Recurrent Escherichia coli Urinary Tract Infection Triggered by Gardnerella vaginalis Bladder Exposure in Mice
Summary
A mouse model of uropathogenic E. coli (UPEC) transurethral inoculation to establish latent intracellular bladder reservoirs and subsequent bladder exposure to G. vaginalis to induce recurrent UPEC UTI is demonstrated. Also demonstrated are the enumeration of bacteria, urine cytology, and in situ bladder fixation and processing for scanning electron microscopy.
Abstract
Recurrent urinary tract infections (rUTI) caused by uropathogenic Escherichia coli (UPEC) are common and costly. Previous articles describing models of UTI in male and female mice have illustrated the procedures for bacterial inoculation and enumeration in urine and tissues. During an initial bladder infection in C57BL/6 mice, UPEC establish latent reservoirs inside bladder epithelial cells that persist following clearance of UPEC bacteriuria. This model builds on these studies to examine rUTI caused by the emergence of UPEC from within latent bladder reservoirs. The urogenital bacterium Gardnerella vaginalis is used as the trigger of rUTI in this model because it is frequently present in the urogenital tracts of women, especially in the context of vaginal dysbiosis that has been associated with UTI. In addition, a method for in situ bladder fixation followed by scanning electron microscopy (SEM) analysis of bladder tissue is also described, with potential application to other studies involving the bladder.
Introduction
Urinary tract infections (UTI) impose a significant healthcare burden worldwide, impacting the quality of life of millions of people each year, especially women1. Uropathogenic Escherichia coli (UPEC) are the most frequent cause of UTI1. Many patients (approximately 20-30%) who develop UTI will experience a recurrent UTI (rUTI) within 6 months despite antibiotic-mediated clearance of the initial infection2. Unfortunately, as many as 5% of premenopausal women suffer from 3 or more rUTI each year3,4. Sequential episodes of rUTI can be caused by persistence of the same UPEC strain from the index case5,6,7,8. Data from human samples and mouse models suggest that same-strain rUTI could be caused by UPEC residing within quiescent reservoirs in the bladder. In humans, UPEC were detected in epithelial cells and bladder biopsies of patients with UTI9,10,11,12,13. Studies in C57BL/6 mice have demonstrated that some strains of UPEC can establish quiescent intracellular reservoirs in the bladder, as detected by fluorescence microscopy and by homogenization and culture of bladder tissue, that are maintained for months following resolution of bacteriuria14,15,16. Treatment of the bladder with agents that induce exfoliation of the bladder epithelium (urothelium), e.g. protamine sulfate17 or chitosan18, trigger emergence of UPEC from reservoirs to cause rUTI. These data suggest that in women harboring bladder UPEC reservoirs from a prior infection, bladder exposures that lead to urothelial exfoliation may trigger rUTI.
There is mounting evidence that the vaginal microbiota contributes to urinary tract infection19,20. Gardnerella vaginalis is a frequent member of both the vaginal and urinary microbiota21,22,23,24,25,26,27,28,29. In the vagina, the presence of high levels of G. vaginalis is associated with a microbial dysbiosis known as bacterial vaginosis (BV), which affects ~30% of women30,31,32. Women with BV are at a higher risk of experiencing UTI compared to women with a vaginal community dominated by Lactobacillus33,34,35,36,37. In mouse models, G. vaginalis causes epithelial exfoliation both in the vagina38 and in the bladder39. In C57BL/6 mice harboring UPEC bladder reservoirs, two sequential bladder exposures to G. vaginalis – but not to PBS – result in reemergence of UPEC from reservoirs to cause UPEC rUTI. The emergence is evidenced by the appearance of UPEC titers in urine from mice that had previously resolved UPEC bacteriuria and a subsequent decrease in UPEC bladder homogenate titers at sacrifice compared to PBS-exposed control animals39. Interestingly, there is not a lasting colonization by G. vaginalis in the bladder. In the vast majority of cases, two short exposures, each with less than 12 (h) of viable G. vaginalis in urine, are sufficient to elicit urothelial exfoliation and promote rUTI.
This protocol describes a mouse model of rUTI caused by UPEC residing in intracellular bladder reservoirs, using G. vaginalis bladder inoculation to trigger the recurrence. The advance achieved by this model is that G. vaginalis is a clinically relevant biological trigger of rUTI compared to previously used chemical agents. Further, the relatively short-lived survival of G. vaginalis in the mouse urinary tract allows examination of the impact of transient microbial exposures on the urothelium, as might occur after sexual activity. In addition to outlining the rUTI model, this protocol also describes methods for urine cytology and in situ bladder fixation and imaging of the urothelium by scanning electron microscopy (SEM).
This protocol of G. vaginalis-induced recurrent UPEC UTI uses UPEC strain UTI89 bearing a kanamycin resistance cassette (UTI89kanR)40. Not all strains of UPEC tested were able to form intracellular bacterial communities during the acute infection stage in mice41 and it is not yet known if all strains of UPEC have the ability to form latent intracellular reservoirs. Reservoir formation should be confirmed prior to use of other UPEC strains in the model. This protocol uses a spontaneous streptomycin-resistant G. vaginalis isolate, JCP8151BSmR38. Induction of rUTI by JCP8151BSmR requires two sequential G. vaginalis inoculations, given either 12 h or 7 days (d) apart39. Whether or not other G. vaginalis strains induce exfoliation and/or UPEC rUTI remains to be determined with this model. It is essential to use UPEC and G. vaginalis strains with known antibiotic resistance (such as kanamycin or spectinomycin for UPEC and streptomycin for G. vaginalis) because antibiotics can be added to agar plates to prevent growth of endogenous mouse microbiota that could otherwise interfere with enumerating colony-forming units (CFU) to monitor infection. This is especially important for culturing urine specimens, because mouse urine frequently contains other bacteria that can overgrow on culture plates without antibiotics. The origin of these endogenous bacteria in mouse urine is unknown but likely reflects periurethral and urogenital bacteria picked up during urine collection.
G. vaginalis is a facultative anaerobic bacterium and, therefore, this protocol describes growing G. vaginalis JCP8151BSmR in an anaerobic chamber. If an anaerobic chamber is not available, other methods for maintaining anaerobic growth conditions (such as a GasPak pouch in an airtight container) can be utilized. Alternatively, some strains of G. vaginalis (including JCP8151BSmR) will grow in a standard tissue-culture incubator (5% CO2). Just as using G. vaginalis strains other than JCP8151BSmR requires testing to ensure that the bacteria behave similarly in this model, changing growth conditions requires empirical determination of ideal durations for culture (on plates and in liquid) and optical density (OD)600 equivalents to achieve desired viable inoculum concentrations. Moreover, it is not known whether growth conditions influence the pathobiology of G. vaginalis.
Finally, when considering whether to utilize this model, researchers should be aware that it can require larger numbers of animals per group than do typical UTI mouse models. This is in part because induction of rUTI requires that the mice resolve the UPEC bacteriuria caused by the initial infection of the bladder. Thus, any mouse that fails to clear bacteriuria (a phenotype usually indicative of ongoing kidney infection) is not included in the rUTI phase of the protocol. The number of mice needed to power these studies is also influenced by the rate of "spontaneous" UPEC emergence into urine (12-14% on average). Finally, different mouse strains have different propensities for developing chronic bacteriuria versus intracellular reservoir formation42,43. If using mouse strains other than C57BL/6 in this model, it must be confirmed that the animals develop quiescent UPEC intracellular reservoirs.
Protocol
The Washington University Institutional Animal Care and Use Committee (IACUC) approved all mouse infections and procedures as part of protocol number 20170081, which expired 06/09/2020, and 20-0031, which expires 03/18/2023. Overall care of the animals was consistent with The Guide for the Care and Use of Laboratory Animals from the National Research Council and the USDA Animal Care Resource Guide. Euthanasia procedures are consistent with the AVMA Guidelines for the Euthanasia of Animals: 2020 Edition.
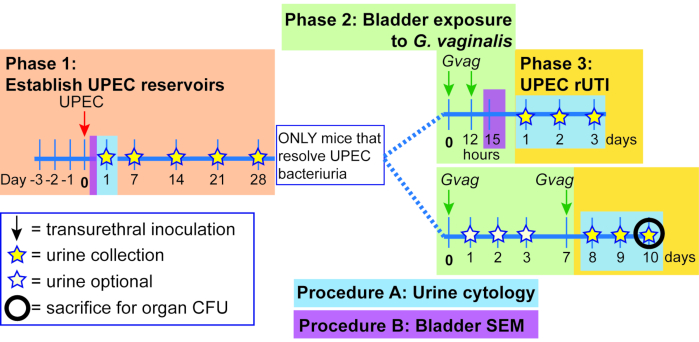
Figure 1. Schematic of Mouse Model. The timeline is highlighted to reflect the phases or procedures of the model outlined in the protocol. Phase 1 (orange): Establishing intracellular UPEC reservoirs. Mice are transurethrally inoculated with UPEC and urine samples are collected and monitored for clearance of bacteriuria. Only mice clearing bacteriuria proceed to the subsequent phases. Phase 2 (green): Bladder exposure to G. vaginalis. Mice are inoculated transurethrally with G. vaginalis two times. The duration of time between the two sequential exposures is either 12 h (top panel) or 1 week (wk; bottom panel), depending on the desired downstream analysis. Phase 3 (yellow): UPEC rUTI. Urine is collected daily following G. vaginalis exposure and monitored for UPEC bacteriuria. Additionally, bladders and kidneys can be collected at the experimental endpoint to measure UPEC tissue titers. In the 1 wk exposure model, G. vaginalis-induced emergence of UPEC from intracellular reservoirs and subsequent clearance from the urinary tract are also reflected in a decrease in UPEC bladder tissue titers (compared to PBS-exposed mice, see Figure 3D). This decrease in bladder titers was not evident in the 12 h exposure model, presumably because more time is required for sufficient reservoir emergence and clearance to occur to significantly reduce tissue titers. Procedure A: Urine cytology is typically performed 1 dpi (or even earlier) during Phase 1 to examine acute UPEC infection and during Phase 3 to assess the urine PMN content, which correlates with UPEC emergence. Urine samples collected at other timepoints can be similarly analyzed. Procedure B: Bladder scanning electron microscopy (SEM) to examine urothelial exfoliation is typically performed in the 12 h model at 3 h after the second G. vaginalis exposure (15 h after administering the first exposure at time 0). Other timepoints can also be assessed, such as 6-24 h after UPEC inoculation as shown in Phase 1. Please click here to view a larger version of this figure.
1. Establish UPEC quiescent intracellular reservoirs in mice
- Prepare urinary catheters (refer to 44,45,46,47 for videos of this step).
- Thread 30 Gauge needles with a length of PE10 tubing extending from the needle base to several mm beyond the needle tip. Take care to not puncture the tubing with the needle tip. Alternatively, use pediatric intravenous cannulas46.
- Place prepared catheters in a petri dish and sterilize with UV light for at least 30 min. Replace petri dish lid and secure for storage until needed.
- Prepare UPEC inoculum (Day -3 to 0)
- Day -3: Streak UTI89kanR from -80 °C freezer stock onto a Luria-Bertani (LB) agar plate. Incubate plate at 37 °C for 18-24 h.
NOTE: It is not necessary to add kanamycin to the inoculum growth media because the kanamycin resistance is stably integrated in UTI89kanR. - Day -2: Inoculate 20 mL of LB broth in a sterile 125 mL flask with a single colony of UTI89kanR. Do not use a smaller flask because this culture method is important to induce expression of the UPEC type 1 pilus that is necessary for bladder adhesion.
- Incubate statically (without shaking) at 37 °C for 18-24 h. Do not add antibiotics to the growth medium. Only use fresh colonies on LB plates (18-24 h old) to start liquid cultures.
- Day -1: Subculture UTI89kanR by removing 20 µL of culture (gently swirl the flask to resuspend settled bacteria) and adding to 20 mL of fresh LB broth in a sterile 125 mL flask. Incubate as in step 2, except for a firm 18 h duration. Do not add antibiotics to the growth medium.
- Day 0: Transfer entire culture into a 50 mL tube and spin at 3200 × g in a tabletop centrifuge for 10 min to pellet bacteria. Aspirate supernatant and resuspend the bacterial pellet in 10 mL of PBS.
- Add 100 µL of the concentrated bacterial suspension from step 4 to 900 μL of PBS in a cuvette and determine the optical density at 600 nm (OD600) using a spectrophotometer that has been blanked with PBS. Multiply the spectrophotometer value by 10 (to account for the dilution) to determine the OD600 of the suspension (ODsuspension).
- To achieve the desired inoculum concentration of 1 x 107 CFU in 50 µL, dilute (or concentrate) the UTI89kanR suspension using the following equation, in which the desired ODinoculum is 0.35 (value may vary for other UPEC strains) and Y is the volume of inoculum required (100 µL per mouse to allow extra for eliminating bubbles and filling the catheters):
X mL x ODsuspension = Y mL x ODinoculum
For example, if the ODsuspension value is 4.7 and 5 mL of inoculum are required:
X mL × 4.7 = 5 × 0.35
X = (5 × 0.35) / 4.7
X = 0.372 mL
Therefore, add 372 μL of bacterial suspension to make 5 mL (final volume) - Use a multi-channel pipette to make 1:10 serial dilutions of the inoculum out to 10-6 in sterile PBS in a 96-well plate. Spot five 10 µL replicates of all 6 dilutions onto an LB and LB+kan plate, allow the spots to dry, and incubate at 37 °C overnight. The LB plate without antibiotics is used to ensure the inoculum was not contaminated by another organism (which would appear as an additional colony morphology not present on the kan antibiotic selection plate). Both plate types should yield the same result.
NOTE: Plates should be allowed to dry on the benchtop for a day prior to use so that they will absorb the plated liquid without spots coalescing. - Count the total number of colonies in all spots of the dilution with distinguishable colonies and use the value to calculate the actual inoculum dose used in each experiment. Do not simply rely on the OD600 values.
- Day -3: Streak UTI89kanR from -80 °C freezer stock onto a Luria-Bertani (LB) agar plate. Incubate plate at 37 °C for 18-24 h.
- Inoculate UTI89kanR into the bladders of anaesthetized female mice (Day 0)
NOTE: Video recordings of this procedure have been published previously44,46. Refer to these papers for a more thorough description. See section 5 of this protocol for more detail on mouse catheterization.- Anesthetize mice with isoflurane inhalation according to IACUC-approved methods.
- While awaiting mice to become anesthetized, fill tuberculin syringe with UTI89kanR inoculum and then affix a prepared catheter. Depress the plunger to void air from the catheter, then dab the catheter into sterile surgical lubricant.
- Position the mouse on its back and confirm anesthetization by firmly squeezing the mouse footpad and observing the absence of a reflex or response. Locate the bladder (feels like a pea in the lower abdomen) between the forefingers of each hand. Express urine by moving fingers toward each other to apply a gentle squeezing pressure to the bladder.
- Insert the catheter through the mouse urethra into the bladder and slowly deliver 50 µL of inoculum.
- Wait a few seconds and then gently remove the catheter by pulling straight out. Return the mouse to its cage and monitor until it recovers from anesthesia.
- Repeat steps 1.3.1 – 1.3.5 with additional mice, changing the catheter between each cage (5 mice). If desired, the same procedure can be used to inoculate a control group of mice with PBS, for example to show another strain of G. vaginalis elicits rUTI (over the spontaneous/background level).
2. Monitoring clearance of UPEC bacteriuria (Days 1 to 28)
NOTE: Video of the urine collection procedure has been published previously44.
- Collect urine (minimum 10 µL) from all mice by bladder palpation as described44 at 1 d post infection and weekly for 4 wk (7, 14, 21 and 28 d post infection). Urine should be cultured within a few hours of collection in order to monitor UPEC infection. Store urine at 4 °C until plated. Urine can also be used for cytology (see Section 4). Occasionally if the bladder is very inflamed, 10 µL of urine cannot be obtained; in this case PBS can be added up to 10 µL, but the urine bacterial titer and cytology scores must be adjusted accordingly (e.g., if only 5 µL urine is collected and 5 µL PBS is added, multiply titers and scores by 2).
- With a multi-channel pipette, make 1:10 serial dilutions out to 10-6 in sterile PBS in a 96-well plate. Use a P10 multi-channel pipette to spot 10 μL of all 6 dilutions from column 1 in a vertical orientation on the left edge of an LB plate containing the relevant antibiotic selection marker. Discard tips.
- Repeat the plating with the remaining samples (column 2, then column 3, etc.). A single plate can accommodate 5 samples side-by-side. This produces a plate with a 5 × 6 spot matrix, with increasing dilutions from top to bottom and increasing sample numbers from left to right (Figure 2A).
- Allow the spots to dry on the benchtop, then incubate at 37 °C overnight. The next day, count the number of colonies in the least diluted spot in which the colonies are distinct (Figure 2B) and use this number to calculate CFU/mL:
# of colonies in single urine spot × dilution factor × 100 = CFU/mL urine - Plot UTI89kanR urine titers using graphing software (Figure 2C). Identify mice that have no detectable UTI89kanR in urine at 28 d (~65-80% of C57BL/6 mice). These mice harbor quiescent intracellular reservoirs and are used in the subsequent experimental phase to examine induction of recurrent UTI. Those with bacteria in urine at 28 d are not included in the subsequent steps.
3. Bladder exposures to G. vaginalis
- Assign mice to exposure groups (Day 29). The primary goal of this step is to avoid having all of the mice with more prolonged bacteriuria together in the same exposure group, since it is unknown whether this affects the likelihood of rUTI.
- Using the urine CFU data (Figure 2D), categorize the mice based on the time point at which UTI89kanR bacteriuria was no longer detectable (Figure 2E).
- Randomize the mice from each category into either the G. vaginalis or PBS inoculation groups; e.g., half the mice who cleared before day 7 get G. vaginalis and half will get PBS; half the mice who cleared between days 8 and 14 will get G. vaginalis and half will get PBS, etc. (as in Figure 2E).
- Prepare G. vaginalis inoculum (all steps performed in an anaerobic chamber)
NOTE: Ideal culture incubation times vary among different strains of G. vaginalis, with some strains entering the stationary phase and even beginning to die more quickly than others. This is particularly important given that killed G. vaginalis (JCP8151B) was unable to trigger rUTI39. Thus, incubation times should be determined empirically for a given strain prior to performing experiments in mice. It is unknown whether other/all strains of G. vaginalis will trigger the same effects in this model.- Streak G. vaginalis strain from -80 °C freezer stock onto an NYCIII plate (without antibiotics). Incubate plate at 37 °C anaerobically for 24 h.
- In the anaerobic chamber, inoculate 5 mL of anaerobic NYCIII media with a 1 μL loopful of cells (a single colony is insufficient) from the NYCIII plate and incubate culture statically at 37 °C under anaerobic conditions for 18 h. Do not include antibiotics in the growth medium.
- Determine the OD600 of the culture using a spectrophotometer.
- Centrifuge a defined volume (X) of culture at 9600 × g for 1 min and aspirate the media. Calculate the volume (Y) of PBS to re-suspend the pellet to achieve the desired inoculum OD to achieve 108 CFU in 50 μL using the following equation:
X mL × ODculture = Y mL × ODinoculum solve for Y
Y = (X ml × ODculture) / ODinoculum
NOTE: The ODinoculum for JCP8151BSmR is 5 but this must be determined empirically for other G. vaginalis strains. For example, if spinning 3 mL of an JCP8151BSmR overnight liquid culture with ODculture = 2.0: Y= (3 mL × 2.0) / 5.0; therefore resuspend pellet in 1.2 mL PBS - Resuspend the bacterial pellet in PBS to the desired concentration. Serially dilute and plate the inoculum (as described in CFU plating protocol above) to determine the actual inoculum dose that has been used in each experiment. Do not simply rely on the OD values.
- Centrifuge a defined volume (X) of culture at 9600 × g for 1 min and aspirate the media. Calculate the volume (Y) of PBS to re-suspend the pellet to achieve the desired inoculum OD to achieve 108 CFU in 50 μL using the following equation:
- On Day 29-31 following UPEC inoculation, inoculate anesthetized mice with G. vaginalis or PBS as described in step 1.3 above. A PBS control group is essential, as the act of catheterizing the bladder could possibly induce damage and urothelial exfoliation that could elicit some degree of UPEC reservoir reemergence. PBS-inoculated mice therefore serve as the control to which G. vaginalis-inoculated mice are compared.
NOTE: The final UPEC bacteriuria determination at 28 d requires overnight incubation of the CFU plate. Therefore, the earliest this step can be performed is 29 days following the initial UPEC inoculation. If necessary, the exposure could be given as late as day 31. Researchers should be consistent between experiments. - Repeat the inoculum preparation to administer a second G. vaginalis (or PBS control) inoculation at the desired time point, such as 12 h or 1 wk after the first inoculation. A second exposure is necessary because a single inoculation with G. vaginalis does not result in significant UPEC emergence39.
4. Monitoring UPEC recurrent UTI
- Collect urine from mice at desired time points following each G. vaginalis inoculation (1, 2, and 3 d post-inoculation recommended).
- Serially dilute and plate urine on selective plates (e.g., LB+kanamycin) to determine UTI89kanR CFU/mL. If desired, urine dilutions can also be plated on selective plates (e.g., NYCIII + 1 mg/mL streptomycin) to determine G. vaginalis CFU/mL. However, G. vaginalis JCP8151BSmR was cleared from the urine of most mice by 12 h 39. Therefore, earlier timepoints would be necessary to detect G. vaginalis in most mice.
- At the experimental endpoint (e.g., 3 d after the second G. vaginalis inoculation), sacrifice the mice according to approved methods (e.g., cervical dislocation under isoflurane anesthesia or CO2 inhalation) and collect bladders and kidneys for CFU enumeration, as described previously 44,46.
5. Urine cytology
NOTE: This procedure can be performed at any timepoint at which visualization of the cells and/or bacteria present in urine is desired. As indicated in Figure 1, urine cytology is typically performed at 1 dpi (or even earlier) during Phase 1 to examine acute UPEC infection and during Phase 3 to assess the presence of polymorphonuclear (PMN) cells in urines that display UPEC emergence.
- Add 10 µL of urine to 90 µL of PBS in a cytofunnel cassette with attached filter and slide. (The simplest method is to use the remainder of the 1:10 dilutions from the 96-well plate used for urine culturing; these samples can be used up to 24 h after urine culturing if stored at 4 °C). Place cassettes in cyto-centrifuge and spin at 600-800 x g for 6 min with high acceleration.
- Remove slides and allow to dry overnight. The next day, stain with a hematology staining kit (e.g., Wright's, Giemsa, including fixative) according to the manufacturer's protocol.
- Analyze the slides by light microscopy for the presence of PMNs and epithelial cells. If desired, these can be scored using a qualitative scoring metric based on the abundance of each cell type present in each high-powered field of view (e.g., 0=none, 1=few, 2= moderate, 3=robust). Ensure that the individual analyzing the slides is blinded to the experimental groups to minimize potential bias.
6. Imaging bladders by scanning electron microscopy
NOTE: This procedure can be performed at any timepoint at which visualization of the urothelium is desired. As indicated in Figure 1 (purple boxes), UPEC-urothelial interactions are best visualized between 6 h and 24 h post UPEC inoculation during the reservoir formation phase, and urothelial exfoliation triggered by G. vaginalis is best visualized between 3 h and 12 h after the second G. vaginalis exposure.
- In situ bladder fixation
- Prepare fixative immediately before bladder harvest by adding glutaraldehyde (2.5% final) and paraformaldehyde (2% final) in 0.15 M sodium cacodylate buffer with 2 mM of CaCl2 at pH 7.4. Use paraformaldehyde and glutaraldehyde from newly opened glass ampules, as both fixatives oxidize over time in opened containers.
CAUTION: Glutaraldehyde is toxic, a respiratory irritant, and corrosive; paraformaldehyde is flammable, carcinogenic, an irritant and a reproductive toxin; sodium cacodylate is toxic and carcinogenic. - To make 50 mL of fixative solution, add 6.25 mL of 16% paraformaldehyde, 2 mL of 50% glutaraldehyde, and 16.75 mL of ultrapure water to 25 mL of a 0.3 M solution of sodium cacodylate at pH 7.4 with 4 mM CaCl2.
- Warm the prepared fixative to 37 °C prior to administering to bladders.
- Fill tuberculin slip-tip syringe with fixative and affix a catheter to the end, bevel facing opposite syringe markings. Snip off the excess tubing 1-2 mm from the end of the needle, taking care not to expose the needle tip. Flick the syringe to remove bubbles and push the plunger to void air and fill the catheter with fixative over a microcentrifuge tube to collect any fixative for proper disposal.
- Anesthetize and sacrifice the mouse using an approved method (e.g., cervical dislocation under anesthesia). Place the mouse on dissecting surface with the legs secured (with rubber bands or pins). Open the mouse pelvic area with forceps and a pair of surgical scissors to expose the bladder. Carefully push aside the adjacent fat but leave the bladder in place.
- Hold the syringe with the dominant hand with the needle pointing down and the needle bevel and syringe markings facing away from you. Dip the catheter tip into sterile lubricant.
- Position the catheter tip at the urethral opening, holding the syringe barrel away positioned at a 30-45° angle over the mouse body.
- Apply downward pressure using a very small clockwise motion with the tip and gently insert the catheter into the urethra. As the catheter tip enters the urethra, hinge the syringe toward the tail of the mouse while continuing to slide the catheter further into the urethra until the syringe barrel is parallel to the working surface. The entire catheter needle shaft (not including the base) should enter the mouse, positioning the catheter tip within the bladder lumen.
- Slowly deliver 50-80 µL of fixative, causing the bladder to inflate like a balloon. Keep the catheter in place and raise the syringe slightly, tilting the tip up.
- With the other hand, open a hemostat and slide one prong under the catheter needle at the intersection of the urethra. Partially close the hemostat until it just makes contact with the needle.
- Gently slide the catheter needle out of the bladder while simultaneously clamping down and locking the hemostat completely to prevent loss of the fixative.
- Grip the hemostat so that it is parallel to the working surface with the bladder resting on top. Lift up gently and carefully cut under the hemostat (opposite side of the bladder) to remove the bladder with the hemostat still attached.
- Place bladder and attached hemostat into a Falcon tube containing warmed fixative. Ensure that the bladder is fully submerged in the fluid and not pressed against the walls of the tube. Incubate at 4 °C for 24 h.
- Prepare fixative immediately before bladder harvest by adding glutaraldehyde (2.5% final) and paraformaldehyde (2% final) in 0.15 M sodium cacodylate buffer with 2 mM of CaCl2 at pH 7.4. Use paraformaldehyde and glutaraldehyde from newly opened glass ampules, as both fixatives oxidize over time in opened containers.
- Bladder processing and imaging with scanning electron microscopy (SEM)
- Sagittally bisect the bladder with a cleaned, double-sided razor blade, and make a second cut tangential to the hemostat to release the bladder. This results in 2 half-bladder "cups." If any remaining fat pads exist on the exterior of the bladder, gently remove them.
- Rinse the bladder halves three times (10 min each) in sodium cacodylate buffer (0.15 M, pH 7.4).
- Stain the tissue with 1% osmium tetroxide in 0.15 M cacodylate buffer for 1 h at room temperature. Osmium is sensitive to light; therefore, perform this step with the staining vessel wrapped in foil to maintain a dark environment.
CAUTION: Osmium tetroxide is toxic and corrosive to skin. Do this step in the fume hood with gloves. - Rinse the bladder halves three times (10 min each) in ultrapure water. During these steps, osmicated oil can sometime be seen on the surface of the water. Aspirate or wick this off to prevent contamination during the drying steps.
- Dehydrate tissues by submerging in a graded ethanol series (50, 70, 90, 100, and 100%) for 10 min each.
- Dry the fixed tissue using a critical-point dryer performing 12 CO2 exchanges at the slowest speed. Set all additional settings to slow, except for the venting step which is set to fast.
- Bisect each bladder half again with a clean double-sided razor to generate 4 total pieces to reduce curvature of the specimen for more efficient coating, for ease of imaging in the SEM, and to expose tissue that may have curled during drying.
- Adhere the bladder pieces to a conductive carbon adhesive tab on an aluminum stub and paint a small amount of silver adhesive around the bottom contact with a toothpick, taking care to prevent excess adhesive from wicking onto the inner surface of the bladder.
- Use a high vacuum sputter coater to sputter coat the sample stubs with 6 nm of iridium. If the samples continue to charge, ensure a conductive path is painted to the surface with silver paint and coat with an additional 4 nm of iridium.
- Image the samples with a scanning electron microscope. While conditions may vary depending on the microscope used, an accelerating voltage of 3 KeV with a beam current of 200 pA and a working distance of 12-13 mm worked well on a Zeiss Merlin FE-SEM when using the Everhart-Thornley (SE2) electron detector.
Representative Results
Following inoculation, UPEC titers are detectable in urine (Figure 2B). Failure to plate urine samples on selective media containing kanamycin will likely result in overgrowth of endogenous mouse microbiota contaminating the urine. The level of UPEC bacteriuria will likely be high on day 1 and may increase during the first week before decreasing at later timepoints (Figure 2C). Approximately 65-80% of mice will have no detectable UPEC in the urine by 28 dpi (Figure 2C, green circle). These mice can be used in the subsequent steps of the model. Mice that remain bacteriuric (Figure 2C, red ellipse) should be eliminated from the experiment.
Two sequential G. vaginalis exposures given 12 h (Figure 3A) or 1 wk apart (Figure 3B) result in the emergence of UPEC from intracellular reservoirs to cause recurrent bacteriuria. Both the level of UPEC bacteriuria (Mann-Whitney test) and the fraction of mice displaying UPEC rUTI (Fisher's exact test) are significantly higher in mice exposed to G. vaginalis compared to the PBS control group. Urine cytology analysis detects PMNs in urine from G. vaginalis-exposed mice that displayed UPEC emergence (Figure 3C). In the model with two exposures given 1 wk apart, UPEC titers in bladder tissue are lower in G. vaginalis-exposed mice compared to PBS (Figure 3D), presumably due to emergence of UPEC from reservoirs and subsequent clearance.
Visualization of in situ-fixed bladder tissue by SEM reveals large superficial umbrella urothelial cells lining the bladder surface in control mice exposed only to PBS (Figure 4A). Urothelial exfoliation is evidenced by a loss of superficial umbrella cells, revealing smaller underlying transitional epithelial cells in mice exposed to G. vaginalis (Figure 4B). Early after UPEC inoculation during the establishment of intracellular reservoirs, UPEC are visible on the urothelium and filamenting out of exfoliating cells (Figure 4C).
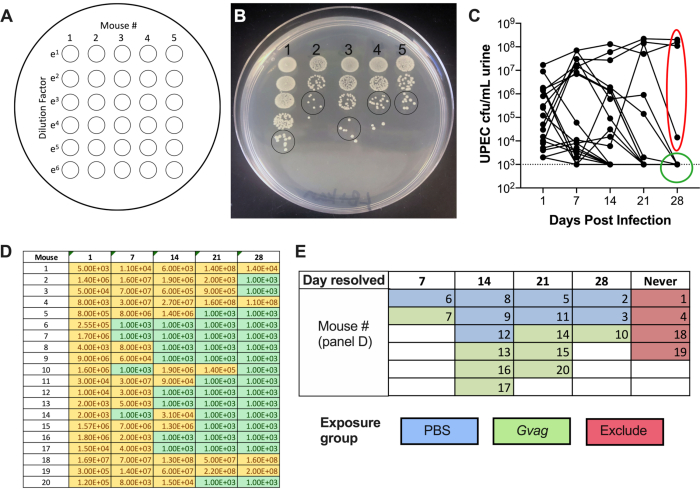
Figure 2. Monitoring UPEC titers in urine during Phase 1 (reservoir formation). (A) Schematic of colony-forming units (CFU) plating. (B) Representative image of UPEC titers in urine on LB+kanamycin. Black circles indicate urine sample spots that should be counted to calculate CFU/mL. (C) Time course of UPEC bacteriuria in C57BL/6 mice. Each line represents an individual mouse, tracing the UPEC urine titers over time. Dotted line indicates the limit of detection (1000 CFU/mL). Red ellipse indicates four mice (out of 20) that failed to resolve UPEC bacteriuria and would therefore not be used for the G. vaginalis-induced rUTI model. Conversely, green circle indicates mice that resolved UPEC bacteriuria and proceeded to subsequent phases. (D) Table of data used to generate graph in panel C. Yellow, detectable CFU; green, no CFU. (E) Randomization of mice into exposure groups based on the time point at which UPEC CFU were no longer detected in urine ("Day resolved"). The mouse numbers in the left column of panel D are the same mouse numbers given in panel E. Please click here to view a larger version of this figure.
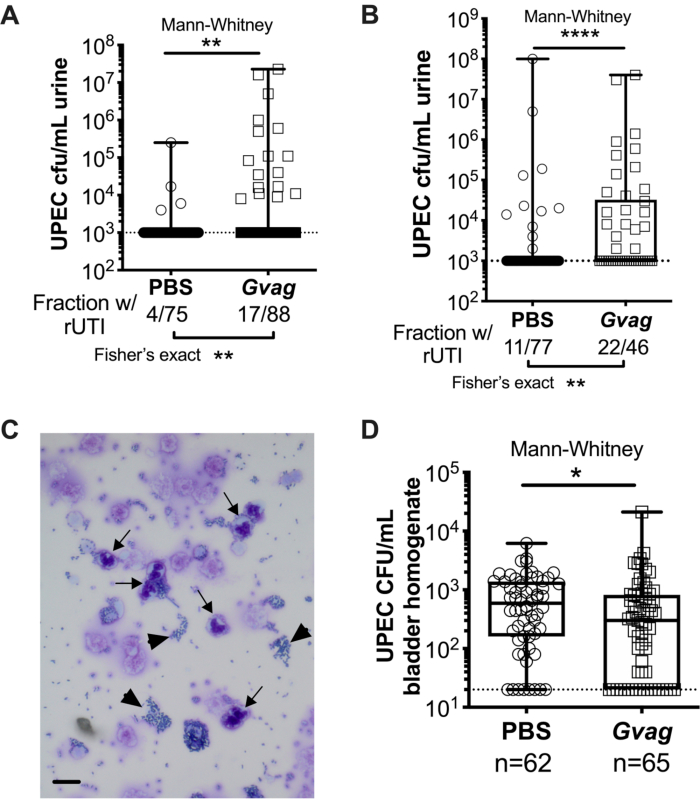
Figure 3. G. vaginalis triggers UPEC rUTI. UPEC titers in urine following two sequential urinary tract exposures to PBS (circles) or G. vaginalis (Gvag; squares) given 12 h (A) or 1 wk (B) apart. Each symbol represents an individual mouse. The highest CFU/mL UPEC detected from each mouse between 1-3 d following the second exposure are plotted. Mice with no detectable bacteriuria are plotted at the limit of detection (dotted line). (C) Urine cytology analysis showing UPEC (arrowheads) and polymorphonuclear (PMN) cells (arrows). Scale bar = 20 μm. (D) UPEC titers in bladder tissues collected 3 d following two sequential urinary tract exposures given 1 wk apart. Each symbol represents a different mouse and zeros are plotted at the limit of detection (dotted line). In A, B, and D, boxes are at the first and third quartile with the median marked and whiskers from min to max. Mann-Whitney U tests * P < 0.05; ** P < 0.01; **** P < 0.0001. Please click here to view a larger version of this figure.
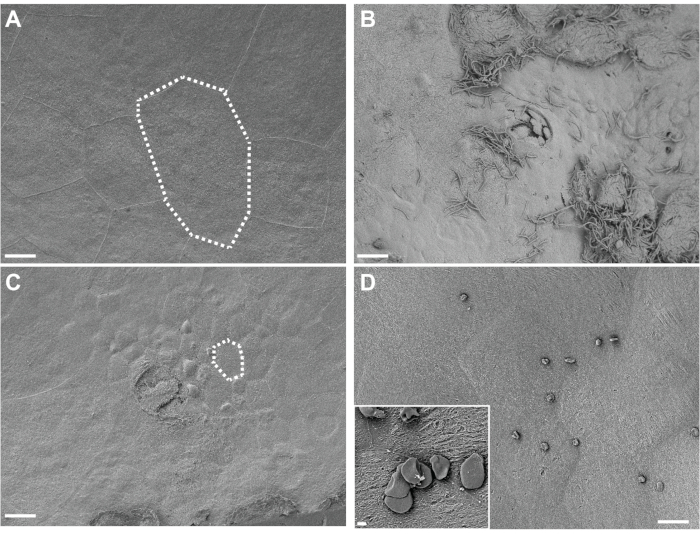
Figure 4. SEM analysis of bladders fixed in situ. Bladders were collected from mice 3 h after two exposures (12 h apart) to PBS (A) or G. vaginalis (C). Dotted lines illustrate a single urinary epithelial cell, which is smaller in G. vaginalis-exposed bladders because the large superficial cells have exfoliated away revealing the underlying transitional epithelium. (B) Bladder collected 6 h after initial inoculation with UPEC, during Phase 1 of the model, showing urothelial exfoliation and extracellular UPEC. (D) Example of insoluble fat droplets present on the bladder surface. Scale bars are 20 μm in the main images and 2 μm in the inset. Please click here to view a larger version of this figure.
Discussion
The first critical step in this model to identify mice that have not cleared UPEC bacteriuria during the primary UTI phase. These mice must be removed from the experiment as they would otherwise confound the rates of UPEC bacteriuria after G. vaginalis exposure. After the initial UPEC inoculation, urine should be collected weekly to monitor bacterial clearance. Approximately 65-80% of C57BL/6 mice will clear a UTI89kanR infection within 4 weeks. Other inbred mouse strains have different propensities for UPEC clearance42,43 and reservoir formation and thus may not be suitable for this model. The second critical point is that empirical studies have determined that two sequential inoculations of G. vaginalis (either 12 h or 1 wk apart) are necessary to trigger significant reservoir emergence above the background spontaneous emergence that occurs in control mice exposed only to PBS. Other durations of time between the two sequential exposures have not been tested but could yield similar results. It is important to note that a reduction in UPEC bladder titers was only observed in the model in which G. vaginalis exposures were given 1 wk apart39. While more than two exposures can be administered, empirical evidence suggests that repeated catheterization alone increases emergence, which may confound the interpretation of the results or require larger numbers of animals to distinguish differences between exposure groups and controls. Finally, the in situ bladder fixation method has several critical steps. Some skill is required to ensure that the fixative remains inside the clamped bladders. Deflated bladders will be more difficult to image by SEM. It is also essential to be very gentle when inoculating the fixative into the bladder, as scraping the urothelium with the fixative-containing catheter can induce urothelial exfoliation independent of what is triggered by G. vaginalis. All concentrations mentioned in the fixative cocktail are final concentrations. Improper ratios of these can result in insufficient fixing and swelling or shrinkage of the cells. Fixatives should be warmed to physiological temperatures to avoid temperature shock in cells and tissues. Warming also provides a slight improvement to the diffusion rate of fixatives through plasma membranes. While osmium staining can often be omitted for samples prepared for SEM analysis, it is an essential step in this protocol to stabilize lipids and prevent cracking of cellular membranes during critical point drying.
This protocol can be modified to test other UPEC and/or G. vaginalis strains for their ability to form reservoirs and to trigger their emergence, respectively. Other experimental factors can also be added, such as exposure to other vaginal bacteria (e.g., Lactobacillus crispatus PVAS100) or heat-killed G. vaginalis, neither of which demonstrate pathology in this model39. When selecting other bacterial strains to test, it is important to demonstrate consistent growth such that a standard inoculum concentration can be used in all experiments. The growth of JCP8151BSmR has been optimized in an anaerobic chamber. This strain could likely be cultivated in an anaerobic GasPak system, but this would require optimization to ensure robust bacterial growth. Finally, it may be possible to modify the timing of certain steps in the model. For instance, urine can be collected at earlier timepoints during the UPEC reservoir formation phase to monitor CFU or host responses. An adverse effect of collecting urine samples at early timepoints (3, 6, 12 hpi) on the progression of infection or establishment of reservoirs has not been observed in this model. Emergence of UPEC reservoirs has been reported to occur after two JCP8151BSmR doses given 12 h or 1 wk, but other time intervals have not yet been tested. It also may be possible to reduce the overall length of time for the model by reducing the UPEC reservoir formation phase to 2 weeks (rather than 4 weeks), since many of the mice clear bacteriuria by this time. Previous studies examining UPEC emergence following bladder exposure to chemical exfoliants used a 1 or 2 wk UPEC reservoir formation phase17,18. However, decreasing the amount of time for UPEC bacteriuria clearance may come at the cost of requiring more animals to be culled from the experiment. Finally, SEM analysis of the bladder can be performed at additional time points to observe the duration of the effect of G. vaginalis on the urothelium.
Regarding troubleshooting, there are some important considerations specifically with respect to the bladder SEM analysis. Depending on the mouse background used and amount of inflammation present, some bladders will present with very thin walls. These bladders tend to curl more during critical point drying and can result in a cowrie shell-like shape. If this occurs, the best method is to cut the shell-shaped bladder in half along the curled interface and then a second time to remove the bulk of the overhanging tissue. Cutting works best with a PTFE-coated double-edged razor blade. Excess fat can sometimes solubilize during the osmium staining steps. This can result in unwanted insoluble fat droplets that may not wash off during the rinsing and dehydration steps and that can settle on the bladder surface during subsequent drying. These droplets can appear as either small spheres or disc-like structures scattered over the sample (Figure 4D). This can be mitigated by ensuring that as much adipose tissue is removed from around the bladder as possible. Platinum can be substituted for iridium coating, but thicknesses should be kept to a minimum to reduce the masking of fine structural details. The use of a rotating stage during coating is highly recommended.
One limitation of this model is that it requires a large number of mice. Only 65-80% of C57BL/6 mice will clear their UPEC bacteriuria and be suitable for subsequent G. vaginalis or PBS inoculation (see Figure 2C). To obtain 10-12 mice per group (G. vaginalis inoculation vs. PBS), ~30 mice should be initially infected with UPEC. Further, multiple experiments are likely required to achieve the biological replicates necessary to detect statistical significance. When exposures were given 1 wk apart, UPEC emergence occurred in 14% of mice exposed to PBS (Figure 3B). Thus, detecting a significant increase in UPEC rUTI in G. vaginalis exposed mice relative to PBS controls (powered at 0.8; alpha=0.05 [one sided]) requires testing a cumulative total of at least 40 mice for each exposure group. An additional consideration is that these experiments are expensive and labor-intensive. Mice must be monitored weekly for UPEC clearance and the experimental time course is 4-5 wk depending on whether G. vaginalis is given twice in a 12 h timeframe or twice 1 wk apart. SEM is labor-intensive and may be costly, depending on microscope availability and service charges. Preparing the entire bladder for SEM provides abundant material for analysis but the drawback is that analyzing each bladder can be time-consuming. Thus, it is likely that only a limited number of bladders can be analyzed by SEM compared to the higher animal numbers used for urine and tissue titers. In addition, obtaining high-quality images of the curved surfaces of the bladder "cups" requires skill due to shadows that can impede visibility. Although bladder SEM is a useful tool for visualizing urothelial exfoliation, this method is largely qualitative. Because the sample is fixed in a round shape, and due to the use of glutaraldehyde in the fixative, screening for fluorescently expressing bacteria via light microscopy is not possible. Immunostaining and chemical dyes are incompatible with this process due to the use of glutaraldehyde that will crosslink most antigens and osmium and that will mask antigen sites and darken the tissue. That said, the SEM technique is useful for parameters that can be evaluated quantitatively without the use of additional probes, such as cell size48,49.
This model offers several advantages beyond previously described methods. It allows the examination of mechanisms of UPEC rUTI caused by emergence from bladder reservoirs, as opposed to reintroduction into the bladder from an outside source. Other models of rUTI due to emergence from bladder reservoirs use chemical agents (protamine sulfate or chitosan) to cause urothelial exfoliation17,18, which would not be triggers of rUTI in women. G. vaginalis is a prevalent urogenital bacterium that has been detected in urine collected directly from the bladder via catheterization or suprapubic aspiration in some women23,26. This fact, coupled with the known association between BV (in which G. vaginalis overgrows in the vagina) and UTI, suggests that G. vaginalis is a clinically plausible trigger of rUTI. Finally, the in situ bladder fixation method preserves bladder ultrastructure and limits damage, ensuring that the bladder layers do not separate from one another. Previous methods for visualizing the urothelium traditionally have the user aseptically harvest, bisect, stretch, and pin the bladder onto a dissection tray before submerging the stretched bladder in fixative48. This method results in a very flat sample but does not ensure even or natural stretching of the tissue and can result in areas that are over and under stretched (resulting in highly wrinkled tissue) and can cause bladder layer separation. Additionally, these physical manipulations of the bladder to stretch and pin the tissue can cause damage, including urothelial exfoliation. Another method is to submerge intact bladders in fixative before embedding in paraffin and acquiring thin sections with a microtome. Thin sections are invaluable for immunohistochemistry experiments to examine bacteria and host protein localization but a thin section does not allow visualization of the urothelial surface. This SEM method allows the surface of the entire bladder to be examined at once.
As described, future applications of this model include testing other UPEC strains to determine whether they form intracellular reservoirs and testing other G. vaginalis strains to assess whether they elicit exfoliation and UPEC emergence to cause rUTI. Other mouse strains beyond C57BL/6 mice may also be tested, although mice with a high propensity for developing chronic cystitis (such as mice on the C3H background) are not recommended, since too many mice would need to be culled from the experiment. An additional advantage of C57BL/6 mice is that many genetic knockout strains are commercially available. Such strains provide an opportunity for interrogating the host factors involved in reservoir formation and/or emergence.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors thank Lynne Foster for technical assistance in infection experiments, James Fitzpatrick at the Washington University Center for Celluar Imaging (WUCCI) for access to the SEM, Scott Hultgren for the UTI89kanR UPEC strain, and David Hunstad for critical reading of the manuscript.
This work was supported by the National Science Foundation (Graduate Research Fellowship to VPO#DGE – 1143954), by the Center for Women's Infectious Disease Research at Washington University School of Medicine (Pilot Research Award to NMG), by the American Heart Association: #12POST12050583 (NMG) and #14POST20020011 (NMG), and by the National Institutes of Health, NIAID: R01 AI114635 (ALL) and NIDDK: R21 DK092586 (ALL), P50 DK064540-11 (SJH, project II PI:ALL) and K01 DK110225-01A1 (NMG). Some of the animal studies were performed in a facility supported by NCRR grant C06 RR015502. The Washington University Center for Cellular Imaging (WUCCI; where SEM was performed) and MSJ were supported by the Washington University School of Medicine, the Children's Discovery Institute of Washington University and the St. Louis Children's Hospital (CDI-CORE-2015-505), the Foundation for Barnes-Jewish Hospital (3770), and the National Institute for Neurological Disorders and Stroke (NS086741). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Materials
| 30G x 1/2 needles | BD | 305106 | for catheters |
| 5 1/2" straight forcep hemostat | McKesson | 487377 | in situ bladder fixation |
| ACE 600 Sputter coater | Leica | SEM sample processing | |
| aluminum SEM stub | Ted Pella | 16111 | SEM sample processing |
| Calcium chloride | EMS | 12340 | in situ bladder fixation |
| conductive carbon adhesive tab | Ted Pella | 16084-1 | SEM sample processing |
| Conductive silver paint | Ted Pella | 16034 | SEM sample processing |
| CPD 300 Critical Point Drier | Leica | SEM sample processing | |
| Cytofunnel metal clip | Simport | M964B | cytospun urinalysis |
| Ethanol | EMS | 15050 | SEM sample processing |
| Glucose | Sigma | G7528 | for NYCIII G. vaginalis growth media |
| glutaraldehyde | EMS | 16320 | in situ bladder fixation |
| Hema 3 staining kit | Fisher | 23123869 | cytospun urinalysis |
| HEPES | Cellgro | 25-060-Cl | for NYCIII G. vaginalis growth media |
| iridium | Ted Pella | 91120 | SEM sample processing |
| isofluorane | mouse anaesthesia | ||
| kanamycin | Gibco | 11815024 | add to UPEC LB selective plates (50 ug/mL) |
| Luria-Bertani agar | BD | DF0445174 | UPEC growth plates |
| Luria-Bertani broth | BD | DF0446173 | UPEC growth media |
| Merlin FE-SEM | Zeiss | scanning electron microscope | |
| Milli-Q Water Purifier | Millipore | IQ-7000 | SEM sample processing |
| NaCl | Sigma | S3014 | for NYCIII G. vaginalis growth media |
| Olympus Vanox AHBT3 microscope | Olympus | cytospun urinalysis | |
| osmium tetroxide | EMS | 19170 | SEM sample processing |
| paraformaldehyde | EMS | 15710 | in situ bladder fixation |
| polyethylene tubing | Intramedic | 427401 | for catheters |
| Proteose Peptone #3 | Fisher | DF-122-17-4 | for NYCIII G. vaginalis growth media |
| PTFE coated double edge razor blade | EMS | 72000 | cutting bladders for SEM |
| Shandon Cytocentrifuge | Thermo Scientific | A78300002 | cytospun urinalysis |
| Shandon cytofunnel filter | Simport | M965FWDV | cytospun urinalysis |
| Shandon Double cytofunnel | Simport | M964-1D | cytospun urinalysis |
| Shandon double cytoslides (coated) | Thermo Scientific | 5991055 | cytospun urinalysis |
| sodium cacodylate trihydrate | EMS | 12310 | in situ bladder fixation |
| spectrophotometer | BioChrom | 80-3000-45 | measuring bacterial OD600 |
| streptomycin | Gibco | 11860038 | add to G. vaginalis NYCIII selective plates (1 mg/mL) |
| tuberculin slip tip syringe | BD | 309659 | for catheters |
| Yeast Extract | Fisher | DF0127-17-9 | for NYCIII G. vaginalis growth media |
Referências
- Foxman, B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infectious Disease Clinics of North America. 28 (1), 1-13 (2014).
- Foxman, B. Recurring urinary tract infection: incidence and risk factors. American Journal of Public Health. 80 (3), 331-333 (1990).
- Foxman, B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. American Journal of Medicine. 113, 5-13 (2002).
- Foxman, B. The epidemiology of urinary tract infection. Nature Reviews Urology. 7 (12), 653-660 (2010).
- Ikaheimo, R., et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clinical Infectious Diseases. 22 (1), 91-99 (1996).
- Russo, T. A., Stapleton, A., Wenderoth, S., Hooton, T. M., Stamm, W. E. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. Journal of Infectious Diseases. 172 (2), 440-445 (1995).
- Luo, Y., et al. Similarity and divergence of phylogenies, antimicrobial susceptibilities, and virulence factor profiles of Escherichia coli isolates causing recurrent urinary tract infections that persist or result from reinfection. Journal of Clinical Microbiology. 50 (12), 4002-4007 (2012).
- Schreiber, H. L. t., et al. Bacterial virulence phenotypes of Escherichia coli and host susceptibility determine risk for urinary tract infections. Science Translational Medicine. 9 (382), (2017).
- Rosen, D. A., Hooton, T. M., Stamm, W. E., Humphrey, P. A., Hultgren, S. J. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4 (12), 329 (2007).
- Elliott, T. S., Reed, L., Slack, R. C., Bishop, M. C. Bacteriology and ultrastructure of the bladder in patients with urinary tract infections. Journal of Infection. 11 (3), 191-199 (1985).
- Robino, L., et al. Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathogens and Disease. 68 (3), 78-81 (2013).
- Robino, L., et al. Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in children. Clinical Infectious Diseases. 59 (11), 158-164 (2014).
- De Nisco, N. J., et al. Direct Detection of Tissue-Resident Bacteria and Chronic Inflammation in the Bladder Wall of Postmenopausal Women with Recurrent Urinary Tract Infection. Journal of Molecular Biology. 431 (21), 4368-4379 (2019).
- Mulvey, M. A., Schilling, J. D., Hultgren, S. J. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infection and Immunity. 69 (7), 4572-4579 (2001).
- Kerrn, M. B., Struve, C., Blom, J., Frimodt-Moller, N., Krogfelt, K. A. Intracellular persistence of Escherichia coli in urinary bladders from mecillinam-treated mice. Journal of Antimicrobial Chemotherapy. 55 (3), 383-386 (2005).
- Eto, D. S., Sundsbak, J. L., Mulvey, M. A. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cellular Microbiology. 8 (4), 704-717 (2006).
- Mysorekar, I. U., Hultgren, S. J. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proceedings of the National Academy of Sciences of the United States of America. 103 (38), 14170-14175 (2006).
- Blango, M. G., Ott, E. M., Erman, A., Veranic, P., Mulvey, M. A. Forced resurgence and targeting of intracellular uropathogenic Escherichia coli reservoirs. PLoS One. 9 (3), 93327 (2014).
- Gilbert, N. M., Lewis, A. L. Covert pathogenesis: Transient exposures to microbes as triggers of disease. PLoS Pathogens. 15 (3), 1007586 (2019).
- Lewis, A. L., Gilbert, N. M. Roles of the vagina and the vaginal microbiota in urinary tract infection: evidence from clinical correlations and experimental models. GMS Infectious Diseases. 8, (2020).
- Janulaitiene, M., et al. Prevalence and distribution of Gardnerella vaginalis subgroups in women with and without bacterial vaginosis. BMC Infectious Diseases. 17 (1), 394 (2017).
- Fredricks, D. N. Molecular methods to describe the spectrum and dynamics of the vaginal microbiota. Anaerobe. 17 (4), 191-195 (2011).
- Hilt, E. E., et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. Journal of Clinical Microbiology. 52 (3), 871-876 (2014).
- Klein, S., et al. Significant increase in cultivation of Gardnerella vaginalis, Alloscardovia omnicolens, Actinotignum schaalii, and Actinomyces spp. in urine samples with total laboratory automation. European Journal of Clinical Microbiology Infect Dis. 37 (7), 1305-1311 (2018).
- Pearce, M. M., et al. The female urinary microbiome in urgency urinary incontinence. American Journal of Obstetrics and Gynecology. 213 (3), 341 (2015).
- Pearce, M. M., et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 5 (4), 01283 (2014).
- Gottschick, C., et al. The urinary microbiota of men and women and its changes in women during bacterial vaginosis and antibiotic treatment. Microbiome. 5 (1), 99 (2017).
- Malki, K., et al. Genomes of Gardnerella Strains Reveal an Abundance of Prophages within the Bladder Microbiome. PLoS One. 11 (11), 0166757 (2016).
- Kramer, H., et al. Diversity of the midstream urine microbiome in adults with chronic kidney disease. International Urology and Nephrology. 50 (6), 1123-1130 (2018).
- Allsworth, J. E., Peipert, J. F. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstetrics & Gynecology. 109 (1), 114-120 (2007).
- Ravel, J., et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America. 108, 4680-4687 (2011).
- Hillier, S. L. Diagnostic microbiology of bacterial vaginosis. American Journal of Obstetrics and Gynecology. 169 (2), 455-459 (1993).
- Amatya, R., Bhattarai, S., Mandal, P. K., Tuladhar, H., Karki, B. M. Urinary tract infection in vaginitis: a condition often overlooked. Nepal Medical College Journal. 15 (1), 65-67 (2013).
- Harmanli, O. H., Cheng, G. Y., Nyirjesy, P., Chatwani, A., Gaughan, J. P. Urinary tract infections in women with bacterial vaginosis. Obstetrics & Gynecology. 95 (5), 710-712 (2000).
- Sharami, S. H., Afrakhteh, M., Shakiba, M. Urinary tract infections in pregnant women with bacterial vaginosis. Journal of Obstetrics and Gynaecology. 27 (3), 252-254 (2007).
- Hillebrand, L., Harmanli, O. H., Whiteman, V., Khandelwal, M. Urinary tract infections in pregnant women with bacterial vaginosis. American Journal of Obstetrics and Gynecology. 186 (5), 916-917 (2002).
- Sumati, A. H., Saritha, N. K. Association of urinary tract infection in women with bacterial vaginosis. Journal of Global Infectious Diseases. 1 (2), 151-152 (2009).
- Gilbert, N. M., Lewis, W. G., Lewis, A. L. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One. 8 (3), 59539 (2013).
- Gilbert, N. M., O’Brien, V. P., Lewis, A. L. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathogens. 13 (3), 1006238 (2017).
- Wright, K. J., Seed, P. C., Hultgren, S. J. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infection and Immunity. 73 (11), 7657-7668 (2005).
- Garofalo, C. K., et al. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infection and Immunity. 75 (1), 52-60 (2007).
- Hannan, T. J., Mysorekar, I. U., Hung, C. S., Isaacson-Schmid, M. L., Hultgren, S. J. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathogens. 6 (8), 1001042 (2010).
- Hopkins, W. J., Gendron-Fitzpatrick, A., Balish, E., Uehling, D. T. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infection and Immunity. 66 (6), 2798-2802 (1998).
- Conover, M. S., Flores-Mireles, A. L., Hibbing, M. E., Dodson, K., Hultgren, S. J. Establishment and Characterization of UTI and CAUTI in a Mouse Model. Journal of Visualized Experiments. (100), e52892 (2015).
- Hannan, T. J., Hunstad, D. A. A Murine Model for Escherichia coli Urinary Tract Infection. Methods in Molecular Biology. 1333, 159-175 (2016).
- Zychlinsky Scharff, A., Albert, M. L., Ingersoll, M. A. Urinary Tract Infection in a Small Animal Model: Transurethral Catheterization of Male and Female Mice. Journal of Visualized Experiments. (130), e54432 (2017).
- Thai, K. H., Thathireddy, A., Hsieh, M. H. Transurethral induction of mouse urinary tract infection. Journal of Visualized Experiments. (42), e2070 (2010).
- O’Brien, V. P., et al. A mucosal imprint left by prior Escherichia coli bladder infection sensitizes to recurrent disease. Nature Microbiology. 2, 16196 (2016).
- O’Brien, V. P., Dorsey, D. A., Hannan, T. J., Hultgren, S. J. Host restriction of Escherichia coli recurrent urinary tract infection occurs in a bacterial strain-specific manner. PLoS Pathogens. 14 (12), 1007457 (2018).

