Maintaining Laboratory Cultures of Gryllus bimaculatus, a Versatile Orthopteran Model for Insect Agriculture and Invertebrate Physiology
Summary
This paper outlines basic methods to standardize important factors such as density, feed availability, hydration source, and environmental controls for the long-term rearing of laboratory cultures of the edible cricket, Gryllus bimaculatus.
Abstract
Gryllus bimaculatus (De Geer) is a large-bodied cricket distributed throughout Africa and Southern Eurasia where it is often wild-harvested as human food. Outside its native range, culturing G. bimaculatus is feasible due to its dietary plasticity, rapid reproductive cycle, lack of diapause requirement, tolerance for high-density rearing, and robustness against pathogens. Thus, G. bimaculatus can be a versatile model for studies of insect physiology, behavior, embryology, or genetics.
Cultural parameters, such as stocking density, within-cage refugia, photoperiod, temperature, relative humidity, and diet, all impact cricket growth, behavior, and gene expression and should be standardized. In the burgeoning literature on farming insects for human consumption, these crickets are frequently employed to evaluate candidate feed admixtures derived from crop residues, food-processing byproducts, and other low-cost waste streams.
To support ongoing experiments evaluating G. bimaculatus growth performance and nutritional quality in response to variable feed substrates, a comprehensive set of standard protocols for breeding, upkeep, handling, measurement, and euthanasia in the laboratory was developed and is presented here. An industry-standard cricket feed has proven nutritionally adequate and functionally appropriate for the long-term maintenance of cricket breeding stocks, as well as for use as an experimental control feed. Rearing these crickets at a density of 0.005 crickets/cm3 in screen-topped 29.3 L polyethylene cages at an average temperature of 27 °C on a 12 light (L)/12 dark (D) photoperiod, with moistened coconut coir serving both as hydration source and oviposition medium has successfully sustained healthy crickets over a 2-year span. Following these methods, crickets in a controlled experiment yielded an average mass of 0.724 g 0.190 g at harvest, with 89% survivorship and 68.2% sexual maturation between stocking (22 days) and harvest (65 days).
Introduction
As typified by the iconic insect, the fruit fly Drosophila melanogaster, the use of insects as laboratory model organisms provides distinct advantages for studies in genetics, toxicology, and physiology1. The small size of insects reduces the space needed for cultures and the amount of feed and consumable materials required. Many insects reproduce quickly making them uniquely suited to the creation of specialized genetic lines and studies requiring the evaluation of multiple successive generations.
Many studies focus on holometabolous insects such as Drosophila, which exhibit complete metamorphosis and pupation. However, other models are available, including Gryllus bimaculatus (De Geer), the two-spotted field cricket. G. bimaculatus is a paurometabolous insect that undergoes between 7 and 11 nymphal instars before reaching sexual maturity2. This cricket displays a wide range of behaviors related to sexual selection, including stridulation, territorial displays, and mate-guarding3. Immature crickets are unlike the larvae of holometabolous insect species in that they, similar to many paurometabolous juveniles, are able to regenerate lost and damaged limbs during ecdysis4. Additionally, the fully sequenced genome of G. bimaculatus was published in 20215. These characteristics make these crickets appealing as a target for basic research.
Two-spotted field crickets are widely reared for human food and animal feed. The scale of these operations is often much larger than for laboratory research6,7. Despite the difference in scale, the challenges faced by researchers overlap greatly with those encountered by commercial cricket farmers. These considerations converge in the context of lab-based research aiming to improve edible insect production. As the edible insect industry continues to evolve and grow, optimizing feed inputs and myriad other aspects of production is a primary goal8. Laboratory studies demonstrating measured improvements in rearing efficiency, survivorship, or generation time in these crickets have the potential to help increase the profitability of cricket farming operations long-term.
Standardized rearing protocols enable closer comparison between studies investigating rearing optimization. To-date, few in-depth protocols for rearing G. bimaculatus in the laboratory have been published. An ideal protocol would reflect conditions encountered in real-world cricket farming operations, while maintaining the strictly controlled conditions necessary to accurately measure changes in growth performance arising from experimental treatments and highlighting risk mitigation strategies. The methods described in this paper were developed based on published protocols, techniques, and apparatus used to rear a variety of cricket species at a broad range of laboratory and commercial production scales2,9,10,11,12. These methods are also informed by several non-peer reviewed sources, including unpublished technical bulletins and personal communication with commercial cricket farmers in North America. This protocol was developed with the intention of facilitating the establishment of laboratory cultures of G. bimaculatus specifically for use in trials related to insect agriculture.
Protocol
1. Preparing the oviposition substrate
NOTE: Coconut coir is an ideal oviposition substrate for G. bimaculatus. For detailed methods on how to separate coir from compressed coir brick and a note on respiratory safety, see Supplemental Materials step 1.1.
- Wash hands with soap and water.
- Tare a clean container on a balance and weigh a mass of dry coconut coir approximately the size of a human fist.
- Place coir into a sealable, clean container, which can accommodate expansion up to 6x the original volume.
- With clean hands, gently break up clumps of coir from the piece removed from the larger block.
- Using a 50 mL graduated cylinder, measure the correct volume of deionized (DI) water to achieve a 5:1 ratio by mass of five parts water to one part dry coir.
- Add the measured DI water slowly, evenly hydrating all coir particles. Manually macerate clumps to ensure even hydration.
- Re-tare the container in which the coir was previously weighed.
- Weigh out 75 g of wetted coir.
- Transfer 75 g of the wetted coir into a 100 mm x 15 mm Petri dish using a clean plastic spoon to ensure that the coir is evenly spread around the bottom of the dish and that there are no clumps.
- Label the side of the Petri dish with lab tape with a label denoting natal colony and date of the egg collection event.
- Measure an additional 45 mL of DI water in a graduated cylinder.
- Add water evenly over the surface of the packed coir in the Petri dish to ensure even hydration. Ensure that the coir is saturated to the point that water pools approximately ¼ the way up the sides of the container.
- Once the Petri dish has been packed, seal the remaining wetted coir in an airtight vessel for storage at -20 °C.
NOTE: Following the methods prescribed by this article, G. bimaculatus individuals will reach sexual maturity after an average of 58 days post oviposition. - Collecting eggs
- Place the hydrated oviposition substrate into cages containing the desired parental stocks of crickets as far from the feed as possible due to the potential for crickets to mechanically transfer granules of feed onto the oviposition substrate.
- Document the date and time.
NOTE: The standardized working density for reproductive cricket colonies reared following these methods is n = 150 adult individuals. At that density, a 24 h oviposition window will yield between 800 and 1,500 eggs depending on the colony age, prior oviposition effort, and parent cage sex ratio. - Place a small autoclavable trash receptacle on the work surface to account for the containment risk posed by handling and cleaning egg-rich oviposition substrates.
- Place a clean, empty 29.3 L plastic cage on the bench next to the trash receptacle to serve as the recipient cage for the egg-rich oviposition substrate.
- Place the 29.3 L cage containing the parent cricket stocks and oviposition substrate on the opposite side of the trash receptacle from the empty cage.
- After 24 h, remove the oviposition substrate from the parent cricket cage and locate it over autoclavable waste vessel.
- Inspect the top of the oviposition substrate for any particles of frass or feed that the crickets may have kicked onto the surface of the coir.
NOTE: Any substance, which is not egg mass or coir, can cause mold to form on the substrate during incubation. - Remove coir contaminants into the waste vessel with a clean scoopula or plastic spoon.
- Place the plastic spoon into the waste receptacle.
- Place the cleaned oviposition substrate in the clean 29.3L cage.
- Place the cage in an incubator set to 27 °C at 60% relative humidity on a 12 h D/12 h L photoperiod.
- Return the cage containing the breeding stock to the original location and clear all items from the work surface.
- Place the waste receptacle into an in-facility freezer dedicated to the storage of items potentially contaminated with cricket eggs.
- Sanitize the work surface with 10% bleach solution and let it sit for 60 s.
- Wipe the work surface dry with a clean paper towel. Open the freezer and dispose of the paper towel in the waste receptacle.
- Misting and monitoring egg substrates daily
NOTE: For methods used to calibrate the volume of mist delivered by a spray bottle, see Supplemental Materials step 1.2.- Position a spray bottle over the oviposition substrate so that the water expressed is evenly distributed over the surface of the substrate.
- Perform the number of pump actuations calculated in the Supplemental Materials step 1.2 for each oviposition substrate daily for 11 consecutive days.
- Check each oviposition substrate daily, monitoring for filamentous mold growth on the surface of the coir.
- If fungal growth is observed, use a clean spoon or scoopula to remove spots of surface mold.
- Dispose of the tool and the removed coir in the autoclavable waste container stored in the facility freezer.
NOTE: It is unclear whether the mold adversely impacts cricket development. - At day 11 post oviposition, begin looking closely at the substrate for juvenile crickets.
NOTE: At 27 °C, the eggs of G. bimaculatus require 11-13 days to hatch.
- Setting up natal cages
- Select two unused 30.8 cm x 30.8 cm (12 inches x 12 inches) commercial egg carton flats. With a utility knife or strong shears, cut these into six separate 10.1 cm (4")-wide strips of equal size. Brush off the cut edges with hands to remove dangling particles of cardboard.
- Place the six individual 10.1 cm x 30.8 cm (4 inches x 12 inches) pieces of carton vertically into the bottom of the cage with the longer axis of the carton spanning the narrower horizontal axis of a 29.3L cage. Place a seventh piece of carton flat across the top of the six upright pieces.
- Select three pieces of rough brown paper towel approximately 25 cm x 25cm. Fold each in half. Place two such that they cover the top of the proximal side of the carton structure. Place one over the carton stack on the distal side.
- At day 11 post oviposition, move the oviposition substrate into the proximal righthand corner of the cage.
- Caring for early instars
NOTE: On day 14 post oviposition, most viable eggs will have hatched and early-stage cricket nymphs will require feed and water. Young crickets are unable to break the surface tension of water droplets and can drown if water is pooled in their environment. However, they are also sensitive to desiccation. Providing a consistent relative humidity of around 60% during this stage of development is important to ensuring survival.- When a hatch is observed, mist the paper towels placed over the top of the cartons in step 1.4.3 until they are wetted but not actively shedding water.
- Thoroughly wipe both sides of a 100 mm Petri dish lid with 70% ethanol and allow it to dry. Use it as the receptacle in which cricket feed will be delivered.
NOTE: First-instar crickets require smaller feed particles than crickets at subsequent stages of development. This finer feed should be administered to crickets for the first 20 days post emergence. - Scoop 50 g of the feed into a 60-watt single-serving blender and mill at 10,000 rpm for 1 min.
- Measure 1 g of the feed and shake it onto the Petri dish lid in the cage. Using the clean end of a spoon or scoopula, spread the feed out as uniformly as possible over the bottom of the dish.
- Replace the feed every 2 days. Use the end of the spoon to brush crickets from the feed dish before removing it. Discard the old feed in the autoclavable waste container.
- Monitor for mold growth on feed. If the feed begins to appear white or greenish, discard the Petri dish and feed immediately.
- At 14 days post oviposition, use a 2.54 cm (1 inch) paintbrush to remove crickets clinging to the natal coir substrate by brushing all crickets from the coir surface and sides of the Petri dish into the cage.
- Place the removed oviposition substrate into the autoclavable waste receptacle and store it in the freezer until autoclaving.
- Replace the natal substrate with a fresh coir dish for hydration prepared following steps 1.1.5-1.1.9.
- Use a DI water wash bottle to add water until the surface of the coir glistens but is not pooling.
NOTE: Cricket density strongly impacts growth performance in G. bimaculatus13. Maintaining the breeding stock at excessive density risks introducing unwanted crowding-induced epigenetic effects into experiments in which their progeny is used9. Crickets must be "thinned" from the high densities that emerge from the oviposition substrates and distributed into densities that adhere to the standard of 0.005 crickets/cm3 of space.
2. Caring for instars three to adult
- Setting up cages
NOTE: For details on the technique to construct the screened lid, see Supplemental Materials step 1.3.1.- Repeat step 1.4.1.
- Install five cut egg carton pieces in the distal end of the cage so the egg-shape concavities are facing outward. Ensure that the short ends are about the sides of the cage, the long side sits flat against the bottom, and there is approximately 3 cm of space between each piece of carton.
- Place the last cut carton piece atop the upright carton pieces such as the roof on a house as shown in Supplemental Figure S1.
- Follow steps 1.1.8-1.1.13 to prepare the hydration substrates.
- Place the hydration substrate in the proximal right-hand corner of the cricket cage as shown in Supplemental Figure S2.
- Use a wash bottle to add 6-10 mL of DI water until the coir surface appears wet and reflective, but the coir is not fully submerged.
NOTE: The surface should appear lightly dimpled, with surface tension causing the water to follow the contours of the coir. - Invert the lid of a 100 mm Petri dish and place it on the proximal left-hand side of the cage. Add 2-3 g of standard cricket feed as shown in Supplemental Figure S2.
- Modifying colony density
NOTE: Conduct this step at 20 days post hatching or when the crickets reach an average mass of 0.01 g.- On the work surface, place a large container that can accommodate the floor plan of three 29.3 L cages standing side-by-side.
NOTE: This is secondary containment and will control crickets that escape during their transfer from one colony to another. - Remove the original colony from the rearing rack and place it on the work surface.
- To the right side of the original colony, place an empty cage of the same size.
- To the right side of the empty cage, place a cage that has been set up according to steps 2.1.1-2.1.7.
- Check to make sure that crickets are not clinging to the underside of the screened lid of the cage containing the crickets. If observed, tap the top of the cage to dislodge them.
- Open the screened lid of the cage containing the crickets.
- In one gentle, smooth motion, transfer the "roof" carton and all the crickets adhering to its contours into the middle cage.
- Once the carton is inside the middle cage, gently agitate the cardboard against the sides to dislodge all the crickets.
- Visually inspect that all crickets have been shaken free before returning the carton piece to the proximal side of the cage of origin to allow the remaining crickets to adhere to the carton.
- Repeat steps 2.2.8-2.2.9 with all the carton pieces in the cage, working sequentially from the front to the back of the original cage until all the crickets have been transferred into the middle cage.
- Gently tilt the middle cage containing the crickets so that all the crickets contained are directed toward the bottom corner.
- Lift the cage containing the crickets over the recipient cage.
- Slowly tilt the donor cage so that the crickets begin to gain purchase on the sides and can move in a controlled manner out of the mass in the bottom corner as shown in Supplemental Figure S3.
- If crickets advance too quickly, adjust the angle at which the middle cage is held, causing the crickets to fall back.
- While the cage is tilted, use a 2.54 cm (1 inch) paintbrush to direct the crickets into the recipient cage, counting each until the total count is equal 150 individuals. Use the brush to deter those advancing too swiftly for an accurate count.
- Label the newly stocked cricket cage with date, parental stock, and the number of crickets contained.
- Humanely euthanize excess crickets still in the bottom of the middle and original containers by placing the entire container into a freezer at -20 °C for a minimum of 30 min.
- Inspect the cage of origin to ensure that all crickets have been transferred.
- Position the cricket cages on plant-growing racks 25 cm below light hoods containing full-spectrum fluorescent lights set to a residential-grade outdoor timer programmed to maintain a 12 h L/D photoperiod. See Supplemental Figure S4.
- Transfer all frass, dishes of spent hydration substrate and feed, paper towel, exuviae, and dead crickets remaining in the cage of origin into the autoclavable waste receptacle.
- Unless the waste is to be autoclaved immediately, store it in an in-facility freezer at -20 °C.
- Inspect the floor, worker apparel, secondary containment cage, and work surface for escaped crickets.
- Sanitize the work surface and secondary containment cage with 10% bleach solution, discarding the paper towels into the autoclavable waste receptacle.
- On the work surface, place a large container that can accommodate the floor plan of three 29.3 L cages standing side-by-side.
- Feeding and watering
- Open the airtight feed storage container and fill an empty 100 mL sample cup with cricket feed. Reach into each colony and deposit a quarter of the feed held in the cup into the Petri dish lid holding the feed.
- To water crickets, prepare a Petri dish of coir following steps 1.1.9-1.1.13.
- Increase the feed rate commensurate to the consumption rate to ensure ad libitum feed availability.
NOTE: Cricket feed demand changes throughout development.
- Transferring crickets to clean cages
- Transfer the crickets to clean cages every 2 weeks. Replicate the arrangement of the cages from steps 2.2.1-2.2.4
NOTE: Significant amounts of frass will have accumulated in the concavities of the egg cartons. - Transfer crickets to clean cages, following steps 2.2.5-2.2.23.
- Gently maneuver the cartons so that the majority of frass falls into the cage of origin while allowing crickets to cling to the carton during repetition of steps 2.2.7-2.2.10.
- Use a brush or plastic spoon to encourage any crickets trapped in the frass to move from the middle cage into a clean cage.
- Inspect the cage of origin to ensure that all crickets have been transferred.
- Transfer all frass, dishes of spent hydration substrate and feed, paper towel, exuviae, and dead crickets remaining in the cage of origin into the autoclavable waste receptacle.
- Unless the waste is to be autoclaved immediately, store it in an in-facility freezer at -20 °C.
- Inspect the floor, worker apparel, secondary containment cage, and work surface for escaped crickets.
- Sanitize the work surface and secondary containment cage with 10% bleach solution, discarding paper towels in autoclavable waste receptacle.
- Transfer the crickets to clean cages every 2 weeks. Replicate the arrangement of the cages from steps 2.2.1-2.2.4
- Setting up experimental cages
NOTE: Experimental cages are plastic containers that house fewer crickets. Their setup is identical to the 29.3 L cages that contain breeding stocks but rely on 7.1 L containers stocked with smaller dishes of water, feed, and contain a reduced egg carton surface area.- Place six 10.1 cm x 15.4 cm (4 inch x 6 inch) carton pieces into the distal end of each experimental cage with the long axes of the cartons spanning the width of the narrow dimension of the cage and the short axes of the cartons oriented toward the lid and the floor.
- Pack 10 g of the hydrated coir working mix into a Petri dish.
- Use a wash bottle containing DI water to add approximately 15 mL of DI water, or until a meniscus forms at the surface of the coir.
- Invert the lid of a 60 mm x 15 mm Petri dish to hold the feed.
NOTE: The feeding rate may vary throughout the duration of the trial. For the experimental cage cricket randomization and stocking procedure, see Supplemental Materials step 1.4.
- Terminating insects
- When the crickets are no longer needed for breeding or experimental use, follow step 2.2.17.
- When the crickets are dead, remove the cage from the freezer. Remove the lid and transfer all the contained materials into an autoclavable waste receptacle. Transfer the waste back into the freezer until autoclaving.
- Immerse the empty cage into 10% bleach solution and allow it to sit for a minimum of 5 min.
- Triple-rinse the empty cage with cold tap water to remove the bleach residue, with particular attention to channels at the bottom of the container.
Representative Results
Data demonstrating successful cricket rearing from hatching to 65 days old were collected during a September 2021 feed trial. Crickets were grown from eggs following steps 1.1.1-2.6.1 of these protocols, and six replicate cages were stocked with 24 random 22-day-old (third instar) crickets following step 2.7 above. Crickets were then reared in ambient room conditions; however, due to a malfunctioning facility air handling unit, the average room temperature was 25 ± 1 °C at 20% relative humidity rather than the suggested 27 °C. Cricket mass was measured twice weekly between 22 and 65 days post hatching. The results from this experiment are outlined below and are presented as means plus or minus standard deviation.
Data shown in Figure 1 and Figure 2 represent the six replicate cages administered the standard feed described in this protocol. Crickets were stocked from a population with mean mass of 21 ± 9 mg. At the end of the experiment, the mean mass of all combined juvenile and adult crickets was 0.724 g ± 0.190 g (Figure 1). As G. bimaculatus is sexually dimorphic, we also report adult mass by sex. Sex ratio at harvest was 51% female. Of 30 adult males present at 65 days of age when the experiment ended, the mean mass was 0.721 g ± 0.123 g. Of the 58 adult females present at 65 days of age, the mean mass was 0.841 g ± 0.112 g (Figure 2). Survivorship between stocking and harvest was 89% and was measured weekly by total counts of all individual crickets in all cages. At day 65, 68.2% of all crickets had reached the adult instar (Figure 2).
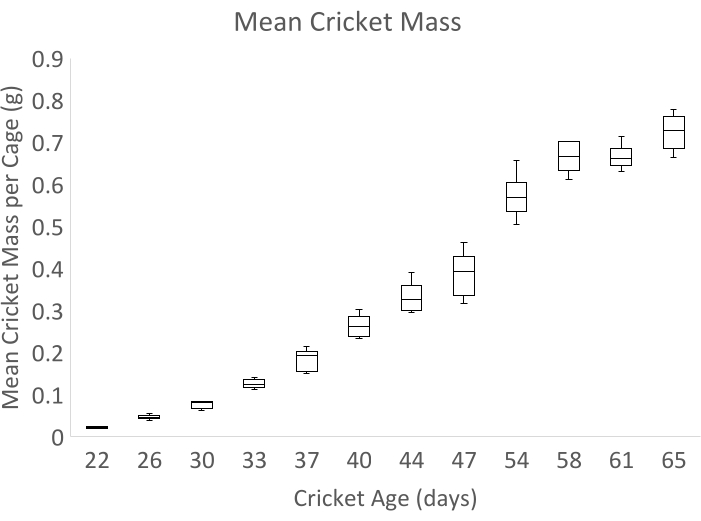
Figure 1: Mean mass of individual crickets 22-65 days post hatching. Bars represent quartiles of mean cricket mass by cage, n = 6 cages. All crickets were counted and weighed 2x per week, except in 1 week of the experiment, in which they were weighed only once. Please click here to view a larger version of this figure.
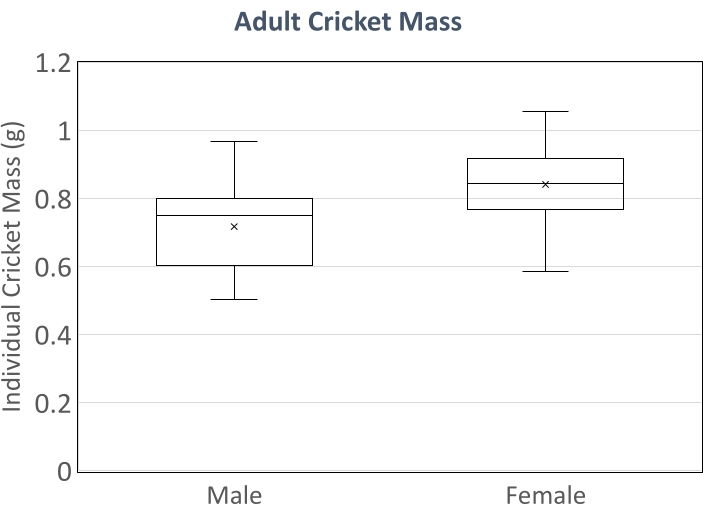
Figure 2: Mean adult cricket mass by sex at the end of the experiment. Male crickets n = 30, female crickets n = 58. Bars represent quantiles of mean mass; 'x' represents mean cricket mass by sex. Please click here to view a larger version of this figure.
Supplemental Materials: (1) Coconut Coir and Respiratory safety, (2) Removing coconut coir material, (3) Calibration of mist delivery, (4) Screened lid construction, (5) Experimental cage stocking, (6) Randomizing crickets to cages, (7) Methods used for feed analysis. Please click here to download this File.
Supplemental Figure S1: Side view of cricket cage containing correct arrangement of cardboard carton refugia, feed, and coconut coir. Please click here to download this File.
Supplemental Figure S2: Top view of Petri dishes containing coconut coir and cricket feed positioned at the bottom of the cage. Please click here to download this File.
Supplemental Figure S3: Crickets being transferred from the bottom of a cage into a new cage by slowly tilting as described in steps 2.2.11-2.2.13. Please click here to download this File.
Supplemental Figure S4: Cages containing crickets positioned on lighted rearing racks. Please click here to download this File.
Supplemental Table S1: (1) Nutritional analyses of commercial feed, (2) Manufacturer's list of feed ingredients. Please click here to download this Table.
Discussion
The simplicity of this approach to cricket rearing can benefit a range of research areas and represents a generic template for successful cricket husbandry, easily adaptable to a variety of experimental needs. Compared to several other studies of G. bimaculatus, the individual body adult size is smaller and maturation is slower14, which we attribute to sub-optimal rearing temperature imposed on us by circumstance. The methods described above have been used and refined over the course of 2 years. Robust cultures have been maintained without evidence of problems sometimes observed in commercial cricket farming, including widespread mortality from pathogens with classical clinical signs (e.g., internal liquification due to densoviruses in Acheta domesticus) or excessive cannibalism15. It is likely that the lack of cricket introductions following colony establishment greatly reduced the likelihood of disease burden.
Preventing crowding is important to ensure cricket health. Wild G. bimaculatus are solitary, and males defend their territories through aggressive displays and fighting16. Successful captive care requires keeping colony density within an appropriate range to reduce antagonistic behavior and overall stress responses13. This is accomplished by providing crickets with abundant within-cage refuge and by thinning breeding stocks of crickets to 150 individuals per 29.3 L cage at 20 days after hatching when they reach an average size of 0.01 g, during the third or fourth juvenile instar. This rearing density is identical with that used in the G. bimaculatus feed optimization trials of Sorjonen et al.14. A consideration of particular relevance during the transfer of crickets from one container to another is the high degree of escape risk. Secondary containment, controlled movements, preparation to apprehend escapees, and vigilance are crucial tools to prevent cricket escape during this process. Such measures reflect the United States Department of Agriculture designation of Gryllus spp. crickets as potential crop pests, requiring federal and state permits for rearing in the United States17.
Environmental controls and feed quality during egg and early nymphal stages are important to the health of all captive cricket cultures, including G. bimaculatus. To lay viable eggs, female G. bimaculatus require a moist substrate in which to oviposit18. Coconut coir is widely used in the commercial cricket production industry as a medium for oviposition. These methods rely on coconut coir moistened with DI water as a substrate for both oviposition and hydration of crickets throughout their life cycle. Similarly, the use of damp paper towels in juvenile cages to absorb excess water droplets and provide humidity gradients within the natal environment has proven highly effective in reducing the number of <1-week-old crickets succumbing to either dehydration or drowning, as indicated by the presence or absence of deceased juvenile crickets in cage bottoms. Juvenile nutrition is known to play an outsized role in predicting successful growth performance in crickets. Ensuring that fresh full-nutrition feed is of a particle size suitable for <0.01 g crickets will lead to higher survivorship, as younger crickets will be more susceptible to the impacts of variability in feed quality19.
The commercially available cricket feed used in this study was selected due to its widespread use in the North American cricket farming industry. First, from personal communications with three commercial cricket farmers, two in the Upper Midwest US, and one in the Southern US it is clear that this feed (Mazuri) is widely applied in the edible insects industry to-date. Cricket farmers find it amenable for desirable growth performance, fecundity, development, and weight gain metrics. Second, for technicians tasked with administering feed to large numbers of crickets in laboratory settings, utilizing one powdered pre-mixed feed throughout the insects' lifespan is convenient. Third, protein demand is known to be an important factor in cricket development and although many other specific nutritional needs for G. bimaculatus are not fully understood, this preformulated mix contains a crude protein percentage, which falls within the reported optimum range of 22%-30%14,20.
Space in incubators is often limited. Cricket farms typically start their early-instar crickets in incubators and transfer more mature stock to open-air environments, where facility-wide air handling systems regulate temperature and humidity. For these reasons, these methods are designed to emulate such arrangements on a smaller scale. After 20 days inside the incubator, the density is reduced, and crickets are transferred either to experimental treatments or to ambient conditions for use as breeding stock. When air handling systems function properly, rearing facility temperature should be a stable 27 ± 1 °C with relative humidity between 20% and 25%. Crickets are allowed ad libitum access to water and feed. The feed referenced throughout these methods is Mazuri Cricket Feed used widely by cricket farmers in North America. For full nutritional analysis, see Supplemental TableS1.
Per the methods of Donoughe and Extavour (2016), cotton wool may be used in lieu of coconut coir as an oviposition medium or as a casing material atop the coir to prevent particles of frass or feed from contaminating the surface of the oviposition medium18. They recommend a thin layer of cotton wool be placed over the substrate during the oviposition period and subsequently removed once oviposition is complete, along with the accumulated frass and detritus. Although data measuring the impact of substrate contamination on egg viability or cricket development are not available, the protocols outlined here yield satisfactory results in both production of juvenile crickets and growth. This may be attributable to the putative antimicrobial qualities of coconut coir and is grounds for future research in the arena of edible insect production21.
Due to a malfunction of the unit regulating the temperature of the cricket rearing facility in which these methods were developed, the trial for which we are reporting data was conducted at 25 °C at 20% relative humidity, which is 2° cooler than these protocols dictate. Furthermore, these narrowly feed-based research aims result in limited availability of data on certain metrics of interest such as fecundity, endocrine responses, pathogen load, and gene expression. Once breeding crickets were consistently producing abundant viable cricket eggs and juvenile mortality was observed to be negligible, efforts were focused primarily on experimentation directly relevant to research questions. Thus, this report offers only anecdotal accounts of the long-term growth performance impacts of these methods across >10 generations. Finally, the use of plant-derived materials such as coir and cardboard in experimental cages likely leads to incidental ingestion by crickets. This is acceptable within the design of these studies but may compromise the validity of study designs where findings rely on precise measurements of total ingested biomass.
The protocol described here is intended to be both basic and thorough, with clear and easy-to-follow steps for feasibly rearing crickets in a laboratory setting fed with a commercially available standard feed. Utilizing such a standardized procedure with optimal cleaning, stocking density, and environmental controls allows for maintenance of uniform and healthy cricket colonies long-term; moreover, it will contribute to the growing research on G. bimaculatus as a farmable edible insect with implications for human health. It may also be useful for studies on insect physiology, growth optimization, and genetics.
Declarações
The authors have nothing to disclose.
Acknowledgements
Funding for this project was made possible through University of Wisconsin-Madison internal grants. Sincerest thanks to Kevin Bachhuber of Bachhuber Consulting Inc. for access to his unpublished guide for commercial cricket rearing and to Michael Bartlett Smith for his assistance in refining and troubleshooting these methods.
Materials
| 31-qt (29.3 L) Snap-lid tote bin with lid | HOMZ | 3430CLBL | Used to house breeding stock |
| 3-tier/12-tray Grow Light Stand | Fischer Scientific | NC1938548 | |
| 50-gal (189.27L) tote bin with lid | Sterilite | #14796603 | Used as secondary containment when handling crickets |
| 50 mL polypropylene graduated cylinder | Fischer Scientific | S95171 | |
| 7.5-qt (7.1 L) snap-lid tote bin with lid | HOMZ | 3410CLBL | Used to house exprimental stock |
| Accuris 500 g x 0.01 g Balance | Manufactured by Accuris, a subsidieary of Benchmark Scientific | W3300-500 | Purchased from Dot Scientific through University of Wisconsin system purchasing service "ShopUW+" |
| Ace Premier 1 Inch Flat Chip Brush | Ace Hardware | #1803261 | |
| Bel-Art SP Scienceware deionized water wash bottle | Fischer Scientific | 03-421-160 | |
| Bright aluminum window screen | Phifer | UNSPSC# 11162108 | Mesh size 18 x 16" |
| Clear Disposable Plastic Portion Cups 5.5 oz w/ lids | Wal-Mart | N/A | |
| Deionized water | |||
| Diablo 4-4/8" x 13 TPI Ultra Fine Finish Bi-Metal Jigsaw Blade | Home Depot | #313114935 | |
| Egg Filler Flats-Paper, 12 x 12" | Uline | S-5189 | |
| Fisherbrand Petri Dishes with Clear Lid 100 x 15mm | Fischer Scientific | FB0875714 | |
| Fisherbrand Petri Dishes with Clear Lid 60 x 15mm | Fischer Scientific | FB0875713A | |
| Georgia-Pacific Envision Brown Paper Towels | Home Depot | #205675843 | |
| Infinity Tough Guy high performance hot-melt glue sticks | Infinity Bond | Infinity IM-Tough-Guy-12 | |
| Mazuri Cricket Diet | Land O' Lakes International | SKU# 3002219-105 | |
| Stanley TimeIt Twin 2-outlet Grounded Mechanical 24 Hour Timer | Wal-Mart | N/A | |
| Vermont Organics Reclamation Soil 11 lb Coir Block | Home Depot | #300679904 |
Referências
- Hales, K. G., Korey, C. A., Larracuente, A. M., Roberts, D. M. Genetics on the fly: a primer on the Drosophila model system. Genética. 201 (3), 815-842 (2015).
- Merkel, G. The effects of temperature and food quality on the larval development of Gryllus bimaculatus (Orthoptera, Gryllidae). Oecologia. 30 (2), 129-140 (1977).
- Bateman, P. W. Mate preference for novel partners in the cricket Gryllus bimaculatus. Ecological Entomology. 23 (4), 473-475 (1998).
- Mito, T., Noji, S. The two-spotted cricket Gryllus bimaculatus: An emerging model for developmental and regeneration studies. Cold Spring Harbor Protocols. 2008, (2008).
- Ylla, G., et al. Cricket genomes: the genomes of future food. BioRxiv. , (2020).
- Halloran, A., Roos, N., Hanboonsong, Y. Cricket farming as a livelihood strategy in Thailand. Geographical Journal. 183 (1), 112-124 (2017).
- Wade, M., Hoelle, J. A review of edible insect industrialization: scales of production and implications for sustainability. Environmental Research Letters. 15, 123013 (2020).
- . Studies on the influence of different diets and rearing conditions on the development and growth of the two-spotted cricket Gryllus bimaculatus de Greer Available from: https://epub.uni-bayreuth.de/310/1/Diss.pdf (2011)
- Ngonga, C. A., Gor, C. O., Okuto, E. A., Ayieko, M. A. Growth performance of Acheta domesticus and Gryllus bimaculatus production reared under improvised cage system for increased returns and food security. Journal of Insects as Food and Feed. 7, 301-310 (2021).
- Behrens, W., Hoffmann, K. -. H., Kempa, S., Gäßler, S., Merkel-Wallner, G. Effects of diurnal thermoperiods and quickly oscillating temperatures on the development and reproduction of crickets, Gryllus bimaculatus. Oecologia. 59 (2-3), 279-287 (1983).
- Collavo, A., Paoletti, M. G., et al. Housecricket smallscale farming. Ecological implications of minilivestock. Potential of insects, rodents, frogs and snails. , (2005).
- Simmons, L. W. Competition between larvae of the field cricket, Gryllus bimaculatus (Orthoptera: Gryllidae) and its effects on some life-history components of fitness. Journal of Animal Ecology. 56, 1015-1027 (1987).
- Sorjonen, J. M., et al. The plant-based by-product diets for the mass-rearing of Acheta domesticus and Gryllus bimaculatus. PLOS ONE. 14 (6), 0218830 (2019).
- Maciel-Vergara, G., Jensen, A. B., Lecocq, A., Eilenberg, J. Diseases in edible insect rearing systems. Journal of Insects as Food and Feed. 7 (5), 1-18 (2021).
- Alexander, R. D. Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae). Behaviour. , 130-223 (1961).
- Pet food, fish bait, and animal feed. USDA APHIS Available from: https://www.aphis.usda.gov/aphis/ourfocus/planhealth/import-information/permits/plant-pests/sa_animalfeed/ct_petfood_fishbait_animalfeed (2022)
- Donoughe, S., Extavour, C. G. Embryonic development of the cricket Gryllus bimaculatus. Biologia do Desenvolvimento. 411 (1), 140-156 (2016).
- Dobermann, D., Michaelson, L., Field, L. M. The effect of an initial high-quality feeding regime on the survival of Gryllus bimaculatus (black cricket) on bio-waste. Journal of Insects as Food and Feed. 5 (2), 1-8 (2018).
- Lundy, M. E., Parrella, M. P. Crickets are not a free lunch: Protein capture from scalable organic side-streams via high-density populations of Acheta domesticus. PLOS ONE. 10, 0118785 (2015).
- Mazaya, G., Karseno, K., Yanto, T. Antimicrobial and phytochemical activity of coconut shell extracts. Turkish Journal of Agriculture – Food Science and Technology. 8 (5), 1090-1097 (2020).

