Conducting Respiratory Oscillometry in an Outpatient Setting
Summary
We demonstrate a standard operating protocol to conduct respiratory oscillometry, highlighting key quality control and assurance procedures.
Abstract
Respiratory oscillometry is a different modality of pulmonary function testing that is increasingly used in a clinical and research setting to provide information regarding lung mechanics. Respiratory oscillometry is conducted through three acceptable measurements of tidal breathing and can be performed with minimal contraindications. Young children and patients who cannot perform spirometry due to cognitive or physical impairment can usually complete oscillometry. The main advantages of respiratory oscillometry are that it requires minimal patient cooperation and is more sensitive in detecting changes in small airways than conventional pulmonary function tests. Commercial devices are now available. Updated technical guidelines, standard operating protocols, and quality control/assurance guidelines have recently been published. Reference values are also available.
We conducted oscillometry test audits before and after implementing a formal respiratory oscillometry training program and standard operating protocol. We observed improvement in the quality of tests completed, with a significant increase in the number of acceptable and reproducible measurements.
The current paper outlines and demonstrates a standard operating protocol to conduct respiratory oscillometry in an outpatient setting. We highlight the key steps to ensuring acceptable and reproducible quality measurements according to the recommended European Respiratory Society (ERS) guidelines, as quality control is critical to measurement accuracies. Potential problems and pitfalls are also discussed with suggestions to resolve technical errors.
Introduction
Respiratory oscillometry measures the impedance of the lung and is exquisitely sensitive to changes in respiratory mechanics1, particularly to the peripheral lung and small airways, regions of the lung that are not well assessed by traditional pulmonary function tests.
Over the last several years, the availability of commercial devices and updated technical and quality control/assurance standards2,3 have led to increasing use of oscillometry for clinical and research purposes. However, to date it is not a routine test in the repertoire of pulmonary function modalities, but the technique is anticipated to become more widely used with increasing recognition of its clinical utility. The overall goal of respiratory oscillometry is to provide measurement of respiratory mechanics during normal breathing and assessment of lung function, that is not discernible by current methods of spirometry and plethysmography. Oscillometry offers other advantages over traditional pulmonary function tests as it can be performed in the very young, the elderly, or in patients with cognitive impairment where forced expiratory maneuvers needed for spirometry are impossible. Furthermore, oscillometry can be conducted in anyone who can breathe spontaneously while wearing a nose clip. Unlike standard pulmonary function tests, it is not contraindicated following cataract, intra-abdominal or cardiothoracic surgery, nor following acute myocardial infarction and heart failure. Lastly, several of the oscillometry devices currently available are portable, and can be used in settings outside of a diagnostic laboratory, including clinic and office settings, bedside, or in workplaces.
Oscillometry measures the total respiratory impedance (Zrs) to multi-frequency oscillatory pressure waves1,2,4,5,6. Impedance is composed of the complex sum of respiratory resistance (Rrs) and reactance (Xrs). Rrs reflects the resistance of airways and is largely frequency-independent in health4,7,8. In small airway diseases, Rrs becomes frequency-dependent and increases more in the lower frequencies5,9,10, so that a difference in Rrs at frequencies between 5 and 19 Hz (R5–19) or 5 and 20 Hz (R5–20) indicates small airway obstruction and heterogeneity of ventilation in different regions of the lung 10,11,12. Xrs measures the balance of elastic and inertial impedances of the respiratory system. At lower frequencies (e.g., 5 to 11 Hz), Xrs reflects the stiffness or elastance of the pulmonary and chest wall tissues13,14. At higher frequencies, Xrs is dominated by the inertia of the air column in the conducting airways. The resonance frequency (Fres) is the point at which the magnitudes of elastic and inertive reactance are equal. AX is an integrative index of Xrs and is calculated as the area under the Xrs versus frequency graph between 5 Hz and Fres. AX has the units of elastance and is inversely related to the volume of the lung in communication with ventilation. AX increases with restrictive processes and peripheral inhomogeneity. X5 becomes increasingly negative while AX and Fres are increased in both obstructive and restrictive lung diseases4,5. See Figure 1 for depiction of these metrics.
While initially focused on measurement of lung function in children, emerging data show that oscillometry provides useful clinical information in adults as well. It is increasingly used in the clinical setting15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45. Oscillometry has been most extensively studied in obstructive lung diseases where it has been found to offer better diagnostic information than spirometry with respect to asthma control31,32,33,34,35, better correlation with symptoms23,34, and earlier detection36,37,38 of chronic obstructive lung disease (COPD). Our group has shown oscillometry to be more sensitive than spirometry for tracking graft injury following lung transplant46. Several studies have shown that Xrs, specifically the difference in the mean inspiratory and expiratory reactance at 5 Hz, can distinguish restrictive defects in interstitial lung disease (ILD) from asthma and COPD47, and can differentiate combined pulmonary fibrosis and emphysema from ILD-only48,49. Figure 2 demonstrates the typical oscillometry patterns for normal, restrictive, and obstructive lung diseases. There has been increasing interest to implement oscillometry as another routine modality of pulmonary function testing to supplement and potentially replace some of the current testing modalities for lung function monitoring50,51.
We suggest that oscillometry is useful for screening of lung diseases, in follow-up of patients with known obstructive and restrictive lung diseases, and following lung transplant. The commercial devices are suitable for use in children as young as 2 years old. There is ongoing research with even younger populations52, and as the field grows it may be possible to evaluate infants and newborns.
The goal of the current manuscript is to provide a training manual for clinicians, technologists, and research personnel on the appropriate conduct of oscillometry, following international standard operating protocols and quality control guidelines. Due to the small footprint of most commercial oscillometers, oscillometry can be implemented in multiple settings. The protocol outlined is appropriate for pulmonary function laboratories, physician offices, clinic settings, and other outpatient settings such as workplace occupational health units.
Protocol
The respiratory oscillometry studies were approved by the University Health Network Research Ethics Board (REB# 17-5373, 17-5652 and 19-5582). Written informed consent was obtained from participants prior to oscillometry test.
NOTE: This video outlines the standard operating procedure for oscillometry. Our laboratory uses a device manufactured by Thorasys Thoracic Medical Systems Inc but the technique is the same regardless of the manufacturer. The software programs are different for each manufacturer, in the same way that different commercial spirometers have unique proprietary software for data collection and display. The protocol below is applicable for all respiratory oscillometry devices. Readers are directed to manuals of their commercial devices and refer to specific instructions regarding software of their device.
1. Pre-test patient screening/preparation
- Ensure that the patient is free of any active or suspected transmissible respiratory infection, such as coronavirus or tuberculosis.
- Ensure that the patient has not had any recent dental or facial surgeries, such as tooth extractions, and can form a proper tight seal around the mouthpiece.
- Ensure that the patient is as relaxed as possible, is not wearing tight-fitted clothing, and withholds from tobacco use and vigorous exercise at least 1 h prior to testing.
- Perform oscillometry prior to conventional PFTs, such as spirometry, if requested by a referring physician.
NOTE: Refer to Supplemental Table 1 for contraindications for spirometry/PFTs. - Ensure that the patient withholds bronchodilators prior to testing, unless instructed by a referring physician to continue bronchodilator medication.
NOTE: Refer to Supplemental Table 2 for bronchodilator withholding times for PFTs and Supplemental Table 3 for bronchodilator withholding times for the methacholine challenge test.
2. Equipment/materials preparation
- Equipment preparation
- Verify the resistance load of the oscillometry device by using a valid factory calibrated mechanical test load prior to patient testing.
- Remove the dust caps at both ends of the mechanical test load and attach onto the oscillometry device.
- Select calibration from the oscillometry software menu and proceed with impedance test load verification.
NOTE: The recommended tolerance for the verification is ≤ ±10% or ±0.1 cmH2O·s/L, whichever is met first. - Upon successful verification, save and proceed with testing.
- Materials preparation
- Have multiple 'single-patient-use-bacterial/viral' filters and nose clips readily available.
- Have personal protection equipment (PPE), such as gloves and masks, and disinfectant wipes available.
NOTE: Refer to laboratory policies for donning and doffing of PPE and infection control guidelines.
3. Patient preparation
- Anthropometry
- Verify the patient's information: first and last names, date of birth, birth sex, height, weight, and gender identity, if applicable.
- Measure the patient's height without shoes, with feet together, standing as tall as possible with the eyes level and looking straight ahead, and the back flush against a wall or flat surface.
NOTE: For patients unable to stand erect, height may be estimated using arm span. For patients aged 25 years or older, where height measurement has been made previously in the same laboratory, remeasuring height at subsequent visits within 1 year may not be necessary. - Update weight measurement at each visit.
- Record patient's usage of bronchodilators, dosage, time/date of last administration, and any medication allergies, such as salbutamol.
- Oscillometry test preparation
- Ask the patient to sanitize their hands prior to entering the testing station.
- Outline the test duration of 30 s and the minimum requirement of three trials.
- Explain the sensation generated by the oscillations such as 'vibrations' or 'fluttering.'
- Ensure that the patient is seated properly in a slight 'chin-up' position with both feet on the floor. Avoid slouching against the back of the chair or leg crossing.
- Instruct the patient to breathe normally while holding their cheeks with their palm and fingers and using their thumbs to support the soft tissue of the jaw during measurements.
NOTE: Cheek and floor of the mouth support is enforced to avoid upper airway shunt. If the cheeks and soft tissues of the mouth are not supported, flow measured at the mouth is lost in the motion of the upper airway wall. - Explain to the patient that swallowing should be avoided and the tongue must be below the mouthpiece during the test.
NOTE: The instructions above apply to both children and adults. Depending on the age of the child, holding a picture or other forms of visual distraction in front of the child can help ensure that the head posture is maintained during the oscillometry recording period. For adults with cognitive impairment, consider having an accompanying person nearby to coach and calm the patient to breath normally. For patients with physical impairment, some oscillometry devices are portable, and can be brought to the patient bedside or wheelchair. Also consider asking the accompanying or another person to provide cheek and jaw support during tests.
4. Software setup
NOTE: Please refer to the manufacturer's instruction manual for individual instructions.
- New patient setup
- Select New Patient and enter the patient's information such as first and last names, date of birth, birth sex, height, weight, ethnicity (if applicable), and smoking history.
- Check that all information entered is correct prior to selecting Standard Test.
- Ensure that the correct wavelength setup is selected. In this demonstration, select Airwave Oscillometry from the Template dropdown menu. The choice of specific wavelengths and wavelength combinations will be different amongst the different manufacturers. Follow the software instruction manual for the specific device.
- Ensure the appropriate set of reference values is selected: Oostveen et al.56 or Brown et al.57 for adults, and Nowowiejska et al.58 for children ages 3 to 17.
NOTE: The preferred and availability of reference values may differ depending on each laboratory's policy and oscillometry device manufacturer.
- Existing patient setup
- Click on Select Patient and choose the correct patient's file by verifying their information such as first and last names and date of birth.
- Ensure patient's weight and height (if applicable) are updated prior to the start of testing.
- Select Standard Test and choose Airwave Oscillometry from the Template dropdown menu. See also section 4.1.3.
5. Testing procedure
- Oscillometry device setup
- Attach a 'single-patient-use-bacterial/viral' filter to the oscillometry device.
- Ensure the oscillometry device is ready in the testing mode.
- Spectral measurement
NOTE: Airwave oscillometry 5-37 Hz in the device shown in the video.- Remind the patient of the 30 s test duration and the minimum requirement of three measurements.
- Instruct the patient to wear a nose clip and provide instructions described in step 3.2.4 and step 3.2.5.
- Adjust the oscillometry device to the patient's head level.
- Instruct the patient to wet his/her lips before wrapping them around the mouthpiece to form a proper, tight seal. Instruct the patient to begin to breathe normally.
NOTE: Inspect for potential air leaks around the mouthpiece and nose clip. Supplementary oxygen must be turned off during measurements to avoid any drift into the oscillometry device. - Observe the patient's breathing pattern and start recording following a minimum of three stable tidal breaths.
NOTE: (Optional): During the test, inform the patient of the time remaining during each measurement. - Provide adequate rest time in between each measurement and adjust accordingly, based on the patient.
NOTE: Patients on supplemental oxygen may require longer rest intervals. Provide supplemental oxygen as needed during the rest intervals. - Following a minimum of three measurements, proceed to Step 6 to assess acceptability and reproducibility.
- Post-bronchodilator response – optional
- Administer bronchodilator (salbutamol or ipratropium bromide) via a spacer.
- Record the method and number of doses administered.
- Wait for 10 min post-salbutamol/albuterol and 20 min post-ipratropium bromide inhalation.
- Repeat Step 5.2 to assess post-bronchodilator response
- 10 Hz (intra-breath) measurement – optional
- Remind the patient that each test duration is 30 s and a minimum of three measurements will be obtained.
- Ensure that the correct wavelength setup for intra-breath measurement is selected.
- Repeat Steps 5.2.2 to 5.2.6.
6. Access acceptability and reproducibility
- Acceptability
- Ensure that the measurements have a validity greater than 70%.
- Check that the symbol beside the measurements have obtained a checkmark.
NOTE: If a 'Caution' symbol is present, the measurement is unacceptable. - Inspect each measurement for anomalies or artefacts that may be caused by coughing, tongue obstruction, glottis closure, air leakage around the mouthpiece, attempting to talk, swallowing, and taking a deep breath.
NOTE: If the patient is observed taking a deep breath, reset the oscillometry device, as forceful breaths disrupt the motors and the quality of subsequent measurements. To reset, stop testing and then click Zero Channels. - Review measurements automatically excluded by the software; these include anomalies or artefacts such as cough or glottis closure.
- Exclude any unacceptable measurement with anomalies outlined in Step 6.1.3 and repeat Step 5.2 to obtain additional measurements.
- Reproducibility
- Ensure that a minimum of three acceptable measurements are recorded.
- Ensure that the coefficient of variance (CoV) of Rrs (resistance of respiratory system) is ≤10% in adults and ≤15% in children.
- Repeat Step 5.2 to obtain additional measurements if the 3 acceptable measurements have CoV >10% in adults and >15% in children.
- Repeat 6.1 to determine acceptability and report three acceptable measurements with CoV ≤10% in adults and ≤15% in children.
7. Disinfection
- Discard patient's mouthpiece and nose clip into the wastebin.
- Use disinfectant wipes to clean the oscillometry device and patient's chair.
- Doff gloves and sanitize hands.
- Place the red dust cap back onto the oscillometry device to avoid any contamination.
NOTE: Each laboratory's infection control policy may be different.
8. Reporting results
NOTE: Refer to Figure 3 for details.
- Include patient's first and last names, height, weight, age, birth sex, BMI, and smoking history.
- Include device name, model, software version, and manufacturer.
- Include input signal frequencies and duration of individual recordings.
- Report the mean of acceptable and reproducible measurements and the CoV for these reported measurements.
NOTE: If the CoV is higher than the specified upper limit, the results should be flagged so that the interpreting physician may interpret the results with caution. - Select reference equations.
- Include impedance graph demonstrating Rrs and Xrs versus oscillation frequency.
- Include post-bronchodilator response with dosage and method of administration including z-scores and absolute percentage change – optional
9. Quality control/quality assurance
- Perform regular audits (weekly or monthly) depending on the volume of oscillometry testing in the laboratory.
- Assess each operator using a standardized checklist to ensure oscillometry tests are conducted accurately and professionally.
- Provide regular feedback to operators and hold quarterly quality assurance meetings to reflect on laboratory matters.
- Ensure biologic quality controls are conducted weekly with at least two healthy non-smoking subjects, and measurements are within ±2SD of their mean baseline.
NOTE: This is extremely important for validating testing equipment and procedures when there are multiple oscillometry devices in the laboratory. - Conduct quarterly self-inspection and annual factory maintenance of oscillometry devices for calibration and quality checks.
Representative Results
From October 17, 2017 to April 6, 2018, we conducted the first quality assurance/quality control (QA/QC) audit of the 197 oscillometry tests3. Although all of the operators were trained prior to testing patient with a one hour seminar and on-site testing, 10 (5.08%) unacceptable and/or irreproducible measurements were identified. These measurements were excluded due to cough, tongue obstruction, and CoV greater than 15% following the initial suggested ERS guidelines52. Biologic quality control (BioQC) was not conducted regularly. The research personnel underwent additional oscillometry training and developed a standard operating protocol to ensure proper ERS guidelines and medical professionalism were in place. The importance of BioQC, a tool to validate testing equipment and procedures, was highlighted to the research personnel, who were reminded to perform regular BioQC tests.3 Improvements were found in subsequent QA/QC audits. Out of the total of 1930 oscillometry tests conducted from April 9, 2018 to June 30, 2019, only three (0.0016%) tests were invalid measurements; these had CoV greater than 15%. Between July 2, 2019 and March 12, 2020, there were 1779 oscillometry tests performed and nine (0.005%) were considered unacceptable, including measurements that had glottis closure, air leakage, and CoV greater than 15%. Refer to Table 1 for additional information.
Since the reinforcement of BioQC in April 2018, research personnel conducted BioQC regularly. At our center, four healthy non-smoking individuals conducted oscillometry daily for the initial 2 weeks to gather a minimum of 10 measurements with the mean with the upper and lower limit (±2SD or standard deviation) with coefficient of variation ≤10% between Rrs in the two oscillometry devices in our laboratory. On August 30, 2021, we observed a BioQC measurement that fell outside the individual's mean ±2SD. The individual's observed R5 was 3.36 cmH2O·s/L (open circle), while R5 mean from the 20 most recent recordings was 4.95 cmH2O.s/L ±2SD (dotted line with lower limit at 4.03 and upper limit at 5.86; Figure 4). A second individual conducted their BioQC oscillometry on the same day with the same oscillometry device, and the observed R5 measurement was also outside the mean ±2SD. These findings indicate problems related to the instrument rather that than the procedure. Subsequently, the manufacturer was contacted and the device was sent for repair. Upon return of the device, BioQC was repeated on October 15, 2021 to ensure it was within the individual's R5 measurement range prior to redeployment of device in our laboratory.
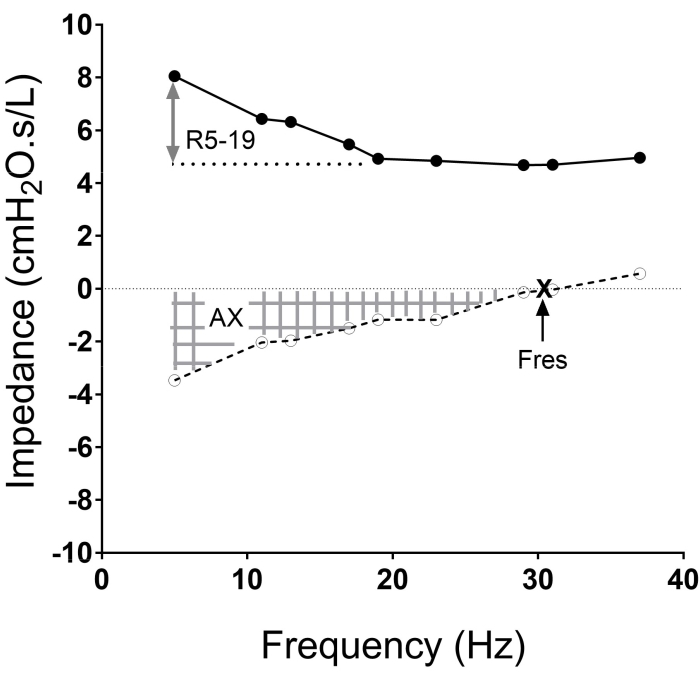
Figure 1: The impedance versus frequency oscillogram with the resistance curve (solid line) and reactance curves (dotted line), and frequencies at which the measurements are made (solid and open circles in each curve) shown. The area of reactance (AX, hatched area), resonant frequency (Fres. X), and resistance between 5 Hz to 19 Hz (R5-19; two-sided arrow) are illustrated. Please click here to view a larger version of this figure.
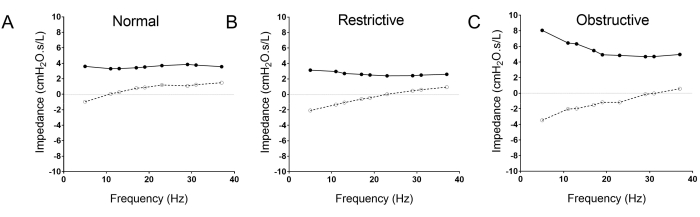
Figure 2: The typical oscillometry pattern differences between normal (A), restrictive (B) and obstructive (C) lung diseases. Note the rightward shift of the reactance curve (open circle, dotted line) in the restrictive disease (B), and the trumpet shaped pattern of the obstructive oscillogram (C) with upward shift of the resistance curve (solid circle and line), increased R5-19, and the downward and rightward shift of the resistance curve (broken line; open circles). Please click here to view a larger version of this figure.
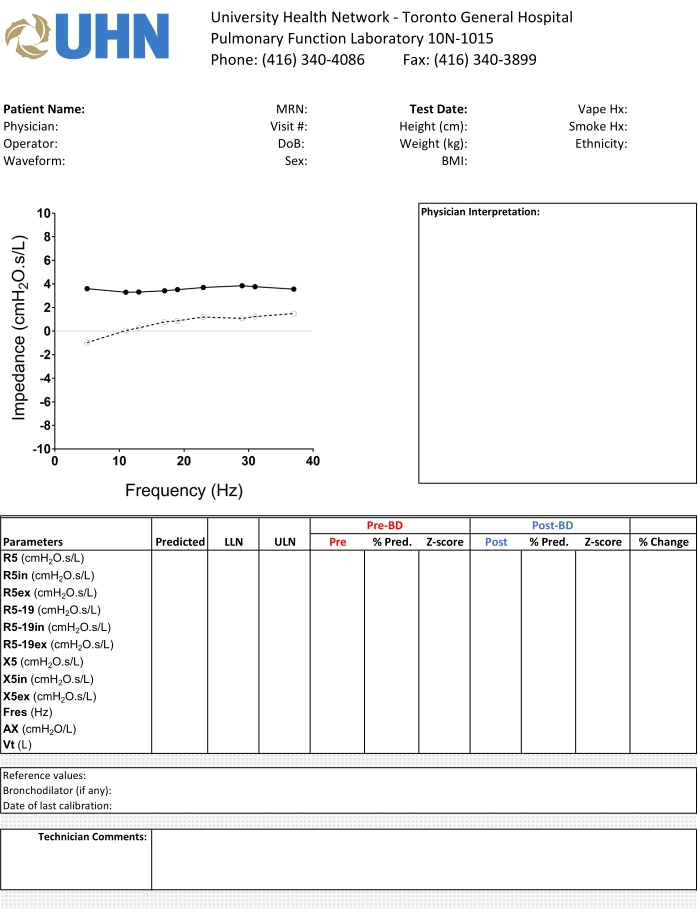
Figure 3: The standard template for reporting of oscillometry in our institution. We display the oscillogram using a standardized X-Y axis, and highlight the relevant pre- and post-bronchodilator measurements in different colors to facilitate interpretation of the results. Please click here to view a larger version of this figure.
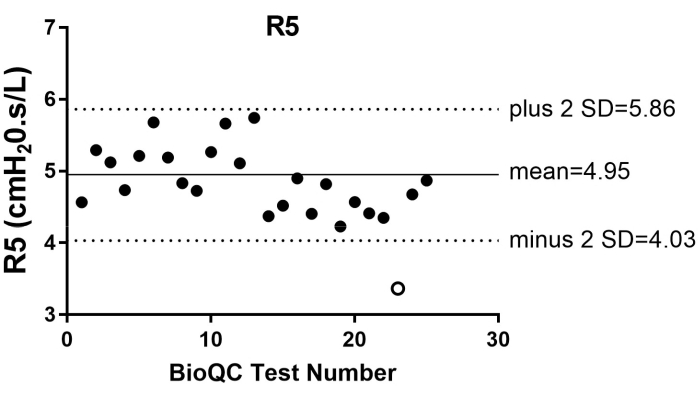
Figure 4: The biologic quality control (BioQC) summary of R5 measurements from one individual from May 2020 to November 2021. The measurement that fell outside (open circle) the individual's mean (solid grey line) ±2SD (dotted line) was observed in August 30, 2021. Please click here to view a larger version of this figure.
| First Audit | Second Audit | Third Audit | |
| October 17, 2017 to April 6, 2018 | April 9, 2018 to June 30, 2019 | July 2, 2019 to March 12, 2020 | |
| Valid | 187 | 1927 | 1770 |
| Invalid | 10 | 3 | 9 |
Table 1: Comparison of oscillometry tests acceptability at three time-points
Personnel underwent refresher training in conduct of oscillometry following the first audit. We also implemented a standard operating protocol for conduct of oscillometry in the pulmonary function laboratory. Significant improvements in the percentage of tests meeting acceptable quality control occurred and were sustained over time. These results demonstrate the effectiveness of developing and adhering to standard operating protocols and quality control guidelines.
Supplemental Table 1. Contraindications for Spirometry53,54 Please click here to download this Table.
Supplemental Table 2. Bronchodilators Withholding Times for Pulmonary Function Tests53,54 Please click here to download this Table.
Supplemental Table 3. Bronchodilators Withholding Times for Bronchial Challenge Test53,55 Please click here to download this Table.
Discussion
The critical steps in a high quality oscillometry measurement can be categorized into the domains of patient, equipment, and operator. Ensuring that the patient is relaxed and comfortable so that the measurements collected are at resting functional residual volume is key. The patient posture is very important; ensure that the patient is sitting upright with both feet on ground with no crossing of legs. The enforcement of cheek and jaw support, good placement of the nose clip, and ensuring lips are sealed around the mouthpiece will eliminate shunting and air leaks1,2,3. The equipment must be calibrated and verified prior to use. The operator must be able to recognize acceptable and unacceptable recordings and able to troubleshoot the underlying cause of unacceptable readings or artefacts to ensure reported measurements have CoV ≤10%1,2,3. Quality control and assurance must be maintained to not only ensure the oscillometry device is validated, but also the quality of tests.
Training of the operator to recognize the patterns produced by common artefacts such as swallowing, leaks, and shunting will allow for timely repeated measurements to obtain quality tests. There are instances when oscillometry is performed at different lung volumes, (e.g., in supine position). Under these circumstances, all the steps described in the protocol can still be applied.
While oscillometry is an easier and more rapid modality of pulmonary function testing, errors in measurements, and thus interpretation, will occur if deviations from the standardized protocol and quality control steps occur. Our protocol is based on the device used at our center. The conduct of oscillometry will be the same across devices. However, there will be differences in the technical aspect of calibration and software applications. Readers are advised to follow the manual for the different instruments.
Oscillometry is faster and easier to perform than spirometry. Moreover, young children and adults with language, physical, and/or cognitive impairment that impede the ability to perform the forced expiratory maneuvers needed for spirometry can still perform oscillometry as it is conducted during normal breathing. In some centers, oscillometry has supplanted spirometry as the initial screening tool for lung disease. Enhancing training in the conduct of oscillometry will facilitate its wider application as a diagnostic tool and ensure quality control of the tests conducted.
Although oscillometry is a fast and easy technique, quality controls are needed to ensure accurate and reproducible measurements. By following international guidelines, research and clinical oscillometry data can be interpreted appropriately so that findings can be applied across different patient populations.
Declarações
The authors have nothing to disclose.
Acknowledgements
The study was funded by CIHR-NSERC Collaborative Health Research Projects (CWC), Pettit Block Term grant (CWC), The Lung Health Foundation, and Canadian Lung Association – Breathing as One: Allied Health Grant (JW). We thank the many participants of our oscillometry research studies who have allowed us to develop expertise in conduct of oscillometry.
Materials
| Accel Prevention Disinfectant wipes – 160/canister | Diversey Care | 100906721 | https://diversey.com/en/ |
| clearFlo F-100 – 100 Airwave Oscillometry filters | Thorasys | 101635 | https://www.thorasys.com/ |
| Noseclip w/cushions, "Snuffer", bx/1000 | McArthur Medical Sales Inc. | 785-1008BULK | https://mcarthurmedical.com/ |
| Tremoflo C-100 Airwave Oscillometry System | Thorasys | 101969 | https://www.thorasys.com/ Software verison: 1.0.43 build 43 Signal Type: Pseudo-random, relative primes Frequencies (Hz): 5, 10, 11, 14, 17, 19, 23, 29, 31, 37 |
| Tremoflo C-100 Calibrated Reference Load 15 cm H2O. s/L | Thorasys | 101059 | https://www.thorasys.com/ |
Referências
- Bates, J. H., Irvin, C. G., Farre, R., Hanto, s. Z. Oscillation mechanics of the respiratory system. Comprehensive Physiology. 1 (3), 1233-1272 (2011).
- King, G. G., et al. Technical standards for respiratory oscillometry. European Respiratory Journal. 55 (2), 1900753 (2020).
- Wu, J., et al. Development of quality assurance and quality control guidelines for respiratory oscillometry in clinical studies. Respiratory Care. 65 (11), 1687-1693 (2020).
- Pride, N. B. Forced oscillation techniques for measuring mechanical properties of the respiratory system. Thorax. 47 (4), 317-320 (1920).
- Clement, J., Landser, F. J., Van de Woestijne, K. P. Total resistance and reactance in patients with respiratory complaints with and without airways obstruction. Chest. 83 (2), 215-220 (1983).
- Leary, D., Bhatawadekar, S. A., Parraga, G., Maksym, G. N. Modeling stochastic and spatial heterogeneity in a human airway tree to determine variation in respiratory system resistance. Journal of Applied Physiology. 112 (1), 167-175 (2012).
- Landser, F. J., Clement, J., Van de Woestijne, K. P. Normal values of total respiratory resistance and reactance determined by forced oscillations: influence of smoking. Chest. 81 (5), 586-591 (1982).
- Wouters, E. F., Polko, A. H., Schouten, H. J., Visser, B. F. Contribution of impedance measurement of the respiratory system to bronchial challenge tests. Journal of Asthma. 25 (5), 259-267 (1988).
- Wouters, E. F., Landser, F. J., Polko, A. H., Visser, B. F. Impedance measurement during air and helium-oxygen breathing before and after salbutamol in COPD patients. Clinical and Experimental Pharmacology & Physiology. 19 (2), 95-101 (1992).
- Grimby, G., Takishima, T., Graham, W., Macklem, P., Mead, J. Frequency dependence of flow resistance in patients with obstructive lung disease. The Journal of Clinical Investigation. 47 (6), 1455-1465 (1968).
- Cavalcanti, J. V., Lopes, A. J., Jansen, J. M., de Melo, P. L. Using the forced oscillation technique to evaluate bronchodilator response in healthy volunteers and in asthma patients presenting a verified positive response. Journal Brasileiro de Pneumologia. 32 (2), 91-98 (2006).
- Cavalcanti, J. V., Lopes, A. J., Jansen, J. M., Melo, P. L. Detection of changes in respiratory mechanics due to increasing degrees of airway obstruction in asthma by the forced oscillation technique. Respiratory Medicine. 100 (12), 2207-2219 (2006).
- Bates, J. H., Maksym, G. N. Mechanical determinants of airways hyperresponsiveness. Critical Reviews in Biomedical Engineering. 39 (4), 281-296 (2011).
- Dellaca, R. L., Aliverti, A., Lutchen, K. R., Pedotti, A. Spatial distribution of human respiratory system transfer impedance. Annals of Biomedical Engineering. 31 (2), 121-131 (2003).
- Dandurand, R., Li, P., Mancino, P., Bourbeau, J. Oscillometry from the CanCOLD Cohort: correlation with spirometry and patient reported outcomes. European Respiratory Society International Congress. , (2018).
- Jabbal, S., Manoharan, A., Lipworth, J., Lipworth, B. Utility of impulse oscillometry in patients with moderate to severe persistent asthma. Journal of Allergy and Clinical Immunology. 138 (2), 601-603 (2016).
- Lipworth, B. J., Jabbal, S. What can we learn about COPD from impulse oscillometry. Respiratory Medicine. 139, 106-109 (2018).
- Manoharan, A., Anderson, W. J., Lipworth, J., Lipworth, B. J. Assessment of spirometry and impulse oscillometry in relation to asthma control. Lung. 193 (1), 47-51 (2015).
- Manoharan, A., Morrison, A. E., Lipworth, B. J. Effects of adding tiotropium or aclidinium as triple therapy using impulse oscillometry in COPD. Lung. 194 (2), 259-266 (2016).
- Manoharan, A., von Wilamowitz-Moellendorff, A., Morrison, A., Lipworth, B. J. Effects of formoterol or salmeterol on impulse oscillometry in patients with persistent asthma. Journal of Allergy and Clinical Immunology. 137 (3), 727-733 (2016).
- Wei, X., et al. Impulse oscillometry system as an alternative diagnostic method for chronic obstructive pulmonary disease. Medicina. 96 (46), 8543 (2017).
- Tse, H. N., Tseng, C. Z., Wong, K. Y., Yee, K. S., Ng, L. Y. Accuracy of forced oscillation technique to assess lung function in geriatric COPD population. International Journal of Chronic Obstructive Pulmonary Disease. 11, 1105-1118 (2016).
- Eddy, R. L., Westcott, A., Maksym, G. N., Parraga, G., Dandurand, R. J. Oscillometry and pulmonary magnetic resonance imaging in asthma and COPD. Physiological Reports. 7 (1), 13955 (2019).
- Yamagami, H., et al. Association between respiratory impedance measured by forced oscillation technique and exacerbations in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease. 13, 79-89 (2018).
- Kitaguchi, Y., Yasuo, M., Hanaoka, M. Comparison of pulmonary function in patients with COPD, asthma-COPD overlap syndrome, and asthma with airflow limitation. International Journal of Chronic Obstructive Pulmonary Disease. 11, 991-997 (2016).
- Jetmalani, K., et al. Peripheral airway dysfunction and relationship with symptoms in smokers with preserved spirometry. Respirology. 23 (5), 512-518 (2018).
- Robinson, P. D., King, G. G., Sears, M. R., Hong, C. Y., Hancox, R. J. Determinants of peripheral airway function in adults with and without asthma. Respirology. 22 (6), 1110-1117 (2017).
- Short, P. M., Anderson, W. J., Manoharan, A., Lipworth, B. J. Usefulness of impulse oscillometry for the assessment of airway hyperresponsiveness in mild-to-moderate adult asthma. Annals of Allergy, Asthma & Immunology. 115 (1), 17-20 (2015).
- Zimmermann, S. C., Tonga, K. O., Thamrin, C. Dismantling airway disease with the use of new pulmonary function indices. European Respiratory Review. 28 (151), (2019).
- Lundblad, L. K. A., Siddiqui, S., Bossé, Y., Dandurand, R. J. Applications of oscillometry in clinical research and practice. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine. 5 (1), 1-15 (2019).
- Shi, Y., et al. Relating small airways to asthma control by using impulse oscillometry in children. Journal of Allergy and Clinical Immunology. 129 (3), 671-678 (2012).
- Pisi, R., et al. Small airway dysfunction by impulse oscillometry in asthmatic patients with normal forced expiratory volume in the 1st second values. Allergy & Asthma Proceedings. 34 (1), 14-20 (2013).
- Saadeh, C., Saadeh, C., Cross, B., Gaylor, M., Griffith, M. Advantage of impulse oscillometry over spirometry to diagnose chronic obstructive pulmonary disease and monitor pulmonary responses to bronchodilators: An observational study. SAGE Open Medicine. 3, (2015).
- Foy, B. H., et al. Lung computational models and the role of the small airways in asthma. American Journal of Respiratory and Critical Care Medicine. 200 (8), 982-991 (2019).
- Tang, F. S. M., et al. Ventilation heterogeneity and oscillometry predict asthma control improvement following step-up inhaled therapy in uncontrolled asthma. Respirology. 25 (8), 827-835 (2020).
- Frantz, S., et al. Impulse oscillometry may be of value in detecting early manifestations of COPD. Respiratory Medicine. 106 (8), 1116-1123 (2012).
- Aarli, B. B., et al. Variability of within-breath reactance in COPD patients and its association with dyspnoea. European Respiratory Journal. 45 (3), 625-634 (2015).
- Dean, J., Kolsum, U., Hitchen, P., Gupta, V., Singh, D. Clinical characteristics of COPD patients with tidal expiratory flow limitation. International journal of Chronic Obstructive Pulmonary Disease. 12, 1503-1506 (2017).
- Kotoulas, S. C., et al. Acute effects of e-cigarette vaping on pulmonary function and airway inflammation in healthy individuals and in patients with asthma. Respirology. 25 (10), 1037-1045 (2020).
- Berger, K. I., Goldring, R. M., Oppenheimer, B. W. POINT: Should oscillometry be used to screen for airway disease? Yes. Chest. 148 (5), 1131-1135 (2015).
- Berger, K. I., et al. Distal airway dysfunction identifies pulmonary inflammation in asymptomatic smokers. ERJ Open Research. 2 (4), (2016).
- Oppenheimer, B. W., et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest. 132 (4), 1275-1282 (2007).
- Lappas, A. S., et al. Short-term respiratory effects of e-cigarettes in healthy individuals and smokers with asthma. Respirology. 23 (3), 291-297 (2018).
- Vardavas, C. I., et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 141 (6), 1400-1406 (2012).
- Antoniewicz, L., Brynedal, A., Hedman, L., Lundback, M., Bosson, J. A. Acute effects of electronic cigarette inhalation on the vasculature and the conducting airways. Cardiovascular Toxicology. 19 (5), 441-450 (2019).
- Cho, E., et al. Airway oscillometry detects spirometric-silent episodes of acute cellular rejection. American Journal of Respiratory and Critical Care Medicine. 201 (12), 1536-1544 (2020).
- Sugiyama, A., et al. Characteristics of inspiratory and expiratory reactance in interstitial lung disease. Respiratory Medicine. 107 (6), 875-882 (2013).
- Mori, K., et al. Respiratory mechanics measured by forced oscillation technique in combined pulmonary fibrosis and emphysema. Respiratory Physiology & Neurobiology. 185 (2), 235-240 (2013).
- Mori, Y., et al. Respiratory reactance in forced oscillation technique reflects disease stage and predicts lung physiology deterioration in idiopathic pulmonary fibrosis. Respiratory Physiology and Neurobiology. 275, 103386 (2020).
- Usmani, O. S. Calling time on spirometry: unlocking the silent zone in acute rejection after lung transplantation. American Journal of Respiratory and Critical Care Medicine. 201 (12), 1468-1470 (2020).
- Calverley, P. M. A., Farré, R. Oscillometry: old physiology with a bright future. European Respiratory Journal. 56 (3), 2001815 (2020).
- Radics, B. L., et al. Effect of nasal airway nonlinearities on oscillometric resistance measurements in infants. Journal of Applied Physiology. 129 (3), 591-598 (2020).
- Toronto General Pulmonary Function Laboratory. . Toronto General Pulmonary Function Laboratory Policies and Procedures Manual. , (2022).
- Graham, B. L., et al. Standardization of Spirometry 2019 Update. American Journal of Respiratory and Critica Care Medicine. 200, 70-88 (2019).
- Coates, A. L., et al. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. European Respiratory Journal. 49, 1601526 (2017).
- Oostveen, E., et al. Respiratory impedance in healthy subjects: baseline values and bronchodilator response. European Respiratory Journal. 42 (6), 1513-1523 (2013).
- Brown, N. J., et al. Reference equations for respiratory system resistance and reactance in adults. Respiratory Physiology and Neurobiology. 172 (3), 162-168 (2010).
- Nowowiejska, B., et al. Transient reference values for impulse oscillometry for children aged 3-18 years. Pediatric Pulmonology. 43 (12), 1193-1197 (2008).

