A Blood-Free Diet to Rear Anopheline Mosquitoes
Summary
A protocol is presented for formulation of a blood-free artificial diet to feed Anopheles mosquitoes in captivity. This diet has a similar performance to vertebrate blood and triggers oogenesis and egg maturation and produces viable adult progeny.
Abstract
Malaria research requires large-scale breeding and production conditions for mosquitoes (Anopheles spp.) in captivity. The sustainable and reliable production of mosquitoes is currently inhibited by the supply of fresh vertebrate blood. Alternatives to blood are required to promote efficient control strategies for malaria and other vector borne diseases that are transmitted by blood feeding insects. With this in mind, artificial liquid diets were formulated as substitutes for fresh vertebrate blood. Herein we report a blood-free artificial liquid diet that delivers feeding rates similar to blood and mimics the physiological effects of a fresh vertebrate blood meal. The diet induces ovarian and egg maturation of Anopheles mosquitoes and also produces good larval survival and development of functional adults. The formulated blood-free liquid diet is an important advance towards sustainable mosquito breeding in captivity and will reduce the maintenance costs of mosquito colonies and eliminate the need for fresh vertebrate blood.
Introduction
Vector-borne diseases affect several million humans worldwide and cause millions of deaths each year. They are transmitted by insects infected with disease-producing microorganisms (protozoan, viruses) acquired when they feed on blood from an infected host. Subsequently, the infected vector will transmit the pathogen to a new host during the next blood meal. Malaria is the deadliest vector-borne disease that is transmitted by several different species of Anopheles mosquito and affects 40% of the world’s population1. The malaria protist parasite is responsible for more than 400,000 deaths every year, of which most are children under 5 years of age (World Health Organization). The female Anopheles mosquito transmits the malaria parasite of the Plasmodium genus between humans and other animals when it feeds on vertebrate blood, a necessary step for egg production and development2.
Current strategies for eradication of malaria and other emerging deadly mosquito vector-borne diseases rely on the development of innovative mosquito control strategies3,4,5, which include the release into the wild of large numbers of mosquitoes bred in insectaries. However, a crucial limiting factor is the dependency on a supply of fresh blood for effective mosquito rearing and breeding. The variable composition of vertebrate blood can negatively impact mosquito fertility and progeny fitness and can limit the reliability and sustainability of captive breeding colonies. Mosquito release and control programs require large-scale mosquito production systems and a regular supply of large quantities of vertebrate blood. This is a major obstacle for mosquito production and raises a series of ethical issues associated with the use of live animals and logistical limitations caused by the associated demanding safety regulations. This makes the costs of maintenance and security of mosquito colonies high and challenges the sustainability of current mosquito rearing practices particularly in low income countries where the threat of malaria is far greater.
Recently research has been focused on the development of blood substitutes that mimic a vertebrate blood meal but so far, only limited success has been achieved6,7,8,9. A successful artificial diet needs to (1) provoke full female mosquito engorgement, (2) trigger vitellogenin production, (3) produce large batches of viable eggs, and (4) generate heathy progeny10. In addition, artificial diets have a standard composition and thus are more reliable for production of mosquitoes for research and control purposes. Successful blood-free diets have been developed for Aedes mosquitoes (reviewed by Gonzales and Hansen11) but not for Anopheles spp. Existing artificial diets contain a phagostimulant (e.g., ATP10), a protein source for egg maturation6,12, carbohydrates as a source of energy, and amino acids (aa)13 that are fundamental for egg production and are a major limiting factor for mosquito fertility14. An artificial blood free diet also needs to provide cholesterol15, which improves egg production. Here we describe an artificial blood-free diet for female Anopheles mosquitoes and demonstrate that it has a consistent and equivalent performance to a high-quality vertebrate blood meal.
Protocol
Mice were obtained from the IHMT animal house. Animal experiments were conducted in strict accordance with the Portuguese law and guidelines for the use of laboratory animals. The Direção-Geral de Veterinária, Ministério da Agricultura do Desenvolvimento Rural e das Pescas, Portugal approved all the study protocols (id approvals: 023351 and 023355).
NOTE: Perform all feeding assays at ~26 °C.
1. Mosquitoes
- Maintain Anopheles coluzzii (former Anopheles gambiae M form) Yaoundé strain mosquitoes at 26 °C, 75% humidity under a 12 h:12 h light:dark cycle. House mosquitoes using standard insectary conditions to guarantee mating.
- Collect mosquito pupae into a small water container. Place the container inside a mosquito cage to let adult mosquitoes emerge and mate. Provide 10% glucose feeding solution. Three days after emergence collect the necessary number of mosquitoes from the stock cage using an aspirator.
- One day before the feeding trials, remove the 10% glucose feeding solution.
NOTE: 3 day-old mosquitoes were used throughout the experiments.
2. Mosquito Feeding
- Preparation of artificial liquid diets
- Prepare the artificial liquid diets under sterile conditions in a laminar flow cabinet. Prepare the rich liquid diet (r-liq_diet) by adding the following to the initial liquid diet (i-liq_diet; Dulbecco's modified Eagle's medium [high glucose with L-glutamine], see Table 1): 0.55 g/L ATP, 1 g/L cholesterol, and 200 g/L bovine serum albumin (BSA). Mix all ingredients thoroughly and filter using a 0.45 µm microfilter.
NOTE: Do not store the diets; prepare diets freshly from the stock solutions for each experiment as they lose quality when stored. Components of the diets are described in Table 1.
- Prepare the artificial liquid diets under sterile conditions in a laminar flow cabinet. Prepare the rich liquid diet (r-liq_diet) by adding the following to the initial liquid diet (i-liq_diet; Dulbecco's modified Eagle's medium [high glucose with L-glutamine], see Table 1): 0.55 g/L ATP, 1 g/L cholesterol, and 200 g/L bovine serum albumin (BSA). Mix all ingredients thoroughly and filter using a 0.45 µm microfilter.
- Mouse blood collection
- Anesthetize 6−8 week-old CD1 female mice (Mus musculus) with ketamine (120 mg/kg) and xylazine (16 mg/kg) using the intraperitoneal route.
- Perform cardiac puncture (Figure 1) when the mouse displays no muscle reaction in response to different physical stimuli (e.g., toe and tail pinches).
- Collect blood using a sterile 1 mL syringe with a 27 G x ½͂ (0.4 x 12 mm2) needle that contains 100 µL of 1 mg/mL heparin (sodium salt) to prevent formation of blood clots. Maintain blood at 37 °C using a water bath.
- Artificial feeding
- Collect approximately 30 female mosquitoes from the stock cage using an aspirator.
- Transfer the female mosquitoes to 500 mL paper cups and cover with a fine mosquito net mesh so they cannot escape. Apply a glass feeder connected to plastic tubes to maintain a constant water flow to the top of each cup (Figure 2). Provide a constant water flow to the cylindrical tubing and feeder so the temperature within is kept at approximately 37.5 °C.
NOTE: A standard glass bell artificial feeding apparatus16 was used to supply the formulated diets to female mosquitoes. - Stretch paraffin film membrane across the mouth of the glass feeder to contain the meal.
- Pre-warm the i-liq_diet and r-liq_diet at 37 °C using a water bath. Apply 1 mL into the glass feeder. Feed the mosquitoes for 60 min in the dark with either i-liq_diet, r-liq_diet or fresh mouse blood. Perform assays at 26 °C.
- Evaluation of the feeding rate.
- After artificial feeding, cold-anesthetize the mosquitoes at -20 °C for 30 s. Place the mosquitoes in a refrigerated Petri dish.
- Record the number of fully engorged (Figure 3) female mosquitoes.
NOTE: The percentage of fed mosquitoes is used as a proxy for feeding success.
3. Life History Traits
- Egg production and fertility
- Transfer the fully engorged females, using a brush, to individual cages (20 cm x 20 cm x 20 cm).
- Keep the mosquitoes at 26 ± 1 °C, 75% humidity and a 12 h:12 h light:dark cycle with 10% glucose ad libitum.
- Forty-eight h after the feeding, add a humidified filter paper at the bottom for egg laying (Figure 4). Count the eggs at 48 h and 72 h after the addition of the egg laying paper using a handheld magnifying glass. Flood the filter paper with distilled water to collect the eggs.
- Larvae mortality
- Collect the eggs into trays (23 cm x 15 cm x 6 cm) filled with distilled water (Figure 5). Maintain the water level in the trays constant during the experiments.
- Feed the larvae daily with approximately 13 mg of ground fish food per tray. Apply a similar feeding regime to all the replicate trays.
- Remove dead pupae and larvae daily. Finalize the experiments when all pupae have developed into adults and count the number of adult males and females.
- Register the dates of hatching and death and calculate the mortality rates.
- Longevity
- Collect 15 adult males and 15 adult females from the F1 generation of each diet group. Keep males and females in the same cage.
- Feed adults with a 10% glucose solution ad libitum. Remove the dead adults daily.
- Maintain the mosquitoes at the same temperature, humidity, light cycle conditions and sugar feeding regime as described above.
- Register the death dates and calculate the longevity.
- Wing length measurement
- Cold-anesthetize five-day-old F1 adult mosquitoes (male and female) from each diet group at -20 °C for 90 s.
- Under a stereoscope, gently grasp the thorax of each mosquito with forceps and place them ventral side up.
- Collect both wings using a scalpel and place them on a clean microscope slide containing a dried drop of mounting medium. With the aid of a 20 G needle add extra mounting media to the borders of the coverslip and slowly lower the coverslip onto the wings.
- Measure the wing length (Figure 6) with a stereoscope using a micrometer.
Representative Results
The results described below compare the performance of female Anopheles mosquitoes fed with the formulated rich artificial meal (r-liq_diet) and mosquitoes fed on the initial liquid diet (i-liq_diet) or a fresh blood meal. The diet was tested following the schematic protocol depicted in Figure 7. The r-liq_diet described herein is part of a patent (PCT/IB2019/052967).
Percentage of fully engorged females
The number of engorged female mosquitoes fed with the r-liq_diet (89%) was significantly higher than the number of engorged females fed on blood (56%) (Figure 8).
Fecundity and fertility
Female fecundity and fertility for the first gonotrophic cycle was used to evaluate the nutritional quality of the i-liq_diet and r-liq_diet. An average of 24 ± 11 eggs was laid by females fed on fresh vertebrate blood, whereas females fed on r-liq_diet laid an average of 25 ± 5 eggs (Table 2). No egg laying was observed by females fed on the i-liq_diet.
F1 mortality
The fitness of F1 mosquitoes was evaluated between colonies fed on vertebrate blood or the r-liq_diet. Larvae, pupae and adult mortality was recorded. Variability (standard error of the mean [SEM]) was higher in the blood-fed mosquitoes (Figure 9 and Table 2) relative to mosquitoes fed the r-liq_diet. The F1 generation of mosquitoes fed on either the blood or the r-liq_diet had comparable mortality and survival rates.
F1 life expectancy
It is estimated by the Center for Disease Control and Prevention that wild adult female mosquitoes live for up to a month but probably do not survive for more than 1−2 weeks and that males live for about a week and feed exclusively on nectar and other sugar sources. It is noted that differences in parental food intake may affect the survival of the mosquito progeny17. In our experiment, adult females and males in the blood (female 24.5 ± 6.8; male 18.5 ± 6.9) and r-liq_diet (female: 22.5 ± 8.1; male: 11.9 ± 6.9) groups had similar average life expectancies average (Table 3) and females showed an increased life span relative to males.
F1 body size
Wing length was used as an indicator of adult body size. When compared to other species, Anopheles adults are small- to medium-sized mosquitoes with a wing length of between 2.8 to 4.4 mm wing length18. Adult body size of F1 Anopheles mosquitoes fed the r-liq_diet was within the expected range and was similar to blood-fed insectary mosquitoes (Figure 10).
Statistical analysis
The data presented represents the mean of at least three independent experiments (unless otherwise stated). Error bars represent the SEM. When data followed a Gaussian distribution, independent groups were compared using the Student's t test, otherwise the Mann-Whitney test was applied. Differences between the artificial diet-fed groups were analyzed using the Fisher's exact test and were considered to be significant at P ≤ 0.05.

Figure 1: CD1 mouse blood collection by intracardiac puncture. Please click here to view a larger version of this figure.

Figure 2: Standard artificial feeding apparatus. The glass feeder contains r-liq_diet that is being fed to female Anopheles mosquitoes. Please click here to view a larger version of this figure.

Figure 3: Anopheles mosquitoes after artificial feeding. From left to right: a fully engorged female that was offered r-liq_diet, non-engorged female that was offered r-liq_diet, male, and fully engorged female that was offered mouse blood. Please click here to view a larger version of this figure.
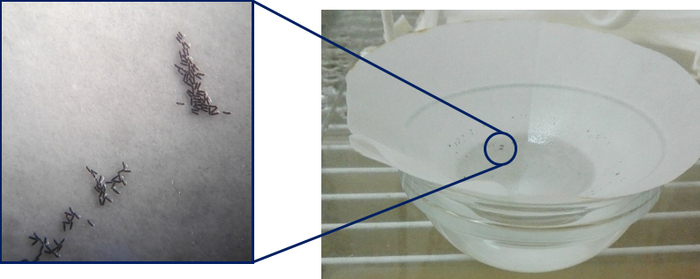
Figure 4: Anopheles eggs laid 48 h post-feeding of females with the r-liq_diet. Please click here to view a larger version of this figure.

Figure 5: L2 larvae stages that developed from the eggs and were collected on filter paper and placed in trays containing distilled water. Please click here to view a larger version of this figure.

Figure 6: Right wing from an F1 generation of an Anopheles coluzzii female mosquito. Please click here to view a larger version of this figure.
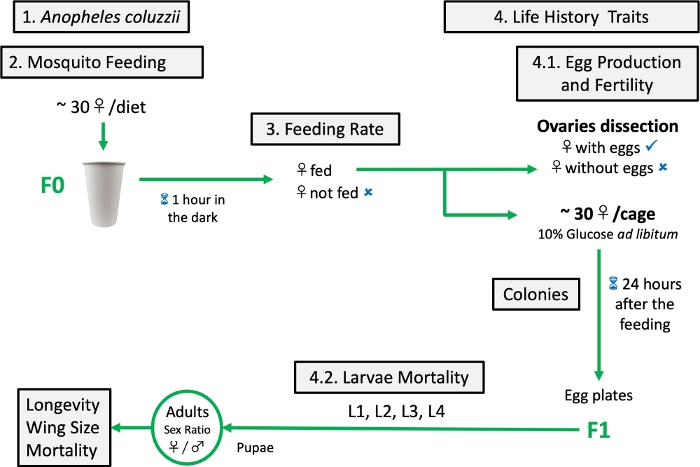
Figure 7: Schematic protocol of artificial diet testing. Please click here to view a larger version of this figure.
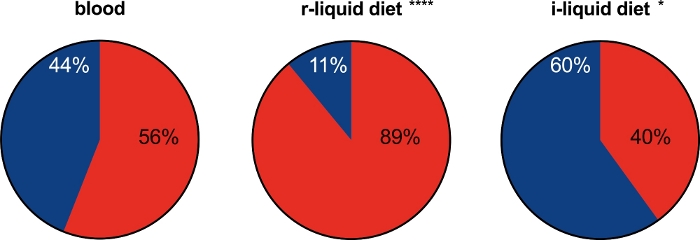
Figure 8: Feeding rates of the female mosquitoes fed either the artificial diets or blood. Asterisks indicate significant differences between the mosquitoes fed the r-liquid and i-liquid diets and the blood-fed control group. Two-sided Fisher's exact test: ****P ≤ 0.0001 (relative risk: 0.4828, 95% confident Level [CL]: 0.3776 to 0.6194) for r-liquid diet versus blood, *P = 0.0335 (relative risk: 1.379, 95% CL: 1.044 to 1.836) for blood versus i-liquid diet. Blue: unfed; red: fed. Please click here to view a larger version of this figure.
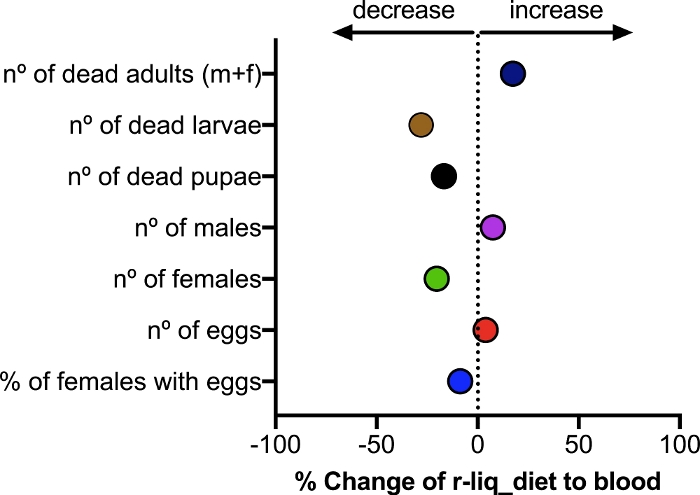
Figure 9: Effect of the formulated blood-free meal on the mortality and male/female ratio of F1 Anopheles coluzzii mosquitoes. Three independent experiments were performed, each using 30 mosquitoes per diet. An unpaired t-test showed no significant differences between the blood-fed group and the r-liq_diet fed group (P values varied from 0.5047 to 0.8491). Please click here to view a larger version of this figure.
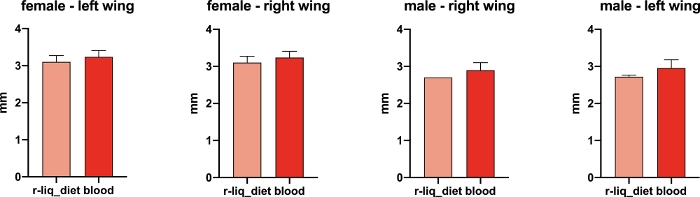
Figure 10: Wing length. The distance from the axial incision to the R4+5 vein excluding the fringe seta was used to determine the wing length. Size was evaluated for 5 females and 5 males from each dietary group (mean ± SEM). Values are represented as the mean ± SEM. Salmon: r-liq_diet; red: vertebrate blood. Unpaired t-test; female left wing: t = 1.300, df = 8, P = 0.2298; male left wing: t = 2.400, df = 8, P = 0.0432; female right wing: t = 1.300, df = 8, P = 0.2298; male right wing: t = 2.277, df = 7, P = 0.0569. Please click here to view a larger version of this figure.
| Components | g/L |
| *Adenosine Triphosphate | 0.55 |
| *Bovine Serum Albumin | 200 |
| *Cholesterol | 1 |
| Calcium chloride anhydrous | 0.2 |
| Choline chloride | 0.004 |
| D-calcium pantothenate (vitamin B5) | 0.004 |
| D-glucose anhydrous | 4.5 |
| Ferric nitrate nonahydrate | 0.0001 |
| Folic acid | 0.004 |
| Glycine | 0.03 |
| I-inositol | 0.007 |
| L-arginine monohydrochloride | 0.084 |
| L-cystine dihydrochloride | 0.063 |
| L-glutamine | 0.584 |
| L-histidine monohydrochloride monohydrate | 0.042 |
| L-isoleucine | 0.105 |
| L-leucine | 0.105 |
| L-lysine monohydrochloride | 0.146 |
| L-methionine | 0.03 |
| L-phenylalanine | 0.066 |
| L-serine | 0.042 |
| L-threonine | 0.095 |
| L-tryptophan | 0.016 |
| L-tyrosine disodium salt dihydrate | 0.104 |
| L-valine | 0.094 |
| Magnesium sulfate anhydrous | 0.098 |
| Niacinamide (nicotinamide) | 0.004 |
| Phenol red | 0.015 |
| Potassium chloride | 0.4 |
| Pyridoxine Monohydrochloride | 0.004 |
| Pyruvic acid sodium salt | 0.011 |
| Riboflavin (vitamin B2) | 0.0004 |
| Sodium bicarbonate | 3.7 |
| Sodium chloride | 6.4 |
| Sodium phosphate monobasic anhydrous | 0.109 |
| Thiamine amonohydrochloride (vitamin B1) | 0.004 |
| *Only in r-liq_diet | |
Table 1: The composition of the i-liquid diet and r-liquid diet.
| Total egg number (± SEM) | Eggs/female (± SEM) | |
| Blood | 733 ± 330 | 24 ± 11 |
| r-liq_diet | 763 ± 164 | 25 ± 5 |
| i-liq_diet | 0 | 0 |
Table 2: Egg batches produced by Anopheles coluzzii females. Three independent experiments were performed for each experimental diet using 30 female mosquitoes in each.
| Females (days ± SEM) | Males (days ± SEM) | |
| Blood | 24.5 ± 6.8 | 18.5 ± 6.9 |
| r-liq_diet | 22.5 ± 8.1 | 11.9 ± 6.9 |
Table 3: Life expectancy of F1 Anopheles mosquitoes. Longevity of F1 mosquitoes from artificially fed F0 was assessed by recording the dates of birth and death of each mosquito coming from the same dietary group (15 females and 15 males were followed). The results are represented as the mean mosquito life span per diet group.
Discussion
The success of our formulated blood-free diet is likely the result of the synergistic physiological effect of all the components added to the i-liq_diet (rich in sugar, amino acids, vitamins and microelements): BSA (protein source), ATP (phagostimulant) and cholesterol (lipid source). Supplementation of the r-liq_diet with the individual components alone was not effective in stimulating egg production (data not shown). One drawback of the protocol might be the cost of some of the components, such as cholesterol. Even so, its presence is fundamental, as insects are unable to synthesize it19 and this molecule is the precursor of the ecdysteroid hormones that regulate yolk synthesis and egg maturation in arthropods20. Lower amounts of cholesterol should be tested in order to optimize the quantity needed with the aim of reducing costs and increasing the benefits of the artificial diet.
Another limitation of the method is that the artificial diet has to be freshly prepared from stock solutions, as once prepared in its final liquid form it loses quality after storage. In the future our formulated diet could be prepared as a dried power, similar to SkitoSnackt, an artificial blood meal replacement for Aedes aegypti mosquitoes21.
Beside supplying the necessary nutrients, an artificial meal needs to attract and stimulate female mosquitoes to feed in the same way as when they feed on vertebrate fresh blood. The artificial blood-free diet herein described resulted in a 20% increase in fully engorged female mosquitoes when compared to the vertebrate blood fed group. This indirect measure of attraction could be further clarified by using olfactometers to confirm that the artificial diet is more attractive and more appealing to mosquitoes than fresh blood.
The highest impact of the diet on larval mortality was observed for larvae derived from mosquitoes fed on blood, suggesting that an artificial diet of stable composition can contribute to reduce mortality and improve mosquito breeding success when compared to fresh blood. The less predictable outcome of a blood meal may arise from host variations in composition17 and the presence in the blood of molecules that may interfere with mosquito physiology22. The preceding facts emphasize the advantages for high-quality mosquitoes rearing of fresh-blood-free diets.
Overall the average number of eggs laid in our study was low relative to those reported in some insectaries, but the mean number of oviposited eggs was comparable to the A. gambiae laboratory-reared strain fed on human blood (22.6 ± 5.5 eggs/female)23. No significant statistical differences were observed between our experimental groups fed on either fresh blood or on the artificial meals (Table 2), suggesting that the implementation of an artificial membrane feeding systems with our formulated diet is sufficient to maintain and propagate Anopheles mosquito colonies in captivity.
Artificial blood-free meals can maintain Aedes colonies22, but when applied to Anopheles mosquitoes they are of limited or no success11. Recently, a plasma-based artificial meal for Anopheles mosquitoes was described24 but feeding rates and reproductive potential was low. Our results represent a substantial advance in the state-of-art (reviewed by Gonzales and Hansen11) as our formulated r-liq_diet had a similar or better performance than the standard vertebrate blood meal. Further improvements on storage stability and cost should widen the scope of its application.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to thank Dinora Lopes (IHMT-NOVA Animal Facility) for technical support, Joana Gomes and Ana Catarina Alves (IHMT-NOVA Insectary Facility) for maintaining Anopheles mosquito colonies. Funded by the Bill and Melinda Gates Foundation (OPP1138841), Fundação para a Ciência e Tecnologia (UID/Multi/04413/201, UID/Multi/04326/2013, SFRH/BPD/89811/2012, CEECIND/00450/2017).
Materials
| Adenosine 5'-triphosphate (ATP) disodium salt hydrate | Sigma Aldrich | A2383 | |
| BSA-Bovine Serum Albumin | Sigma Aldrich | A790G | |
| Cholesterol | MP Biomedicals | 199342 | |
| Dulbecco's modified Eagle's medium (high glucose with L-glutamine) | Lonza Bioscience | BE12-604F | |
| Entellan mounting medium | Merck | 1079610100 | |
| Glassfeeder | Local glazier | by design | |
| Heparin Sodium Salt | Pan Reac AppliChem | A3004,0001 | |
| Imalgène 1000 | Merial, Portugal | 01MER122 | |
| Needle 20 G x 1" 0.9 x 25 mm needle | Terumo Europe | NN-2025R | |
| Parafilm | Sigma Aldrich | P6543-1EA | |
| Rompun | Bayer, Portugal | 7427831 | |
| Sterilization Millex-HV 0,45 | Millipore | SLHVR25KS | |
| Syringe, 1ml, 27 G x ½" 0.4 x 12 mm needle | Terumo Europe | BS-NIN2713 | |
| Teich Mix Astra Pond | Astra | 4030733100957 | |
| Tetra Goldfish Flakes | Tetra | 4004218742642 |
Referências
- WHO. . World Malaria Report. , (2016).
- Hansen, I. A., Attardo, G. M., Rodriguez, S. D., Drake, L. L. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Frontiers in Physiology. 5, 103 (2014).
- Catteruccia, F., Crisanti, A., Wimmer, E. A. Transgenic technologies to induce sterility. Malaria Journal. 8, 7 (2009).
- Dame, D. A., Curtis, C. F., Benedict, M. Q., Robinson, A. S., Knols, B. G. J. Historical applications of induced sterilisation in field populations of mosquitoes. Malaria Journal. 8, 2 (2009).
- Lacroix, R., et al. Open Field Release of Genetically Engineered Sterile Male Aedes aegypti in Malaysia. PLoS One. 7, 42771 (2012).
- Lea, A. O., Knierim, J. A., Dimond, J. B., Delong, D. M. A Preliminary Note on Egg Production from Milk-Fed Mosquitoes. The Ohio Journal of Science. 55, 1-21 (1955).
- Kogan, P. H. Substitute blood meal for investigating and maintaining Aedes aegypti (Diptera: Culicidae). Journal of Medical Entomology. 27, 709-712 (1990).
- Griffith, J. S., Turner, G. D. Culturing Culex quinquefasciatus mosquitoes with a blood substitute diet for the females. Medical and Veterinary Entomology. 10, 265-268 (1996).
- Jason Pitts, R. A blood-free protein meal supporting oogenesis in the Asian tiger mosquito, Aedes albopictus (Skuse). Journal of Insect Physiology. 64, 1-6 (2014).
- Gonzales, K. K., Tsujimoto, H., Hansen, I. A. Blood serum and BSA, but neither red blood cells nor hemoglobin can support vitellogenesis and egg production in the dengue vector Aedes aegypti. PeerJ. 3, 938 (2015).
- Gonzales, K. K., Hansen, I. A. Artificial Diets for Mosquitoes. International Journal of Environmental Research and Public Health. 13 (12), 1267 (2016).
- Cosgrove, J. B., Wood, R. J. Effects of variations in a formulated protein meal on the fecundity and fertility of female mosquitoes. Medical and Veterinary Entomology. 10, 260-264 (1996).
- Attardo, G. M., Hansen, I. A., Shiao, S. -. H., Raikhel, A. S. Identification of two cationic amino acid transporters required for nutritional signalling during mosquito reproduction. Journal of Experimental Biology. 209, 3071-3078 (2006).
- Clements, A. N. . The biology of mosquitoes: Development, nutrition, and reproduction. , (1992).
- Talyuli, O. A., et al. The use of a chemically defined artificial diet as a tool to study Aedes aegypti physiology. Journal of Insect Physiology. 83, 1-7 (2015).
- Lopes, L. F., Abrantes, P., Silva, A. P., Dorosario, V. E., Silveira, H. Plasmodium yoelii: The effect of second blood meal and anti-sporozoite antibodies on development and gene expression in the mosquito vector, Anopheles stephensi. Experimental Parasitology. 115, 259-269 (2007).
- Phasomkusolsil, S., et al. Maintenance of mosquito vectors: effects of blood source on feeding, survival, fecundity, and egg hatching rates. Journal of Vector Ecology. 38, 38-45 (2013).
- Gillies, M. T., De Meillon, B. The Anophelinae of Africa South of the Sahara (Ethiopian zoogeographical region). Publications of the South African Institute for Medical Research. 54, 1 (1968).
- Canavoso, L. E., Jouni, Z. E., Karnas, K. J., Pennington, J. E., Wells, M. A. Fat metabolism in insects. Annual Review of Nutrition. 21, 23-46 (2001).
- Clifton, M. E., Noriega, F. G. The fate of follicles after a blood meal is dependent on previtellogenic nutrition and juvenile hormone in Aedes aegypti. Journal of Insect Physiology. 58, 1007-1019 (2012).
- Gonzales, K. K., et al. The Effect of SkitoSnack, an Artificial Blood Meal Replacement on Aedes aegypti Life History Traits and Gut Microbiota. Scientific Reports. 8, 11023 (2018).
- Vodovotz, Y., Zamora, R., Lieber, M. J., Luckhart, S. Cross-talk between nitric oxide and transforming growth factor-beta1 in malaria. Current Molecular Medicine. 4, 787-797 (2004).
- Sumba, L. A., et al. Daily oviposition patterns of the African malaria mosquito Anopheles gambiae Giles (Diptera: Culicidae) on different types of aqueous substrates. Journal of Circadian Rhythms. 2, 6 (2004).
- Baughman, T., et al. A highly stable blood meal alternative for rearing Aedes and Anopheles mosquitoes. PLoS Neglected Tropical Diseases. 11, 0006142 (2017).

