在啮齿动物中进行体内和离体电阻抗肌图
Summary
本文详细介绍了如何对啮齿动物腓肠肌进行 体内 (使用表面和针状电极阵列)和 离体 (使用介电电池)电阻抗肌图。它将在小鼠和大鼠中演示该技术,并详细说明可用的修改(即肥胖动物,幼崽)。
Abstract
电阻抗肌图(EIM)是一种方便的技术,可用于临床前和临床研究,以评估肌肉组织的健康和疾病。EIM是通过在一系列频率(即从1 kHz到10 MHz)上向感兴趣的肌肉施加低强度,定向聚焦的电流并记录产生的电压来获得的。由此,可以获得几个标准阻抗分量,包括电抗、电阻和相位。当对切除的肌肉进行 离体 测量时,还可以计算组织的固有被动电特性,即电导率和相对介电常数。EIM已被广泛用于动物和人类,以诊断和跟踪各种疾病的肌肉改变,与简单的废用性萎缩有关,或作为治疗干预的措施。在临床上,EIM提供了跟踪疾病随时间发展并评估治疗干预影响的潜力,从而提供了缩短临床试验持续时间和减少样本量要求的机会。由于EIM可以在活体动物模型和人类中进行无创或微创,因此具有作为新型转化工具的潜力,可实现临床前和临床开发。本文提供了有关如何在小鼠和大鼠中进行 体内 和 离体 EIM测量的分步说明,包括使该技术适应特定条件的方法,例如用于幼崽或肥胖动物。
Introduction
电阻抗肌图(EIM)提供了一种评估肌肉状况的强大方法,有可能诊断神经肌肉疾病,跟踪疾病进展和评估对治疗的反应1,2,3。它可以类似地应用于动物疾病模型和人类,允许从临床前到临床研究的相对无缝转换。使用四个线性放置的电极可以轻松获得EIM测量,其中两个外部电极在频率范围内(通常在1 kHz和大约2 MHz之间)施加无痛的弱电流,两个内部电极记录产生的电压1。从这些电压中,可以获得组织的阻抗特性,包括电阻(R),这是电流通过组织的难度的量度,以及组织的电抗(X)或“可充电性”,与组织的电荷存储能力(电容)有关。根据电抗和电阻,相位角(θ)通过以下公式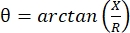 计算:,提供单个求和阻抗测量。这种测量可以使用任何多频生物阻抗设备获得。由于肌纤维本质上是长圆柱体,肌肉组织也是高度各向异性的,电流更容易沿着纤维流动而不是穿过它们4,5.因此,EIM通常在两个方向上进行:阵列沿着纤维放置,使电流平行于它们,并穿过肌肉,使电流垂直于它们流动。此外,在离体测量中,在阻抗测量池中测量已知体积的组织时,可以得出肌肉的固有电特性(即电导率和相对介电常数)6。
计算:,提供单个求和阻抗测量。这种测量可以使用任何多频生物阻抗设备获得。由于肌纤维本质上是长圆柱体,肌肉组织也是高度各向异性的,电流更容易沿着纤维流动而不是穿过它们4,5.因此,EIM通常在两个方向上进行:阵列沿着纤维放置,使电流平行于它们,并穿过肌肉,使电流垂直于它们流动。此外,在离体测量中,在阻抗测量池中测量已知体积的组织时,可以得出肌肉的固有电特性(即电导率和相对介电常数)6。
术语“神经肌肉疾病”定义了导致结构肌肉改变和功能障碍的各种原发性和继发性疾病。这包括肌萎缩侧索硬化症和各种形式的肌肉萎缩症,以及与衰老(例如肌肉减少症)、废用性萎缩(例如由于长时间卧床或微重力)甚至损伤相关的简单变化7。虽然病因很多,可能起源于运动神经元、神经、神经肌肉接头或肌肉本身,但EIM可用于检测由于许多这些过程引起的肌肉早期变化,并跟踪治疗的进展或反应。例如,在杜氏肌营养不良症 (DMD) 患者中,EIM 已被证明可以检测疾病进展和对皮质类固醇的反应8。最近的工作还表明,EIM对不同的废弃状态很敏感,包括部分重力9,就像在月球或火星上经历的那样,以及老化的影响10,11。最后,通过将预测和机器学习算法应用于每次测量获得的数据集(多频率和方向依赖性数据),可以推断组织的组织学方面,包括肌纤维大小12,13、炎症变化和水肿 14,以及结缔组织和脂肪含量15,16。
其他几种非侵入性或微创方法也用于评估人类和动物的肌肉健康,包括针状肌电图17 和成像技术,如磁共振成像,计算机断层扫描和超声18,19。然而,与这些技术相比,EIM显示出明显的优势。例如,肌电图仅记录肌纤维膜的主动电特性,而不记录被动特性,因此无法提供对肌肉组成或结构的真实评估。在某种程度上,成像方法与EIM的关系更密切,因为它们也提供了有关组织结构和组成的信息。但从某种意义上说,它们提供了太多的数据,需要详细的图像分割和专家分析,而不仅仅是提供定量输出。此外,鉴于其复杂性,成像技术也受到所用硬件和软件细节的极大影响,理想情况下需要使用相同的系统,以便可以比较数据集。相比之下,EIM更简单的事实意味着它受这些技术问题的影响较小,并且不需要任何形式的图像处理或专家分析。
以下协议演示了如何使用非侵入性(表面阵列)和微创(皮下针阵列)技术以及在新鲜切除的肌肉上进行体外 EIM,在大鼠和小鼠中进行体内 EIM。
Protocol
Representative Results
Discussion
本文提供了在 啮 齿动物体内和 体外进行EIM的基本方法。为了获得可靠的测量结果,执行一系列步骤至关重要。首先,需要正确识别感兴趣的肌肉,因为每块肌肉对疾病、治疗和病理都有不同的反应。必须注意,在一块肌肉(例如腓肠肌)上获得的数据不会提供与另一块肌肉(例如胫骨前肌)相同的信息。其次,需要仔细选择最佳的电极阵列来进行阻抗测量。虽然每种阵列类型都有?…
Declarações
The authors have nothing to disclose.
Acknowledgements
这项工作得到了查理基金和NIH R01NS055099的支持。
Materials
| 3D Printer | Formlabs Inc. | Form 2 Desktop | 3D printer |
| 3D Printer | Shenzhen Creality 3D Technology Co. LTD | Creality Ender 3 V2 | 3D printer |
| 3M Micropore surgical tape | Fisher | 19-027761 and 19-061655 | models 1530-0 and 1530-1 |
| 3M TRANSPORE surgical tape | Fisher | 18-999-380 and 18-999-381 | models 1527-0 and 1527-1 |
| Connector header vertical 10 POS 1 mm spacing | Digi-Key (Sullins connector solution) | S9214-ND (SMH100-LPSE-S10-ST-BK) | Plastic spacer 1 mm holes for the rat in vivo array displayed in Figure 2A |
| Cotton-tipped applicators | Fisher | 22-363-172 | |
| Dental Wax | Fisher | NC9377103 | |
| Depilatory agent | NAIR | NA | hair remover lotion with softening baby oil |
| Dumont #7b Forceps | Fine Science Tools | No. 11270-20 | Used for dissection, Style: #7b, Tip Shape: Curved, Tips: Standard, Tip Dimensions: 0.17 mm x 0.1 mm, Alloy/Material: Inox, Length: 11 cm |
| Electronic Digital Caliper | Fisher | 14-648-17 | Used to measure out the dimensions of the Gastrocnemius muscle |
| Epoxy adhesive dual cartridge 4 min work life | Devcon | series 14265, model 2217 | Glue used in the rat in vivo array displayed in Figure 2A |
| Ex vivo dielectric impedance cell | Custom | NA | Dielectric cells were 3D printed in the Rutkove laboratory |
| Graefe Forceps | Fine Science Tools | No. 11051-10 | Used for muscle to place and adjust, Length: 10 cm, Tip Shape: Curved, Tips: Serrated, Tip Width: 0.8 mm, Tip Dimensions: 0.8 mm x 0.7 mm, Alloy/Material |
| Hair clipper | Amazon | NA | Wahl professional animal BravMini+ |
| Impedance Animal Device | Myolex | EIM1103 | mView system – investigational electrical impedance myography device for use in animal research |
| In vivo needle arrays | Custom | NA | Custom arrays using 27 G subdermal needles from Ambu. The construction was finalized using a 3D printer in the Rutkove laboratory |
| In vivo surface array | Custom | NA | The in vivo surface array was printed and assembled in the Rutkove laboratory |
| Isoflurane | Patterson Veterinary Supplies | 07-893-8441 (NDC: 46066-755-04) | Pivetal – 250 mL bottle |
| Non-woven gauze | Fisher | 22-028-559 | 2 x 2 inch |
| Polystyrene Weighing Dishes | Fisher | S67090A | Dimensions (L x W x H): 88.9 mm x 88.9 mm x 25.4 mm |
| Razor Blades | Fisher | 12-640 | Used to cut muscle to right dimensions, Single-edge carbon steel blades |
| Student Fine Scissors | Fine Science Tools | No. 91460-11 | Used for dissection, Tips: Sharp-Sharp, Alloy/Material: Student Stainless Steel, Serrated: No, Tip Shape: Straight, Cutting Edge: 20 mm, Length: 11.5 cm, Feature: Student Quality |
| Subdermal needles 27 G Neuroline | Ambu | 745 12-50/24 | Needles used in the rat in vivo array displayed in Figure 2A |
| Surgical Scissors – Sharp | Fine Science Tools | No. 14002-13 | Used to cut skin, Tips: Sharp-Sharp, Alloy/Material: Stainless Steel, Serrated: No, Tip Shape: Straight, Cutting Edge: 42 mm, Length: 13 cm |
| TECA ELITE monopolar needle electrodes | Natus | 902-DMG50-S | 0.46 mm diameter (26 G). Blue hub |
| Teknova 0.9% saline solution | Fisher | S5815 | 1000 mL sterile |
Referências
- Rutkove, S. B., Sanchez, B. Electrical impedance methods in neuromuscular assessment: An overview. Cold Spring Harbor Perspectives in Medicine. 9 (10), 034405 (2019).
- Rutkove, S. B. Electrical impedance myography: Background, Current State, and Future Directions. Muscle & Nerve. 40, 936-946 (2009).
- Sanchez, B., Rutkove, S. B. Present uses, future applications, and technical underpinnings of electrical impedance myography. Current Neurology and Neuroscience Reports. 17 (11), 86 (2017).
- Garmirian, L. P., Chin, A. B., Rutkove, S. B. Discriminating neurogenic from myopathic disease via measurement of muscle anisotropy. Muscle & Nerve. 39 (1), 16-24 (2009).
- Rutkove, S. B., et al. Loss of electrical anisotropy is an unrecognized feature of dystrophic muscle that may serve as a convenient index of disease status. Clinical Neurophysiology. 127 (12), 3546-3551 (2016).
- Wang, L. L., et al. Assessment of alterations in the electrical impedance of muscle after experimental nerve injury via finite-element analysis. IEEE Transactions on Biomedical Engineering. 58 (6), 1585-1591 (2011).
- Katirji, B., Ruff, R. L., Kaminski, H. J. . Neuromuscular Disorders in Clinical Practice. , (2014).
- Rutkove, S. B., et al. Electrical impedance myography for assessment of Duchenne muscular dystrophy. Annals of Neurology. 81 (5), 622-632 (2017).
- Semple, C., et al. Using electrical impedance myography as a biomarker of muscle deconditioning in rats exposed to micro- and partial-gravity analogs. Frontiers in Physiology. 11, 557796 (2020).
- Kortman, H. G. J., Wilder, S. C., Geisbush, T. R., Narayanaswami, P., Rutkove, S. B. Age- and gender-associated differences in electrical impedance values of skeletal muscle. Physiological Measurement. 34 (12), 1611-1622 (2013).
- Clark, B. C., Rutkove, S., Lupton, E. C., Padilla, C. J., Arnold, W. D. Potential utility of electrical impedance myography in evaluating age-related skeletal muscle function deficits. Frontiers in Physiology. 12, 666964 (2021).
- Kapur, K., et al. Predicting myofiber size with electrical impedance myography: A study in immature mice. Muscle and Nerve. 58 (1), 106-113 (2018).
- Kapur, K., Nagy, J. A., Taylor, R. S., Sanchez, B., Rutkove, S. B. Estimating myofiber size with electrical impedance myography: a study in amyotrophic lateral sclerosis mice. Muscle and Nerve. 58 (5), 713-717 (2018).
- Mortreux, M., Semple, C., Riveros, D., Nagy, J. A., Rutkove, S. B. Electrical impedance myography for the detection of muscle inflammation induced by λ-carrageenan. PLoS ONE. 14 (10), 0223265 (2019).
- Pandeya, S. R., et al. Predicting myofiber cross-sectional area and triglyceride content with electrical impedance myography: A study in db/db mice. Muscle and Nerve. 63 (1), 127-140 (2021).
- Pandeya, S. R., et al. Estimating myofiber cross-sectional area and connective tissue deposition with electrical impedance myography: A study in D2-mdx mice. Muscle & Nerve. 63 (6), 941-950 (2021).
- Stålberg, E., et al. Standards for quantification of EMG and neurography. Clinical Neurophysiology. 130 (9), 1688-1729 (2019).
- Theodorou, D. J., Theodorou, S. J., Kakitsubata, Y. Skeletal muscle disease: Patterns of MRI appearances. British Journal of Radiology. 85 (1020), 1298-1308 (2012).
- Simon, N. G., Noto, Y., Zaidman, C. M. Skeletal muscle imaging in neuromuscular disease. Journal of Clinical Neuroscience. 33, 1-10 (2016).
- Kwon, H., Rutkove, S. B., Sanchez, B. Recording characteristics of electrical impedance myography needle electrodes. Physiological Measurement. 38 (9), 1748-1765 (2017).
- Kwon, H., Di Cristina, J. F., Rutkove, S. B., Sanchez, B. Recording characteristics of electrical impedance-electromyography needle electrodes. Physiological Measurement. 39 (5), 055005 (2018).
- Rutkove, S. B., et al. Characterizing spinal muscular atrophy with electrical impedance myography. Muscle and Nerve. 42 (6), 915-921 (2010).
- Schwartz, S., et al. Optimizing electrical impedance myography measurements by using a multifrequency ratio: A study in Duchenne muscular dystrophy. Clinical Neurophysiology. 126 (1), 202-208 (2015).
- Li, J., Pacheck, A., Sanchez, B., Rutkove, S. B. Single and modeled multifrequency electrical impedance myography parameters and their relationship to force production in the ALS SOD1G93A mouse. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 17 (5-6), 397-403 (2016).
- Hu, N., et al. Antisense oligonucleotide and adjuvant exercise therapy reverse fatigue in old mice with myotonic dystrophy. Molecular Therapy – Nucleic Acids. 23, 393-405 (2021).
- Sanchez, B., et al. Non-invasive assessment of muscle injury in healthy and dystrophic animals with electrical impedance myography. Muscle & Nerve. 56 (6), 85-94 (2017).
- Sanchez, B., Li, J., Bragos, R., Rutkove, S. B. Differentiation of the intracellular structure of slow- versus fast-twitch muscle fibers through evaluation of the dielectric properties of tissue. Physics in Medicine and Biology. 59 (10), 2369-2380 (2014).
- Shefner, J. M., et al. Assessing ALS progression with a dedicated electrical impedance myography system. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration. 19 (7-8), 555-561 (2018).
- Lungu, C., et al. Quantifying muscle asymmetries in cervical dystonia with electrical impedance: a preliminary assessment. Clinical Neurophysiology. 122 (5), 1027-1031 (2011).
- Wang, Y., et al. Electrical impedance myography for assessing paraspinal muscles of patients with low back pain. Journal of Electrical Bioimpedance. 10 (1), 103-109 (2019).
- Leitner, M. L., et al. Electrical impedance myography for reducing sample size in Duchenne muscular dystrophy trials. Annals of Clinical and Translational Neurology. 7 (1), 4-14 (2020).
- Rutkove, S. B., et al. Improved ALS clinical trials through frequent at-home self-assessment: a proof of concept study. Annals of Clinical and Translational Neurology. 7 (7), 1148-1157 (2020).

