefore, RNA gel blot hybridization is required to verify the cRT-PCR mapping results, and alternative primers may be necessary to amplify those transcripts detected in Northern blot but not by the first time cRT-PCR.
This protocol includes a RT-qPCR step to verify the cRT-PCR discrimination results. Because the multiple isoforms of some mature mRNAs are very close in size1, it is impossible to confirm of all of the discrimination results by RT-qPCR, and some steady-state transcripts were determined by comparing the cRT-PCR results between the treated and non-treated RNAs only.
In this protocol, the 5' and 3' termini of the target transcripts were mapped by cloning and sequencing of the cRT-PCR products, and it limits the number of single clones to be sequenced. As an alternative method, the cRT-PCR products could be sequenced by next generation sequencing, which sequences thousands of molecules for one set of steady-state transcripts, and the mapping results would be more accurate.
Besides the discrimination of primary and processed 5' termini by cRT-PCR and RT-qPCR, the monophosphate 5' termini could be determined by identification of the surrounding RNA secondary structures1,12. It is well known that RNA secondary structures such as tRNA and t-element could mediate mitochondrial transcript end formation by directing endonucleolytic cleavages of RNase P and/or RNase Z12,18,19,20. Therefore, those 5' termini adjacent to tRNA or t-element should be derived from post-transcriptional processing and contain monophosphates.
By using this protocol, a great part of steady-state transcripts have been determined in maize mitochondria1. However, a few were not differentiated and/or mapped for unknown reason1. Moreover, we think the position of 5' transcript termini is better to be confirmed by primer extension analysis, though it is included in this protocol. Due to the lack of experimental data, we are not sure whether this strategy is suitable for other plant species, such as rice (Oryza sativa) and Arabidopsis. Besides plant mitochondria, both primary and processed transcripts are stably accumulated in plastids21, and it is uncertain whether this strategy could be used to map and discriminate plastid transcripts.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 31600250, Y.Z.), Science and Technology Projects of Guangzhou City (grant no. 201804020015, H.N.), and the China Agricultural Research System (grant no. CARS-04-PS09, H.N.).
Materials
| Acetic acid | Aladdin, China | A112880 | To prepare 1x TAE buffer |
| Applied Biosystems 2720 Thermal Cycler | Thermo Fisher Scientific, USA | 4359659 | Thermal cycler for PCR amplification |
| Ascorbic acid | Sigma-aldrich, USA | V900134 | For preparation of extraction buffer |
| Biowest Agarose | Biowest, Spain | 9012-36-6 | To resolve PCR products and RNAs |
| Bovine serum albumin | Sigma-aldrich, USA | A1933 | For preparation of extraction buffer |
| Bromophenol blue | Sigma-aldrich, USA | B8026 | For preparation of loading buffer for agarose gel electrophoresis and Northern blot |
| DEPC | Sigma-aldrich, USA | V900882 | Deactivation of RNase |
| DIG Northern starter kit | Roche, USA | 12039672910 | For DIG-RNA labeling and Northern blot. This kit contains the reagents for transcription-labeling of RNA with DIG and T7 RNA polymerase, hybridization and chemiluminescent detection. |
| EDTA | Sigma-aldrich, USA | V900106 | For preparation of extraction buffer and 1x TAE buffer |
| EGTA | Sigma-aldrich, USA | E3889 | For preparation of wash buffer |
| Gel documentation system | Bio-Rad, USA | Gel Doc XR+ | To image the agarose gel |
| Glycerol | Sigma-aldrich, USA | G5516 | For preparation of loading buffer for agarose gel electrophoresis |
| GoldView II (5000x) | Solarbio,. China | G8142 | DNA staining |
| Hybond-N+, Nylon membrane | Amersham Biosciences, USA | RPN119 | For Northern blot |
| Image Lab | Bio-Rad, USA | Image Lab 3.0 | Image gel, and compare the abundance of PCR products. |
| KH2PO4 | Sigma-aldrich, USA | V900041 | For preparation of extraction buffer |
| KOH | Aladdin, China | P112284 | For preparation of extraction buffer |
| L-cysteine | Sigma-aldrich, USA | V900399 | For preparation of extraction buffer |
| Millex | Millipore, USA | SLHP033RB | To sterile extraction and wash buffers by filtration |
| Miracloth | Calbiochem, USA | 475855-1R | To filter the ground kernel tissues |
| MOPS | Sigma-aldrich, USA | V900306 | For preparation of running buffer for Northern blot |
| NanoDrop | Thermo Fisher Scientific, USA | 2000C | For RNA concentration and purity assay |
| NaOH | Sigma-aldrich, USA | V900797 | For preparation of wash buffer |
| pEASY-Blunt simple cloning vector | TransGen Biotech, China | CB111 | Cloning of the gel-recovered band. It contains a T7 promoter several bps upstream of the insertion site. |
| Phanta max super-fidelity DNA polymerase | Vazyme, China | P505 | DNA polymerase for PCR amplification |
| Polyvinylpyrrolidone 40 | Sigma-aldrich, USA | V900008 | For preparation of extraction buffer |
| Primer Premier 6.24 | PREMIER Biosoft, USA | Primer Premier 6.24 | To design primers for reverse transcription and PCR amplification |
| PrimeScript II reverse transcriptase | Takara, Japan | 2690 | To synthesize the first strand cDNA |
| PureLink RNA Mini kit | Thermo Fisher Scientific, USA | 12183025 | For RNA purificaion |
| RNA 5' polyphosphatase | Epicentre, USA | RP8092H | To convert 5' triphosphate to monophosphate |
| RNase inhibitor | New England Biolabs, UK | M0314 | A component of RNA self-ligation and 5' polyphosphatase treatment reactions, and it is used to inhibite the activity of RNase. |
| Sodium acetate | Sigma-aldrich, USA | V900212 | For preparation of running buffer for Northern blot |
| Sodium chloride | Sigma-aldrich, USA | V900058 | To prepare 20x SSC |
| SsoFas evaGreen supermixes | Bio-Rad, USA | 1725202 | For RT-qPCR |
| T4 RNA Ligase 1 | New England Biolabs, UK | M0437 | For RNA circularization |
| Tetrasodium pyrophosphate | Sigma-aldrich, USA | 221368 | For preparation of extraction buffer |
| TIANgel midi purification kit | Tiangen Biotech, China | DP209 | To purify DNA fragments from agarose gel |
| Tris | Aladdin, China | T110601 | To prepare 1x TAE buffer |
| TRIzol reagent | Invitrogen, USA | 15596026 | To extract mitochondiral RNA. |
| Universal DNA purification kit | Tiangen Biotech, China | DP214 | To recover linearized plastmids from the restriction enzyme digestion reaction |
| Xylene cyanol FF | Sigma-aldrich, USA | X4126 | For preparation of loading buffer for agarose gel electrophoresis |
Referências
- Zhang, Y., et al. Major contribution of transcription initiation to 5'-end formation of mitochondrial steady-state transcripts in maize. RNA Biology. 16 (1), 104-117 (2019).
- Choi, B. Y., Acero, M. M., Bonen, L. Mapping of wheat mitochondrial mRNA termini and comparison with breakpoints in DNA homology among plants. Plant Molecular Biology. 80 (4-5), 539-552 (2012).
- Calixte, S., Bonen, L. Developmentally-specific transcripts from the ccmFN-rps1 locus in wheat mitochondria. Molecular Genetics and Genomics. 280 (5), 419-426 (2008).
- Kuhn, K., Weihe, A., Borner, T. Multiple promoters are a common feature of mitochondrial genes in Arabidopsis. Nucleic Acids Research. 33 (1), 337-346 (2005).
- Jonietz, C., Forner, J., Holzle, A., Thuss, S., Binder, S. RNA PROCESSING FACTOR2 is required for 5′ end processing of nad9 and cox3 mRNAs in mitochondria of Arabidopsis thaliana. ThePlant Cell. 22 (2), 443-453 (2010).
- Stoll, B., Stoll, K., Steinhilber, J., Jonietz, C., Binder, S. Mitochondrial transcript length polymorphisms are a widespread phenomenon in Arabidopsis thaliana. Plant Molecular Biology. 81 (3), 221-233 (2013).
- Mulligan, R. M., Lau, G. T., Walbot, V. Numerous transcription initiation sites exist for the maize mitochondrial genes for subunit 9 of the ATP synthase and subunit 3 of cytochrome oxidase. Proceedings of the National Academy of Sciences of the United States of America. 85 (21), 7998-8002 (1988).
- Lupold, D. S., Caoile, A. G., Stern, D. B. The maize mitochondrial cox2 gene has five promoters in two genomic regions, including a complex promoter consisting of seven overlapping units. Journal of Biological Chemistry. 274 (6), 3897-3903 (1999).
- Yan, B., Pring, D. R. Transcriptional initiation sites in sorghum mitochondrial DNA indicate conserved and variable features. Current Genetics. 32 (4), 287-295 (1997).
- Rapp, W. D., Lupold, D. S., Mack, S., Stern, D. B. Architecture of the maize mitochondrial atp1 promoter as determined by linker-scanning and point mutagenesis. Molecular and Cellular Biology. 13 (12), 7232-7238 (1993).
- Rapp, W. D., Stern, D. B. A conserved 11 nucleotide sequence contains an essential promoter element of the maize mitochondrial atp1 gene. The EMBO Journal. 11 (3), 1065-1073 (1992).
- Forner, J., Weber, B., Thuss, S., Wildum, S., Binder, S. Mapping of mitochondrial mRNA termini in Arabidopsis thaliana: t-elements contribute to 5′ and 3′ end formation. Nucleic Acids Research. 35 (11), 3676-3692 (2007).
- Binder, S., Stoll, K., Stoll, B. Maturation of 5′ ends of plant mitochondrial RNAs. Physiologia Plantarum. 157 (3), 280-288 (2016).
- Hang, R. L., Liu, C. Y., Ahmad, A., Zhang, Y., Lu, F. L., Cao, X. F. Arabidopsis protein arginine methyltransferase 3 is required for ribosome biogenesis by affecting precursor ribosomal RNA processing. Proceedings of the National Academy of Sciences of the United States of America. 111 (45), 16190-16195 (2014).
- Wang, H. Q., et al. Maize Urb2 protein is required for kernel development and vegetative growth by affecting pre-ribosomal RNA processing. New Phytologist. 218 (3), 1233-1246 (2018).
- Maloney, A. P., et al. Identification in maize mitochondrial 26S rRNA of a short 5′-end sequence possibly involved in transcription initiation and processing. Current Genetics. 15 (3), 207-212 (1989).
- Green, R. M., Sambrook, J. . Molecular Cloning: A Laboratory Manual. , (2012).
- Canino, G., et al. Arabidopsis encodes four tRNase Z enzymes. Plant Physiology. 150 (3), 1494-1502 (2009).
- Gobert, A., et al. A single Arabidopsis organellar protein has RNase P activity. Nature Structural, Molecular Biology. 17, 740-744 (2010).
- Gutmann, B., Gobert, A., Giege, P. PRORP proteins support RNase P activity in both organelles and the nucleus in Arabidopsis. Genes & Development. 26 (10), 1022-1027 (2012).
- Stern, D. B., Goldschmidt-Clermont, M., Hanson, M. R. Chloroplast RNA metabolism. Annual Review of Plant Biology. 61, 125-155 (2010).

