Concomitant Isolation of Primary Astrocytes and Microglia for Protozoa Parasite Infection
Summary
The overall goal of this protocol is to instruct how to extract, maintain, and dissociate murine astrocyte and microglia cells from the central nervous system, followed by infection with protozoa parasites.
Abstract
Astrocytes and microglia are the most abundant glial cells. They are responsible for physiological support and homeostasis maintenance in the central nervous system (CNS). The increasing evidences of their involvement in the control of infectious diseases justify the emerging interest in the improvement of methodologies to isolate primary astrocytes and microglia in order to evaluate their responses to infections that affect the CNS. Considering the impact of Trypanosoma cruzi (T. cruzi) and Toxoplasma gondii (T. gondii) infection in the CNS, here we provide a method to extract, maintain, dissociate and infect murine astrocytes and microglia cells with protozoa parasites. Extracted cells from newborn cortices are maintained in vitro for 14 days with periodic differential media replacement. Astrocytes and microglia are obtained from the same extraction protocol by mechanical dissociation. After phenotyping by flow cytometry, cells are infected with protozoa parasites. The infection rate is determined by fluorescence microscopy at different time points, thus enabling the evaluation of differential ability of glial cells to control protozoan invasion and replication. These techniques represent simple, cheap and efficient methods to study the responses of astrocytes and microglia to infections, opening the field for further neuroimmunology analysis.
Introduction
The CNS is mainly composed of neurons and glial cells1,2,3. Microglia and astrocytes are the most abundant glia cells in the CNS. Microglia, the resident macrophage, is the immunocompetent and the phagocytic glia cell in the CNS3,4, while astrocytes are responsible for maintaining homeostasis and exert supportive functions5.
Despite glial cells being classically known to be responsible for the support and protection of neurons6,7, emerging functions of these cells have been described in the recent literature, including their responses to infections8,9,10,11. Thus, there is a push to develop methods to isolate these glial cells to understand their functions individually.
There are some alternative models to study glial cells rather than primary cultures, like immortalized cell lineages and in vivo models. However, immortalized cells are more likely to undergo genetic drifting and morphological changes, while in vivo studies impose limited manipulation conditions. Conversely, primary cultures are easy to handle, better resemble in vivo cells and also allow us to control experimental factors12,13. Here, we describe guidelines on how to extract, maintain and dissociate murine astrocytes and microglia primary cells in the same protocol. Furthermore, we also provide examples on how to work with protozoa infection in these cultures.
CNS cells extracted from neonatal mice (up to 3 day-old) were cultured for 14 days on differential media that allows the preferential growth of astrocytes and microglia cells. Since microglia rest above the attached astrocytes, cell populations were mechanically dissociated in an orbital incubator. Next, we collected all the supernatant containing microglia and added trypsin to detach astrocytes. Isolated glial cells were phenotypically evaluated by flow cytometry and plated according to the desired experiment.
We also provided examples on how to infect these isolated microglia and astrocytes with protozoa parasites. T. gondii is a highly neurotropic protozoan responsible for toxoplasmosis14, while T. cruzi is responsible for Chagas disease which can leads to development of neurological disorders in the CNS15,16. Furthermore, it has also been reported that infection with T. gondii17,18 or T. cruzi19,20,21 were the presumable cause of death in immunocompromised patients. Therefore, the elucidation of immunologic role of glial cells from the CNS in controlling protozoa infections is of great importance.
Protocol
All experimental procedures involving mice were carried out in accordance to the Brazilian National Law (11.794/2008) and approved by the Institutional Animal Care and Use Committees (IACUC) of the Federal University of São Paulo (UNIFESP).
1. Glial Cells Extraction, Maintenance and Dissociation
NOTE: The number of mice used for the glial cells extraction depends on the quantity of cells required to perform the desired experiments. In this protocol, a total of 2.7 x 107 astrocytes and 4 x 106 microglia were obtained from six neonatal C57BL/6 mice. All procedures were performed under sterile condition in a class II biosafety cabinet.
- Day 1
- Prepare pure Hank's balanced salt solution (HBSS) and HBSS + 10% of heat-inactivated fetal bovine serum (FBS) (see Table of Materials).
- Prepare supplemented Dulbecco's Modified Eagle Medium (DMEM)/F12 (10% FBS + 0.08 mM Penicillin + 0.09 mM Streptomycin + 12.5 mM HEPES + 30 mM sodium bicarbonate, pH 7.2) and filter it with a 0.22 µm filter (see Table of Materials).
NOTE: All culture media should be stored at 4 °C, but it is necessary to prewarm them at 37 °C for 20 min before starting the experiment. - Sterilize all surgical instruments (scissors, spatula and tweezers) in an autoclave and use 70% ethanol during the procedure.
- Put newborns (up to 3 day-old) mice in a sealed chamber containing cotton soaked with isoflurane for 5 min for profound anesthesia.
- Spray the mouse pup with 70% ethanol and decapitate the animal with scissors.
- Make sagittal cut (posterior to anterior) with scissors along the cranium to open it and expose the brain. Separate the skull from the brain using tweezers. Remove the brain using a micro spatula to maintain the brain integrity.
- Place the brain in a dry Petri dish (6 cm diameter). Remove the olfactory bulb and cerebellum using a micro spatula. Move the cortex to another Petri dish (6 cm diameter) containing HBSS + 10% FBS (2 mL/Petri dish).
- Cut the brain tissue into small pieces with sterile scissors and using a p1000 micropipette transfer each brain tissue with HBSS + 10% FBS to different 15 mL conical tubes. Make sure that the final volume is 2 mL/tube. If not, complete with HBSS + 10% FBS.
NOTE: The protocol can be paused here. If so, conical tubes with CNS tissue must be placed on ice up to 1 h. - Wash the cut brain tissue with 3 mL of HBSS + 10% FBS (per tube) and after decantation. Carefully remove the supernatant. Repeat this step 3 times.
NOTE: This step aims to remove the debris and to recover the extracted tissue. - Wash the cut brain tissue with 3 mL of pure HBSS (per tube) and after decantation, carefully remove the supernatant. Repeat this step 3 times.
NOTE: This step aims to remove the remaining serum from the previous washes, as cells will be trypsinized in the next step. - Add 3 mL of trypsin per tube and place in a water bath at 37 °C for 30 min, gently shaking the tubes every 5 min. Avoid bubbles by inverting or abruptly mixing the tubes.
NOTE: This step aims to digest the collected tissue. - To inactivate the trypsin, wash the tissue with 3 mL per tube of HBSS + 10% FBS and, after decantation of the tissue, carefully remove the supernatant. Repeat this step 3 times.
- Wash the tissue with 3 mL per tube of pure HBSS and carefully remove the supernatant. Repeat this step 2 times.
- Add 7 mL per tube of pure HBSS and homogenize the tissue through successive passages in pipettes: first with the 10 mL serological pipette, followed by the 5 mL and finally with the p1000 micropipette.
- After homogenization, centrifuge tubes at 450 x g for 5 min at 4 °C.
- Discard the supernatant and resuspend the pellet with 4 mL of HBSS + 10% FBS per tube.
- Transfer cells to a pretreated T-75 flask for optimal cell culture adhesion (see Table of Materials).
NOTE: Process tissue from each animal per flask. - Place the flask in the incubator at 37 °C and 5% CO2 for 30 min for adherence.
- Add 10 mL of supplemented DMEM/F12 per flask and incubate at 37 °C and 5% CO2.
NOTE: The final volume is 14 mL per flask.
- Day 3
- Remove 7 mL of culture medium from the T-75 flask and add 7 mL of fresh supplemented DMEM/F12.
NOTE: The flasks will contain a lot of cellular debris and a cloudy appearance.
- Remove 7 mL of culture medium from the T-75 flask and add 7 mL of fresh supplemented DMEM/F12.
- Day 5
- Remove all the medium from the T-75 flask and add 14 mL of fresh supplemented DMEM/F12.
NOTE: Medium is replaced from the flasks to remove debris and non-adherent cells. - After each 48 h, remove 6 mL of the supernatant and add 7 mL of fresh supplemented DMEM/F12 medium until day 14 of culture.
- Remove all the medium from the T-75 flask and add 14 mL of fresh supplemented DMEM/F12.
- Day 14
- After removing 6 mL of the supernatant and adding 7 mL of fresh supplemented DMEM/F12 medium, close the T-75 flasks tightly.
- Place them in the floor orbital shaker at 200 rpm and 37 °C overnight to mechanically dissociate microglia from astrocytes.
- Day 15
- Take the flasks from the shaker and vigorously wash them with their own medium in order to optimize cell dissociation and harvest the maximum number of microglia. Then, collect the supernatant (containing microglia) and transfer to a 50 mL conical tube.
- Next, add 4 mL of trypsin into each flask and incubate for 5 min at 37 °C in order to detach the astrocytes.
- Add 5 mL per flask of supplemented DMEM/F12 to inactivate the trypsin. Wash the flasks with their own medium and transfer contents to a 50 mL conical tube.
- Centrifuge all tubes containing dissociated microglia and astrocytes at 450 x g for 5 min at 4 °C.
- Discard the supernatant. Resuspend the microglia pellet in 1 mL and the astrocytes in 10 mL of supplemented DMEM/F12.
- Proceed to cell counting in a Neubauer chamber or an automatic cell counter. Plate the cells with supplemented DMEM/F12 at the desired density in an appropriate flat bottom cell culture plate (pretreated for optimal cell attachment; see Table of Materials). Incubate cells at 37 °C and 5% CO2 for 24 h to allow them to attach.
NOTE: Reserve 1.5 x 106 of each cell population to confirm their purity by flow cytometry. - Perform a flow cytometric analysis to verify the purity of cell populations.
NOTE: We consider microglia CD11b+/CD45+/GFAP– and astrocytes CD11b–/CD45–/GFAP+. Iba1 and TMEM119 staining might be considered in order to fully characterize microglia22. - Add a FMO (Fluorescence Minus One)23 and an unstained sample for each cell population (astrocytes and microglia) as controls of flow cytometry assay.
- For each cell population, reserve 5 x 105 cells for each stained and unstained samples. For FMO, reserve two other tubes of microglia containing 2.5 x 105 cells each and also reserve another tube of 5 x 105 astrocytes.
NOTE: Since microglia numbers can be a limiting factor, it is possible to use fewer cells for unstained and FMO controls. - Centrifuge all microtubes at 450 x g for 5 min at 4 °C. Discard the supernatant and resuspend the pellet with 500 µL/microtube of FACS buffer (phosphate-buffered saline (PBS) + 0.5% bovine serum albumin (BSA) + 2 mM EDTA, pH 7.2).
- Centrifuge all microtubes at 450 x g at 4 °C for 5 min. Discard the supernatant and resuspend the pellet with 100 µL/microtube of anti-CD16/CD32 (clone 93) (1:50) diluted in FACS buffer. Incubate for 10 min at room temperature.
- Add 500 µL/microtube of FACS buffer into all microtubes and centrifuge at 450 x g at 4 °C for 5 min. Discard the supernatant. Resuspend astrocytes and microglia staining tubes and also astrocyte FMO tube with 50 µL/microtube of surface markers mix: anti-CD45 (PE, clone 30-F11) and anti-CD11b (eFluor 450, clone M1/70) (both diluted at 1: 100 in FACS buffer).
- For unstained cells, resuspend with the same volume of FACS buffer. For microglia FMO, incubate each microglia tube with anti-CD45 or anti-CD11b. Incubate all samples at 4 °C for 20 min in the dark.
- Add 500 µL of FACS buffer per microtube. Centrifuge at 450 x g at 4 °C for 5 min. Discard the supernatant and resuspend the pellet with 100 µL/microtube of fixation buffer (see Table of Materials) for 20 min at room temperature in the dark.
- Add 500 µL/microtube of permeabilization buffer 1x (see Table of Materials) and incubate at 4 °C for 5 min in the dark.
- Centrifuge all the microtubes at 450 x g at 4 °C for 5 min. Discard the supernatant. Resuspend astrocytes and microglia staining tubes and also microglia FMO tubes with 50 µL/microtube of intracellular antibody anti-GFAP (APC, clone GA5) in a 1:100 dilution in 1x permeabilization buffer. For unstained cells and astrocyte FMO, resuspend the cells with the same volume of FACS buffer. Incubate all samples at 4 °C for 30 min in the dark.
- Wash all tubes by adding 500 µL/microtube of 1x permeabilization buffer. Centrifuge all the microtubes at 450 x g, for 5 min at 4 °C. Resuspend each microtube in 200 µL of FACS buffer. Acquire 1 x 105 events/sample on the flow cytometer.
- For analysis, cells must be gated first on CD11b x CD45 and then GFAP histogram.
NOTE: Unstained and FMO controls are useful to determine gates.
2. Infection and Evaluation of Infection Rates
- Infection of glial cells with T. gondii
- Plate 3 x 104 microglia or astrocytes with supplemented DMEM/F12 at 37 °C and 5% CO2 for 24 h in a pretreated 96-well flat plate (see Table of Materials).
- Infect each cell population with tachyzoites from T. gondii RH strain expressing yellow fluorescent protein (YFP) at a multiplicity of infection (MOI) of 1:1 (parasite:cell) diluted in 200 µL/well of supplemented RPMI (3% FBS + 0.16 mM Penicillin + 0.18 mM Streptomycin + 12.5 mM HEPES + 30 mM sodium bicarbonate, pH 7.2) (see Table of Materials). Incubate at 37 °C and 5% CO2 for 48 h.
- Then, discard the supernatant and fix the cells by adding 100 µL/well of 1% paraformaldehyde (PFA) diluted in PBS at 4 °C for 24 h.
- Replace PFA with 100 µL/well of PBS at pH 7.2.
- Carefully remove the supernatant and stain cells' nuclei by pipetting 50 µL/well of DAPI (5 mg/mL) diluted in PBS pH 7.2 (1:1,000) for 1 min at room temperature in the dark.
- Replace DAPI staining solution with 100 µL/well of PBS (pH 7.2) and analyze it by fluorescence microscopy.
- Infection of glial cells with T. cruzi
- Plate 3 x 104 microglia or astrocytes with supplemented DMEM/F12 at 37 °C and 5% CO2 for 24 h in a pretreated 96-well flat plate (see Table of Materials).
NOTE: For each time point of infection, use a different plate. - Infect the glial cells with trypomastigotes from T. cruzi Y strain at a MOI of 5:1 (parasites:cell) diluted in 200 µL/well of supplemented RPMI and incubate at 37 °C and 5% CO2 for 2 h.
NOTE: This step is important for the cell invasion by the parasite. - Remove all of the supernatant and wash the wells by adding 200 µL/well of supplemented RPMI to remove all the extracellular parasites. Remove supernatant and add 200 µL/well of fresh supplemented RPMI.
NOTE: This step intends to remove all parasites that did not invade the adherent cells. - Incubate infected cells for 2 h, 48 h and 96 h to evaluate T. cruzi replication in glial cells11.
- After each time point, remove the supernatant and fix the cells with 100 µL/well of methanol for 15 min at room temperature and then replace methanol with 100 µL/well of PBS (pH 7.2).
- After fixation, prepare the cells for the immunofluorescence assay as follows.
- To avoid autofluorescence, treat the cells with 100 µL/well of 50 mM NH4Cl diluted in PBS (pH 8.0) for 15 min. Wash the cells by adding 100 µL/well of PBS and immediately remove it (repeat this step 3 times).
- Next, permeabilize the cells by adding 100 µL/well of 0.5% Triton diluted in PBS for 15 min. Wash cells by adding 100 µL/well of PBS and immediately remove it (repeat this step 3 times).
- Then, incubate the cell culture with 100 µL/well of blocking solution (5% non-fat milk + 2% BSA diluted in PBS [pH 7.2]) for 1 h at room temperature. Wash cells by adding 100 µL/well of PBS and immediately remove it (repeat this step 3 times).
- In order to stain amastigotes, incubate with 30 µL/well of non-commercial monoclonal antibody (mAb) 2C2 anti-Ssp-4 protein (1:200) diluted in blocking solution for 1 h at room temperature.
- Wash cells by adding 100 µL/well of PBS and immediately remove it (repeat this step 3 times). Incubate the plate with 30 µL/well of secondary anti-mouse antibody (1:500) diluted in PBS for 1 h at room temperature.
NOTE: The non-commercial mAb 2C2 anti-Ssp-4 protein was a kind gift from Prof. Dr. Renato A. Mortara from Department of Microbiology, Immunology and Parasitology of Federal University of São Paulo. - Carefully remove the supernatant and stain nuclei by adding 50 µL/well of DAPI (5 mg/mL) diluted in PBS pH 7.2 (1:1,000) for 1 min at room temperature in the dark.
- Replace DAPI staining solution with 100 µL/well of PBS pH 7.2 and analyze it by fluorescence microscopy.
- Plate 3 x 104 microglia or astrocytes with supplemented DMEM/F12 at 37 °C and 5% CO2 for 24 h in a pretreated 96-well flat plate (see Table of Materials).
Representative Results
On the 14th day, glial cells culture (Figure 1A) underwent mechanical dissociation. Isolated cell populations were analyzed by flow cytometry according to CD11b, CD45 and GFAP markers. We could observe a purity of 89.5% for the astrocyte population and 96.6% for the microglia population (Figure 1B). After isolation, cells were plated in a 96-well flat plate and after 24 h they were ready to be infected by T. cruzi or T. gondii according to the respective infection protocols. Here we provided a time-course infection of T. cruzi as an example of infection rate evaluation in these glial cells.
Astrocytes and microglia (3 x 104 cells/well) were infected with T. cruzi at MOI = 5:1 (parasites:cell) for 2–96 h (Figure 2). Infection rate was evaluated by immunofluorescence by counting the number of cells and the number of parasites stained with a DNA intercalator (DAPI). Briefly, we could observe that T. cruzi is able to invade microglia and astrocytes at similar rates. Moreover, T. cruzi replicates in both cell types, reaching the highest infection rate at 96 h post infection (p.i.). Interestingly, at 96 h p.i. the infection rate is more pronounced in astrocytes than in microglia, suggesting that microglia cells are able to control the parasite replication more efficiently than astrocytes, as we previously described11.
It is important to note that the use of genetically modified parasites expressing fluorescent reporters or parasites labeled with specific fluorescent antibodies could improve the immunofluorescence microscopy, since they are better distinguished from cell nucleus (Figure 3). Moreover, the infection rate of fluorescent parasites can be determined by other techniques such as flow cytometry. Here we provided examples of T. gondii RH strain constitutively expressing YFP (Figure 3A) and T. cruzi Y strain stained with non-commercial mAb 2C2 anti-Ssp-4 protein (Figure 3B).
Altogether, this protocol describes how to extract, maintain, dissociate and infect murine microglia and astrocytes primary cells, which could be a powerful tool to study, for example, immune responses of glial cells to protozoa infection as briefly elucidated here.
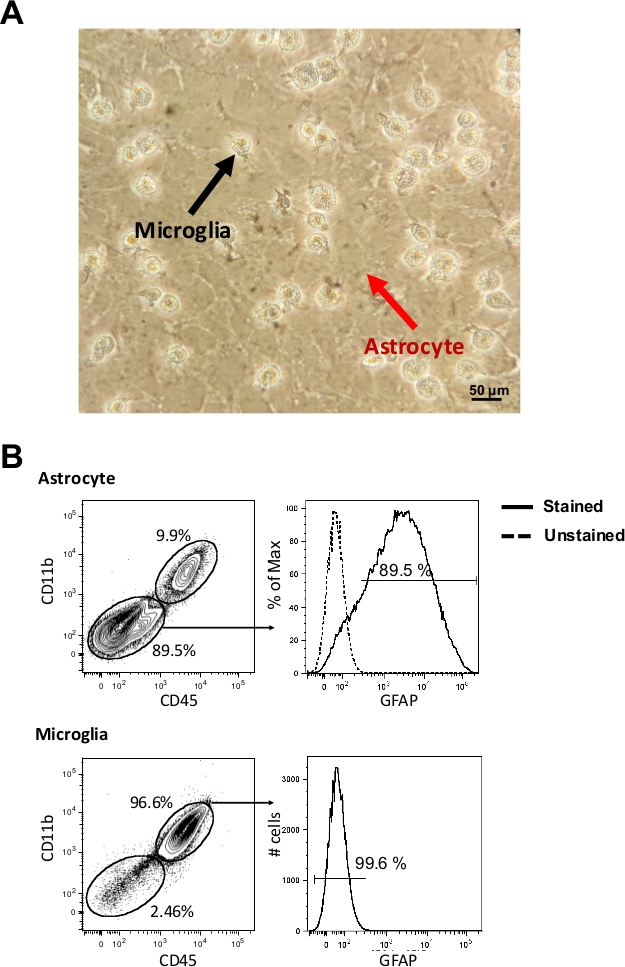
Figure 1: Primary astrocytes and microglia cells. (A) Images of C57BL/6 murine glial cells on the 14th day of culture were obtained by inverted microscope (400x). The red arrow indicates the layer of astrocytes and the black arrow indicates the microglia. (B) After mechanical dissociation, astrocytes and microglia were stained (5 x 105 cells) with fluorescent anti-CD11b, anti-CD45 and anti-GFAP and evaluated by flow cytometry (1 x 105 cells acquisition). Astrocytes are considered as CD11b–/CD45– and GFAP+ cells, whereas microglia are CD11b+/CD45+ and GFAP– cells. Please click here to view a larger version of this figure.
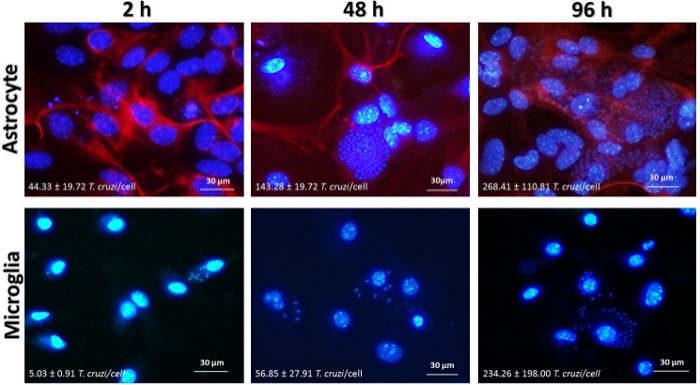
Figure 2: Time-course of T. cruzi infection in glial cells. Astrocytes and microglia from C57BL/6 mice were infected with T. cruzi Y (MOI = 5:1). After 2 h, 48 h and 96 h, chambers were fixed with methanol and stained with cell nucleus marker DAPI (blue) and anti-GFAP (red). Images were obtained by inverted fluorescence microscope (400x). The number of amastigotes inside the cells and the frequency of infected cells were evaluated by the fluorescence microscope software (see Table of Materials). Please click here to view a larger version of this figure.
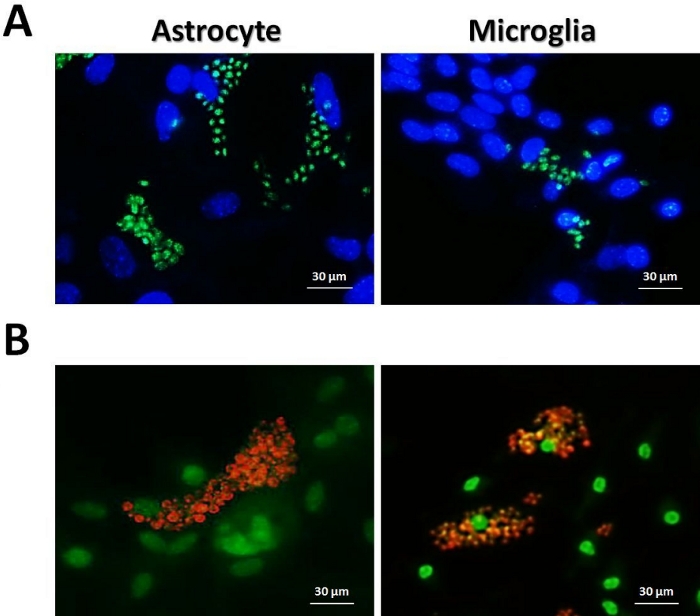
Figure 3: Astrocytes and microglia infection with fluorescent protozoan parasites. (A) 3 x 104 astrocytes and microglia from C57BL/6 mice were infected (MOI = 1:1) with T. gondii RH YFP (green) for 48 h, chambers were fixed with 1% PFA and stained with DAPI (blue). (B) 3 x 104 astrocytes and microglia from C57BL/6 mice were infected (MOI = 5:1) with T. cruzi Y for 96 h, and chambers were fixed with pure methanol and stained with DAPI (green) and mAb 2C2 anti-Ssp-4 (orange). Images were acquired using an inverted fluorescence microscope. Please click here to view a larger version of this figure.
Discussion
The importance of studying isolated glial cells functions in distinct biological contexts has been expanding in the last two decades. Understanding the CNS beyond neurons is still a growing field in cell biology, especially under infections or inflammatory conditions8,9,24. Glial cells are crucial not only for neurons physical support (as it was previously known), but also in many other physiological situations such as neuron energy supply, neurometabolism, immune surveillance, synaptic pruning, shaping and modulation of the tissue, among others3,25,26,27,28,29.
Since astrocytes and microglia can be differentially modulated during infection or sterile inflammation, it is of great importance to understand the individual and relative role of these glial cells. Although microglia are known to represent the mononuclear phagocyte system (MPS) in CNS, astrocytes have also been described as a pivotal player in the CNS immune responses8. In order to compare the role of each glial subset in the context of infection and/or inflammation, it is mandatory to obtain them from the same extraction and conditions. There are few reports about the effector responses of microglia and astrocytes to control infections, especially in regard to protozoan parasites. In this sense, we recently demonstrated the requirement of NLRP3 inflammasome to the control of T. cruzi replication in microglia but not in astrocytes by a mechanism involving nitric oxide (NO) secretion11.
This protocol describes a method that is focused on simultaneously extracting the two most abundant glial cells populations, astrocytes and microglia, from postnatal (up to 3-day-old) mice. Newborn mice older than 3-day-old can be used, but we experienced that the yield of both cells decreases slightly over time (data not shown). Our method differs from previously described protocols for astrocytes or microglia isolation because we focused on obtaining and isolating both cell types in the same extraction, under the same conditions, thus optimizing the usage of experimental animals and improving the study of the relative role for those cells in immunological contexts. Moreover, our protocol also optimized the yield of both cell types. The yield is usually 3–5 x 106 astrocytes and 3–5 x 105 microglia per flask. Compare this to other protocols that result in less cells using more animals per T-75 flask (some protocols use 2–3 animals per flask)30,31.
Another advantage of this protocol is the time required to obtain isolated astrocytes and microglia. Within 14 days it is possible to obtain mature microglia. In fact, it is important to highlight that, as Flode and Combs demonstrated, microglia older than 14 days present diminished ability to secrete cytokines; it is very important to consider this in neuroimmunological contexts30. Despite the fact that the mature makers for astrocyte are not completely understood, GFAP is largely used to characterize responsive and active astrocytes. Some protocols achieve levels of 90% GFAP positive cells only after 21 or 28 days of culture. For that reason, we proposed a method that provided, cells that are immunoresponsive and mostly mature within 7–14 days. Although thepurity can be lower than other methods for both astrocyte31 and microglia30, the main focus was to obtain microglia and astrocytes in greater quantities in a concomitant extraction. We understand that cell populations could be further sorted or isolated with column separation to improve their purities. For this, additional cell markers might be included, such as Iba1 or TMEM119 for microglia; Aquaporin-4 or S100B for astrocytes, CC1 or O4 for oligodendrocytes and NeuN for neurons22,31,32,33. However, an important cell loss at the end of the protocol should be considered.
Thus, we provided a modified method to concomitantly obtain microglia and astrocytes in an efficient and cheap protocol. Moreover, this protocol provide the advantages of a great yield allowing the comparative studies of glial subsets in neuroimmunological contexts, as illustrated here during T. cruzi and T. gondii infection.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to thank professor Dr. Renato A. Mortara from Federal University of São Paulo (UNIFESP) for mAb 2C2 anti-Ssp-4. This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant 2017/25942-0 to K.R.B.), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant 402100/2016-6 to K.R.B.), Instituto Nacional de Ciência e Tecnologia de Vacinas (INCTV/CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001). M.P.A. receives fellowship from CNPq, A.L.O.P. receives fellowship from CAPES, and I.S.F and L.Z.M.F.B. receives fellowship from FAPESP.
Materials
| 70% Ethanol | Dinâmica Química Contemporânea | Cat: 2231 | Sterilize |
| 75 cm2 Flask | Corning | Cat: 430720U | Plastic material |
| 96 well cell culture plate | Greiner Cellstar | Cat: 655090 | Cell culture |
| Ammonium Chloride (NH4Cl) | Dinâmica Química Contemporânea | Cat: C10337.01.AH | Remove autofluorescence |
| Anti-GFAP antibody | Abcam | Cat.: ab49874 | Immunofluorescence antidoby |
| Bottle Top Filter 0.22 mm CA | Corning | Cat: 430513 | Culture medium filter |
| Bovine Serum Albumin (BSA) | Sigma Aldrich | Cat: A7906 | FACS Buffer preparation |
| CD11b (FITC) | BD Pharmigen | Cat.: 553310 | Flow cytometry antibody |
| CD45 (PE) | Invitrogen | Cat.: 12-0451-83 | Flow cytometry antibody |
| Centrifuge | Eppendorf | Cat: 5810R | Centrifugation |
| Centrifuge | Eppendorf | 5415R | Centrifugation |
| Class II biosafety cabinet | Pachane | Cat: 200 | Biosafety cabinet for sterile procedures |
| CO2 Incubator | ThermoScientific | Model: 3110 | Primary cells maintenance |
| Conical tubes 15 mL | Corning | Cat: 430766 | Plastic material |
| Conical tubes 50 mL | Corning | Cat: 352070 | Plastic material |
| Countess automated cell counter | Invitrogen | Cat: C10281 | Cell counter |
| DAPI | Invitrogen | Cat.: D1306 | Immunofluorescence antidoby |
| Digital Microscope Camera | Nikon | Cat: DS-RI1 | Capture images on microscope |
| Dulbecco's Modified Eagle Medium (DMEM) | Gibco | Cat: 12800-058 | Cell culture medium |
| Ethylenediaminetetraacetic acid (EDTA) | Sigma Aldrich | Cat: E9884 | FACS Buffer preparation |
| F12 Nutrient Mixture | Gibco | Cat: 21700-026 | Cell culture medium |
| FACS Canto II | BD Biosciences | Unavaiable | Flow cytometer |
| Fetal Bovine Serum (FBS) | LGC Biotechnology | Cat: 10-bio500-1 | Cell culture medium supplement |
| Flow Jo (software) | Flow Jo | Version: Flow Jo_9.9.4 | Data analysis |
| Fluorescence intenselight | Nikon | Cat: C-HGFI | Fluorescence source |
| GFAP (APC) | Invitrogen | Cat.: 50-9892-82 | Flow cytometry antibody |
| Goat – anti-mouse IgG (FITC) | Kirkeegood&Perry Lab (KPL) | Cat.: 172-1806 | Immunofluorescence antidoby |
| HBSS – Hank's Balanced Salt Solution | Gibco | Cat: 14175079 | Cell culture medium |
| HEPES | Sigma Aldrich | Cat: H4034 | Cell culture medium supplement |
| IC Fixation Buffer | Invitrogen | Cat: 00-8222-49 | Cell fixation for Flow Citometry |
| Inverted microscope | Nikon | Model: ECLIPSE TS100 | Microscope |
| Isoflurane | Cristália | Cat: 21.2665 | Inhaled anesthetic |
| Methanol | Synth | Cat: 01A1085.01.BJ | Fixation for Immunofluorescence |
| Micro spatula | ABC stainless | Unavaiable | Surgical material |
| Microtube 1.5 mL | Axygen | Cat: MCT-150-C | Plastic material |
| Monoclonal antibody (mAb) 2C2 anti-Ssp-4 | Non commercial | Non commercial | Immunofluorescence antidoby |
| Multichannel Pipette (p200) | Corning | Cat: 751630124 | Pipette reagents |
| NIS Elements Software | Nikon | Version 4.0 | Acquire and analyse images |
| Non-fat milk | Nestlé | Cat: 9442405 | Blocking solution for immunofluorescence |
| Orbital Shaker Incubator | ThermoScientific | Model: 481 Cat: 11 | Dissociate microglia from astrocytes |
| Paraformaldehyde (PFA) | Sigma Aldrich | Cat: P6148 | Fixation for Immunofluorescence |
| PBS | Non commercial | Non commercial | Neutral Buffer |
| Penicillin G | Sigma Aldrich | Cat: P-7794 | Cell culture medium supplement |
| Permeabilization Buffer (10X) | Invitrogen | Cat: 00-8333-56 | Cell permeabilization for Flow Citometry |
| Petri dish 60×15 mm (Disposable, sterile) | Prolab | Cat: 0303-8 | Plastic material |
| pH meter | Kasvi | K39-1014B | Calibrate pH solution |
| RPMI 1640 Medium | Gibco | Cat: 31800-014 | Cell culture medium |
| Scissors | ABC stainless | Cat: LO9-W4 | Surgical material |
| Serological pipette 10 mL | Corning | Cat: 4101 | Plastic material |
| Serological pipette 5 mL | Corning | Cat: 4051 | Plastic material |
| Single Channel Pipette (p1000) | Gilson Pipetman | Cat: F123602 | Pipette reagents |
| Single Channel Pipette (p200) | Gilson Pipetman | Cat: F123601 | Pipette reagents |
| Sodium bicarbonate | Sigma Aldrich | Cat: S6297 | Cell culture medium supplement |
| Streptomycin sulfate salt | Sigma Aldrich | Cat: S9137 | Cell culture medium supplement |
| Triton X-100 | Sigma Aldrich | Cat: T9284 | Permeabilization for immunofluorescence |
| Trypsin | Gibco | Cat: 27250-018 | Digestive enzyme |
| Tweezers | ABC stainless | Cat: L28-P4-172 | Surgical material |
| Water Bath | Novatecnica | Model: 09020095 | Digeste tissue at 37 ºC with trypsin |
Referências
- Azevedo, F. A. C., et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. Journal of Comparative Neurology. 513 (5), 532-541 (2009).
- Herculano-Houzel, S. The glia/neuron ratio: How it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 62 (9), 1377-1391 (2014).
- Jäkel, S., Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Frontiers in Cellular Neuroscience. 11, 1-17 (2017).
- Streit, W. J. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 40 (2), 133-139 (2002).
- Sofroniew, M. V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends in Neuroscience. 32 (12), 638-647 (2009).
- Virchow, R. Die Cellularpathologie in ihrer Begründung auf physiologische and pathologische Gewebelehre. Verlag von August Hirschfeld, Berlin. , (1858).
- Nimmerjahn, A., Kirchhoff, F., Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in vivo. Science. 308 (5726), 1314-1318 (2005).
- Samartino, C. G., et al. Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis. American Journal of Pathology. 176 (3), 1323-1338 (2010).
- Jamilloux, Y., et al. Inflammasome activation restricts Legionella pneumophila replication in primary microglial cells through flagellin detection. Glia. 61 (4), 539-549 (2013).
- Freeman, L., et al. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. Journal of Experimental Medicine. 214 (5), 1351-1370 (2017).
- Pacheco, A. L., et al. The impairment in the NLRP3-induced NO secretion renders astrocytes highly permissive to T. cruzi replication. Journal of Leukocyte Biology. 160 (1), 201-207 (2019).
- Stansley, B., Post, J., Hensley, K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. Journal of Neuroinflammation. 9 (1), 115 (2012).
- Lian, H., Roy, E., Zheng, H. Protocol for Primary Microglial Culture Preparation. Bio-Protocol. 6 (21), 1-10 (2016).
- Lüder, C. G. K., Giraldo-Velásquez, M., Sendtner, M., Gross, U. Toxoplasma gondii in primary rat CNS cells: Differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Experimental Parasitology. 92 (1), 23-32 (1999).
- Chagas, C. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Memorias do Instituto Oswaldo Cruz. 1 (2), (1909).
- Berkowitz, A. L., Raibagkar, P., Pritt, B. S., Mateen, F. J. Neurologic manifestations of the neglected tropical diseases. Journal of Neurological Sciences. 349 (1), 20-32 (2015).
- Jones, J. L., et al. Toxoplasmic encephalitis in HIV-infected persons: risk factors and trends. The Adult/Adolescent Spectrum of Disease Group. AIDS. 10 (12), 1393-1399 (1996).
- Luft, B. J., et al. Toxoplasmic Encephalitis in Patients with the Acquired Immunodeficiency Syndrome. New England Journal of Medicine. 329 (14), 995-1000 (1993).
- Madalosso, G., et al. Chagasic meningoencephalitis: Case report of a recently included AIDS-defining illness in Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo. 46 (4), 199-202 (2004).
- Rocha, A., et al. Pathology of patients with Chagas’ disease and acquired immunodeficiency syndrome. American Journal of Tropical Medicine and Hygiene. 50 (3), 261-268 (1994).
- Yasukawa, K., et al. Case report: Trypanosoma cruzi meningoencephalitis in a patient with acquired immunodeficiency syndrome. American Journal of Tropical Medicine and Hygiene. 91 (1), 84-85 (2014).
- Bennett, M. L., et al. New tools for studying microglia in the mouse and human CNS. Proceedings of the National Academy of Sciences of the United States of America. 113 (12), 1738-1746 (2016).
- Roederer, M. Compensation in Flow Cytometry. Current Protocols in Cytometry. 22 (1), (2002).
- Silva, A. A., et al. Priming astrocytes with TNF enhances their susceptibility to Trypanosoma cruzi infection and creates a self-sustaining inflammatory milieu. Journal of Neuroinflammation. 14 (182), (2017).
- Tsacopoulos, M., Evêquoz-Mercier, V., Perrottet, P., Buchner, E. Honeybee retinal glial cells transform glucose and supply the neurons with metabolic substrate. Proceedings of the National Academy of Sciences of the United States of America. 85 (22), 8727-8731 (1988).
- Nagase, M., Takahashi, Y., Watabe, A. M., Kubo, Y., Kato, F. On-site energy supply at synapses through monocarboxylate transporters maintains excitatory synaptic transmission. Journal of Neuroscience. 34 (7), 2605-2617 (2014).
- Buckman, L. B., Thompson, M. M., Moreno, H. N., Ellacott, K. L. J. Regional astrogliosis in the mouse hypothalamus in response to obesity. Journal of Comparative Neurology. 521 (6), 1322-1333 (2013).
- Vesce, S., Bezzi, P., Volterra, A. The active role of astrocytes in synaptic transmission. Cellular and Molecular Life Sciences. 56 (11-12), 991-1000 (1999).
- Arcuri, C., Mecca, C., Bianchi, R., Giambanco, I., Donato, R. The Pathophysiological Role of Microglia in Dynamic Surveillance, Phagocytosis and Structural Remodeling of the Developing CNS. Frontiers in Molecular Neuroscience. 10, 191 (2017).
- Floden, A. M., Combs, C. K. Microglia repetitively isolated from in vitro mixed glial cultures retain their initial phenotype. Journal of Neuroscience Methods. 164 (2), 218-224 (2007).
- Schildge, S., Bohrer, C., Beck, K., Schachtrup, C. Isolation and culture of mouse cortical astrocytes. Journal of Visualized Experiments. (71), e50079 (2013).
- Sarkar, S., et al. Rapid and refined CD11b magnetic isolation of primary microglia with enhanced purity and versatility. Journal of Visualized Experiments. (122), e55364 (2017).
- Tamashiro, T. T., Dalgard, C. L., Byrnes, K. R. Primary microglia isolation from mixed glial cell cultures of neonatal rat brain tissue. Journal of Visualized Experiments. (66), e3814 (2012).

