3D Printing and In Situ Surface Modification via Type I Photoinitiated Reversible Addition-Fragmentation Chain Transfer Polymerization
Summary
The present protocol describes the digital light processing-based 3D printing of polymeric materials using type I photoinitiated reversible addition-fragmentation chain transfer polymerization and the subsequent in situ material post-functionalization via surface-mediated polymerization. Photoinduced 3D printing provides materials with independently tailored and spatially controlled bulk and interfacial properties.
Abstract
3D printing provides facile access to geometrically complex materials. However, these materials have intrinsically linked bulk and interfacial properties dependent on the chemical composition of the resin. In the current work, 3D printed materials are post-functionalized using the 3D printer hardware via a secondary surface-initiated polymerization process, thus providing independent control over the bulk and interfacial material properties. This process begins with preparing liquid resins, which contain a monofunctional monomer, a crosslinking multifunctional monomer, a photochemically labile species that enables initiation of polymerization, and critically, a thiocarbonylthio compound which facilitates reversible addition-fragmentation chain transfer (RAFT) polymerization. The thiocarbonylthio compound, known commonly as a RAFT agent, mediates the chain growth polymerization process and provides polymeric materials with more homogeneous network structures. The liquid resin is cured in a layer-by-layer fashion using a commercially available digital light processing 3D printer to give three-dimensional materials having spatially controlled geometries. The initial resin is removed and replaced with a new mixture containing functional monomers and photoinitiating species. The 3D printed material is then exposed to light from the 3D printer in the presence of the new functional monomer mixture. This allows photoinduced surface-initiated polymerization to occur from the latent RAFT agent groups on the surface of the 3D printed material. Given the chemical flexibility of both resins, this process allows a wide range of 3D printed materials to be produced with tailorable bulk and interfacial properties.
Introduction
Additive manufacturing and 3D printing have revolutionized material manufacturing by providing more efficient and facile routes for the fabrication of geometrically complex materials1. Apart from the enhanced design freedoms in 3D printing, these technologies produce less waste than traditional subtractive manufacturing processes via the judicious use of precursor materials in a layer-by-layer manufacturing process. Since the 1980s, a wide range of different 3D printing techniques has been developed to fabricate polymeric, metal, and ceramic components1. The most commonly employed methods include extrusion-based 3D printing such as fused filament fabrication and direct ink writing techniques2, sintering techniques such as selective laser sintering3, as well as resin-based photoinduced 3D printing techniques such as laser and projection-based stereolithography and masked digital light processing techniques4. Among the many 3D printing techniques in existence today, photoinduced 3D printing techniques provide some advantages compared to other methods, including higher resolution and faster printing speeds, as well as the ability to perform solidification of the liquid resin at room temperature, which opens the possibility to advanced biomaterial 3D printing4,5,6,7,8,9.
While these advantages have allowed the widespread adoption of 3D printing in many fields, the limited ability to independently tailor the 3D printed material properties restricts future applications10. In particular, the inability to easily tailor the bulk mechanical properties independently of the interfacial properties limits applications such as implants, which require finely tailored biocompatible surfaces and often vastly differing bulk properties, as well as antifouling and antibacterial surfaces, sensor materials, and other smart materials11,12,13. Researchers have proposed surface modification of 3D printed materials to overcome these issues to provide more independently tailorable bulk and interfacial properties10,14,15.
Recently, our group developed a photoinduced 3D printing process that exploits reversible addition-fragmentation chain transfer (RAFT) polymerization to mediate network polymer synthesis15,16. RAFT polymerization is a type of reversible deactivation radical polymerization that provides a high degree of control over the polymerization process and allows for the production of macromolecular materials with finely tuned molecular weights and topologies, and broad chemical scope17,18,19. Notably, the thiocarbonylthio compounds, or RAFT agents, used during RAFT polymerization are retained after polymerization. They can thus be reactivated to modify further the chemical and physical properties of the macromolecular material. Thus, after 3D printing, these dormant RAFT agents on the surfaces of the 3D printed material can be reactivated in the presence of functional monomers to provide tailored material surfaces20,21,22,23,24,25,26. The secondary surface polymerization dictates the interfacial material properties and can be performed in a spatially controlled fashion via photochemical initiation.
The present protocol describes a method for 3D printing polymeric materials via a photoinduced RAFT polymerization process and the subsequent in situ surface modification to modulate the interfacial properties independently of the bulk material mechanical properties. Compared to previous 3D printing and surface modification approaches, the current protocol does not require deoxygenation or other stringent conditions and is thus highly accessible for non-specialists. Furthermore, the use of 3D printing hardware to perform both the initial material fabrication and the surface post-functionalization provides spatial control over the material properties and can be performed without the tedious alignment of several different photomasks to make complex patterns.
Protocol
1. Preparation of 3D printing program and 3D printer
- Design the digital model for 3D printing following the steps below.
- Open a computer-assisted design program (see Table of Materials).
- In the x-y plane, create a rectangle centered on the origin having dimensions of 80 mm x 40 mm, then extrude along the positive z-axis for 1.5 mm to make a solid rectangular prism, called the base object.
- Above the base object, i.e., at z = 1.5 mm, draw the desired surface patterns (in this case, two yin-yang symbols) on the surface of the rectangular prism.
- Extrude the surface patterns in selected regions 0.05 mm along the positive z-axis to create a slightly raised pattern relative to the base object.
- Export the 3D model to provide a stereolithography file with .STL file extension.
NOTE: In this work, dog-bone-shaped specimens were designed27. For other desired models to be printed, follow steps 1.1.1-1.1.5. - Open a 3D printer slicing program (see Table of Materials) to enable single-layer settings.
- Open the converted .STL files from the computer hard drive by clicking on File > Open then navigating to the saved .STL file.
- Arrange the 3D models on the build platform using the "Model Rotate" and "Model Move" buttons to fit at least 1 mm between all objects on the build stage.
- By entering text in the entry field boxes in the right-hand panel, change the parameters as mentioned in Table 1.
- Click on the blue Slice button in the bottom left-hand corner and save it as a slice file with an extension of. PWS or other 3D printer readable sliced file.
- Click on the Visualizar button once the pop-up menu appears and navigate through the sliced layers using the scroll bar on the right-hand side. Take careful note of the layer numbers for the last base layer (layer 29 in this case) and the surface pattern layer (30 in this case).
NOTE: The first printed layer is "layer 0" not "layer 1". - In the right-hand panel, select Single-layer settings, then expand the drop-down menu.
- Change the "Exposure Time (s)" for only the surface layer (layer 30) to 180 s, leaving all other layer exposure times as the default value.
- Click on Save button in the top left corner to save the sliced file to a USB.
- Prepare the 3D printer.
- Insert the USB containing the sliced file into the 3D printer (see Table of Materials).
- Before 3D printing, level the build stage and calibrate the z-axis position to the z = 0 by following the specific 3D printer method (manual or automatic calibration following the 3D printer manual).
- Inspect the film of the 3D printer vat to ensure a smooth and clean surface free of defects.
- If the vat film appears damaged, replace it according to the manufacturer's protocol.
2. Preparation of resins
NOTE: Resins are categorized as "Bulk Resin" for the resin used to 3D print the original material (base substrate) and "Surface Resin" for the solution used to perform the surface functionalization (surface pattern).
- Prepare the Bulk Resin.
- For preparing the bulk resin, weigh 0.36 g of 2-(n-butylthiocarbonothioylthio) propanoic acid (BTPA) into a clean 50 mL amber vial.
- Add 13.63 mL of poly (ethylene glycol) diacrylate average Mn 250 (PEGDA) to the amber vial using a micropipette.
- Add 14.94 mL of N, N-dimethylacrylamide (DMAm) to the amber vial using a micropipette.
- In a separate 20 mL clean glass vial covered with aluminum foil, add 0.53 g of diphenyl (2,4,6-trimethyl benzoyl) phosphine oxide (TPO).
- Using a micropipette, add 10 mL of DMAm to the 20 mL glass vial containing the TPO and seal the vial using the cap.
- Thoroughly homogenize the solution of TPO and DMAm by mixing using a vortex mixer for 10 s and then using a standard laboratory sonic bath (~40 kHz) to sonicate the mixture for 1 min at room temperature (Figure 1C, left).
- Using a glass pipette and rubber pipette bulb, transfer the solution from the 20 mL glass vial to the 50 mL amber vial and seal the vial with a cap and moldable plastic film.
- Gently shake the 50 mL amber vial and then place the vial in a sonic bath for 2 min at room temperature to ensure the mixture is homogeneous (Figure 1C, second from left).
- Place the sealed amber vial filled with the bulk resin in a fume hood for later use.
- Prepare the Surface Resin.
- For preparing the surface resin, weigh 0.50 g of TPO into a clean 50 mL amber vial.
- Using a micropipette, add 3.56 mL of DMAm and 11.98 mL of N, N-dimethylformamide (DMF) to the 50 mL amber vial and seal the vial with a cap moldable plastic film.
- Gently shake the sealed amber vial and sonicate for 1 min at room temperature using a standard laboratory sonic bath (~40 kHz).
- To a clean 20 mL vial covered with foil, add 0.29 g 1-pyrenemethyl methacrylate (PyMMA).
- Add 10 mL of DMF to the 20 mL vial and seal the vial with a cap using a micropipette.
- Gently shake the 20 mL glass vial and sonicate in increments of 1 min at room temperature using a standard laboratory sonic bath, visually inspecting between cycles until the PyMMA appears to be dissolved entirely (Figure 1C, third and fourth from left).
- Using a glass pipette and rubber pipette bulb, transfer the solution from the 20 mL glass vial to the 50 mL amber vial.
- Gently shake the 50 mL amber vial and then place the vial in a sonic bath for 2 min at room temperature to ensure the mixture is homogeneous (Figure 1C, right and second from right).
- Place the sealed amber vial filled with the bulk resin in a fume hood for later use.
CAUTION: Some chemicals used in this protocol may cause severe skin and eye irritation and other toxicity to humans and the environment. Ensure safety protocols are followed in line with the safety data sheet and local regulations.
3. 3D printing and surface functionalization
- Perform 3D printing of the base substrate following the steps below.
- Pour the previously prepared bulk resin (step 2.1) into the 3D printer vat (see Table of Materials), ensuring that the solution completely covers the bottom film in the vat without any air bubbles or other inhomogeneities, and then close the 3D printer case.
- Navigate the USB using the 3D printer screen and select the sliced model file by clicking on the triangle Play button to begin the 3D printing process.
- By watching the 3D printer screen, take careful note of the number of layers printed, and pause the printing program by pressing the two vertical lines Pause button during 3D printing of the last layer of the base substrate (layer 29 in this case).
- Remove the entire build stage and gently rinse the build stage and printed material with undenatured 100% ethanol from a wash bottle for 10 s to remove residual bulk resin from the 3D printed material and the build stage.
- Using compressed air, gently dry the 3D printed material and build stage to remove residual ethanol and then reinsert the build stage into the 3D printer.
- Remove the vat from the 3D printer and pour the remaining bulk resin into an amber vial. Store the vial in a cool dark place.
- Using undenatured 100% ethanol from a wash bottle, carefully rinse the vat to remove any residual bulk resin.
- Dry the vat using a stream of compressed air to remove any residual ethanol and reinsert the vat into the 3D printer.
- Perform surface functionalization.
- Pour the previously prepared surface resin (step 2.2) into the 3D printer vat, ensuring that the solution completely covers the bottom film without any air bubbles or other inhomogeneities, and then close the 3D printer case.
- Resume the 3D printing program by clicking on the triangle Play button to allow the predetermined surface patterning to occur.
- Once the printing program has been completed, remove the build stage from the 3D printer and wash for 10 s with undenatured 100% ethanol using a wash bottle to remove residual surface resin from the 3D printed material and the build stage.
- Using compressed air (flow rate, 30 L/min), gently dry the 3D printed material and build stage to remove residual ethanol.
- While still attached to the build stage, post-cure the material by inverting the entire build stage and placing it under 405 nm light for 15 min.
- Gently remove the surface-functionalized 3D printed material from the build stage using a thin metal plate or paint scraper.
- Without further adjustments, analyze the material's mechanical and surface properties.
4. Analysis of 3D printed samples
- Perform the fluorescence analysis.
- Place the 3D printed, surface-functionalized material under a 312 nm UV gas discharge lamp (see Table of Materials) in a dark place, ensuring the surface-functionalized layer is facing up.
- Turn the lamp on to continuously irradiate the surface layer with 312 nm light and observe the fluorescent pattern. Take photographs if required.
NOTE: This is a visual inspection step; time cannot be specified. Irradiation is continuous while observation is occurring. - Place the 3D printed, surface-functionalized material into a Fluorescence imager. Using the provided software, capture digital fluorescence images of the top and bottom surfaces using the Trans-UV (302 nm) gas discharge source (see Table of Materials).
- Perform the tensile property analysis.
- Measure the gauge with and thickness of the dog-bone specimens (in millimeters).
- Place the dog-bone-shaped specimens between the grips of a tensile testing machine, ensuring the 3D printed material is equally placed at a distance specified by the standards document, in this case, 50.3 mm.
- Set the tensile test program; in this case, the lift speed was set to 1.1 mm/min, the number of samples was set at 10 per second.
- Start the program to acquire force (N) vs. travel (mm) data.
- Once the sample gets prepared, stop the machine, and save the data as column-separated data with a .CSV file extension.
- Convert the force (N) data to stress (MPa) by dividing each point of the force column by the gauge area (mm2, obtained by multiplying the gauge width by the gauge thickness).
- Convert the travel data to strain (%) by diving the travel data by the gauge length (50.3 mm) at every point and multiplying each result by 100.
- Calculate toughness (MJ/m3) using the trapezoidal rule to calculate the area under the stress-strain curve.
- Calculate Young's modulus (MPa) by taking the gradient of the stress (MPa) vs. strain (%) curve in the elastic region, in this work from 1%-2% elongation27.
Representative Results
The general procedure for 3D printing and surface functionalization is shown in Figure 1. In this protocol, a network polymer is initially synthesized via a photoinduced RAFT polymerization process15, using a 3D printer to fabricate an object in a layer-by-layer process (Figure 1A). The bulk resin used to form the polymer network contains a photolabile initiating species (TPO), which generates radicals upon exposure to 405 nm light. These radicals can then add to vinyl bonds in the monomer DMAm and the crosslinker PEGDA, which provides a polymer network via a chain-growth polymerization mechanism. The RAFT agent BTPA mediates the network growth via a degenerative chain-transfer mechanism, which provides polymer materials with increased homogeneity28. During the layer-by-layer 3D printing process, a 3D polymer network is formed via photopolymerization for a defined time, called the layer cure time. In this work, the layers were designed to be 50 µm thick, and the layer cure time was 40 s. To ensure the 3D printed material adheres to the 3D printer build stage, the first two layers in the printing process are exposed for a longer time, for 80 s/layer. Once a layer is cured, the build stage rises along the z-axis, allowing the fresh uncured resin to fill the void underneath the 3D printed layers. The build stage lowers into the vat again, and the next layer is cured. The resulting 3D printed object displays the characteristic yellow hue of trithiocarbonate RAFT agents such as BTPA, as visualized in both the bulk resin (Figure 1C, second from left) and the final 3D printed object.
Critically, the trithiocarbonate terminus on the polymer network provides a functional handle from which the surface functionalization can occur. Following the 3D printing of the base substrate, the 3D printing program was paused, and the resin was switched to the surface resin. The surface resin components are shown in Figure 1B. TPO is added to initiate polymerization, while monofunctional vinyl monomers are used for surface functionalization, designed to provide linear polymer chains rather than a crosslinked network. Specifically, the monomers selected in this process are DMAm and the fluorescent PyMMA, which allows the formation of fluorescent polymers from the 3D printed material.
As shown in Figure 2A,B, the designed materials in this protocol include a rectangular prism and several dog-bone-shaped specimens for tensile testing. The general rectangular prism and dog-bone shapes27 are used to print the base substrate, using 30 total layers (layers 0-29 in the 3D printing program) with 50 µm thickness to provide a 1.5 mm thick base substrate. As shown in Figure 2C, the surface pattern is designed to irradiate only the rectangular prism base object in the yin-yang pattern. The surface pattern was designed to have a layer of 50 µm thickness. The layer cure time was increased to 180 s to ensure sufficient polymerization to modify the material surface.
Following 3D printing of the base object and surface functionalization, the objects are post-cured under a 405 nm light source for 15 min. Following post-curing, the materials retained the characteristic yellow hue of the RAFT agent (Figure 3A) and showed well-defined shapes in line with the digital models shown in Figure 2A,B. The 3D printed materials are then removed from the build stage for further analysis. As shown in Figure 3B, the 3D printed and surface functionalized materials are yellow but highly transparent (Figure 3B). The effectiveness of the surface functionalization can be viewed by irradiating the materials under 312 nm light. As shown in Figure 3C,D, the functional materials show no fluorescence in the dark; however, switching the light source on reveals spatially resolved surface fluorescence in the regions irradiated with light during the surface functionalization step. The yin-yang pattern is visible on the material surface under these conditions; however, some imperfections were visible. When viewed under white light, the yin-yang pattern can be seen as a slightly raised structure. This may indicate the presence of unreacted crosslinking units during the surface functionalization or the formation of excess free polymer in solution during the surface functionalization. Further analysis of the material using a fluorescent imager showed that the underside of the material showed no fluorescence under UV light irradiation (Figure 3E); however, the topside of the material showed strong fluorescence in the yin-yang pattern (Figure 3F).
Finally, the mechanical properties of the 3D printed dog-bone shaped samples were analyzed via a tensile testing machine to determine the material strength, ductility, and toughness. A representative stress-strain curve for the duplicate dog-bone-shaped samples is shown in Figure 4. The material initially showed an elastic deformation, providing yield stress (σy) of 24.8 ± 0.2 MPa, and then a plastic deformation before failure. The elongation at break (εb) was 11.7 ± 0.3 %, while the stress at break (σb) was 22.6 ± 0.3 MPa. The Young's modulus (E) was calculated to be 7.1 ± 0.2 MPa, while the toughness was 115.2 ± 3.0 MJ/m3.
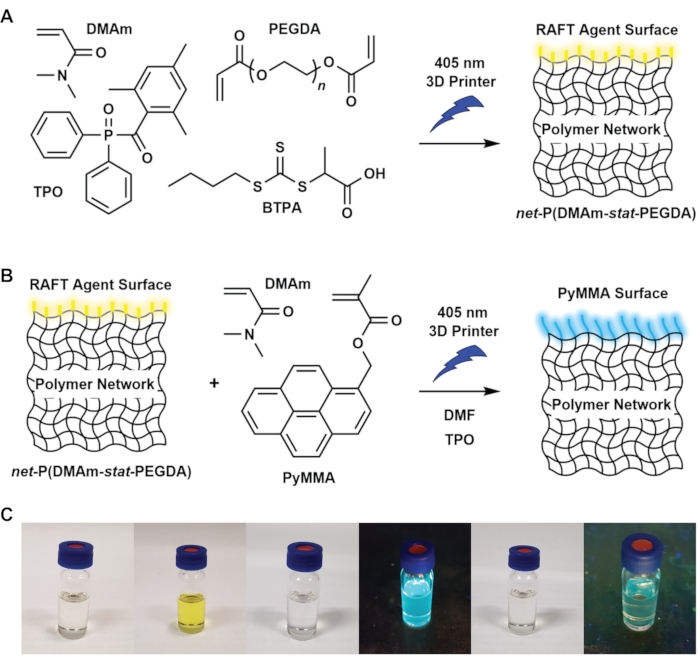
Figure 1: Schematic of the chemical process and illustration of selected resin components. (A) Bulk resin components and reaction schematic showing the synthesis of a net-P(DMAm-stat-PEGDA) polymer network via a 405 nm DLP 3D printer. (B) Surface resin components and reaction schematic showing surface functionalization of net-P(DMAm-stat-PEGDA) in a 405 nm DLP 3D printer. (C) Photographs of (from left to right): TPO in DMAm solution, bulk resin, PyMMA in DMF, PyMMA in DMF under 312 nm irradiation, surface resin, surface resin under 312 nm irradiation. Please click here to view a larger version of this figure.
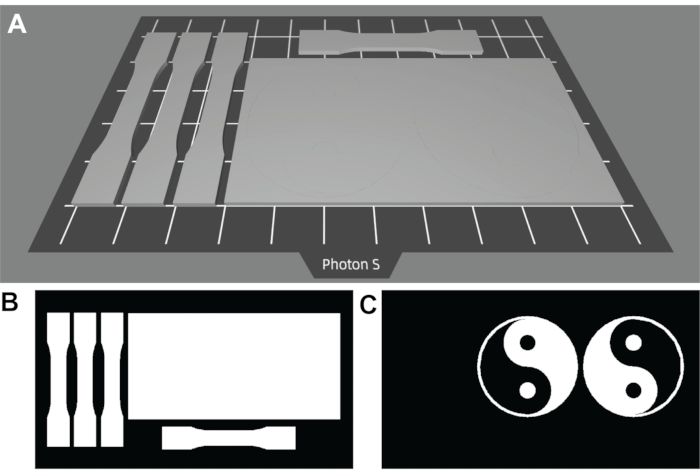
Figure 2: Digital images of the designed object to be 3D printed and surface functionalized. (A) 3D image showing the designed arrangement of 3D materials on the build stage. (B) Projection image showing the desired irradiation pattern in white for making the base object (layers 0-29). (C) Projection image showing the desired irradiation pattern in white for the surface functionalization (layer 30). The rectangular prism model is 80 x 40 x 1.5 mm (X x Y x Z), and the yin-yang symbol diameter is 38 mm. Please click here to view a larger version of this figure.
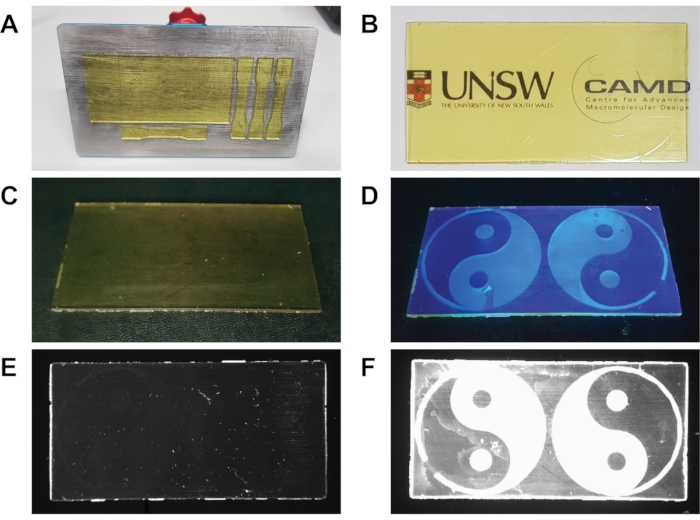
Figure 3: Images showing 3D printed and post-functionalized materials. (A) Photograph of the build stage after printing, post-functionalization, and 15 min post-cure under 405 nm irradiation. (B) Photograph of the functional material on top of the paper with logos, showing transparency. (C) Photograph of functional material in low light before UV irradiation. (D) Illustration of functional material under 312 nm irradiation shows strong fluorescence in the areas irradiated during the surface functionalization step. (E) Fluorescence image of the underside of functional material using a 2 s exposure time, showing no fluorescence. (F) Fluorescence image of the topside of functional material using a 1 s exposure time, showing strong fluorescence in the areas of the region that were irradiated during the surface functionalization step. 3D printed rectangular base substrate is 80 × 40 mm (X x Y), and the yin-yang symbol diameter is 38 mm. Images from (E) and (F) were obtained using a fluorescence imager. Please click here to view a larger version of this figure.
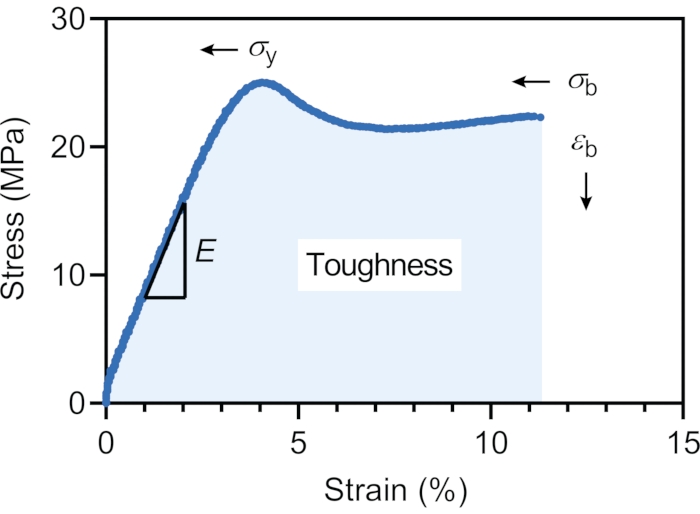
Figure 4: Stress vs. strain curves for 3D printed dog-bone shaped samples without surface functionalization. The yield stress (σy = 24.8 ± 0.2 MPa), elongation at break (εb = 11.7% ± 0.3%), and stress at break (σb = 22.6 ± 0.3 MPa) are indicated on the curve. The Young's modulus (E = 7.1 ± 0.2 MPa) was calculated in the linear elastic region from 1%-2% strain, while the toughness (115.2 ± 3.0 MJ/m3) was calculated based on the area under the stress-strain curve. Please click here to view a larger version of this figure.
| Parameters | Values |
| Layer thickness (mm) | 0.05 |
| Normal Exposure Time (s) | 40 |
| Off Time (s) | 2 |
| Bottom Exposure Time (s) | 80 |
| Bottom layers | 2 |
| Z Lift Distance (mm) | 3 |
| Z Lift Speed (mm/s) | 6 |
| Z Lift Retract Speed (mm/s) | 1 |
| Anti-alias | 1 |
Table 1: Parameters for creating the 3D model.
Discussion
The present protocol demonstrates a process for 3D printing of polymer materials with independently tunable bulk and interfacial properties. The procedure is performed via a two-step method by 3D printing the base substrate and subsequently modifying the surface layer of the 3D printed object using a different functional resin but using the same 3D printing hardware. While the 3D printers used in this work are designed to print crosslinked materials in a layer-by-layer fashion, the surface functionalization can also be performed using the same hardware. As shown in this protocol, the advantage of using the 3D printer hardware for surface functionalization is the ease of applying spatially controlled chemical patterns to the previously 3D printed polymer material.
For the design of the 3D models, a single layer is included above the material, which acts as the surface pattern. Different patterning results will be obtained depending on the concentrations of reagents in the surface resin, the layer thickness, and the layer cure time for the surface layer. For instance, in the current work, the surface layer was 50 µm, and the cure time was 180 s. Under these conditions, the surface pattern shows some minor surface defects, which may have been avoided by selecting a different layer thickness. In particular, a lower layer height for the surface layer may lead to better reproductions of the desired surface patterns due to more limited diffusion of material and light away from the irradiated area.
In addition, the cure time per layer used during 3D printing and surface functionalization is critical in producing well-defined materials. Based on previous work15, the inclusion of RAFT agent in the bulk resin extends the range of cure time per layer for the base substrate. This is due to the delayed onset of gelation, which maintains the print resolution even at extended layer cure times15. For the current system, layer cure times between 30-120 s should yield well-defined objects; however, this is also highly dependent on other reaction parameters such as the concentration of photoinitiator and RAFT agent, the layer thickness, and the light intensity. It is advisable to optimize the critical layer cure times per layer for new systems. If ill-defined materials are obtained, the cure time per layer is a simple parameter to manipulate to provide better results. If the bulk material is incompletely cured, the cure time per layer should be increased, while the cure time per layer should be decreased for over-cured materials5.
The concentration of TPO in both the bulk and surface resins will significantly influence the rate of radical generation and thus the rate of polymerization. Based on previous works15, the bulk material can be effectively fabricated using TPO: RAFT molar ratios in the range of 0.25-2.0. Further increasing the TPO concentration decreases the effective cure depth due to excessive light absorption5, while further reducing the TPO concentration reduces the polymerization rate and restricts effective polymerization. Similar trends will occur for the surface pattern, with suitable concentrations ranging from 0.5-3 wt% under the current conditions. Longer reaction times or thinner surface layer cure depths will decrease the required TPO concentration5.
It should also be noted that the inclusion of RAFT agents in the bulk resin will affect the subsequent surface patterning15,29. As shown previously15, in the absence of a RAFT agent, the surface patterning becomes ill-defined due to the limited attachment of the propagating chain to the material surface. In the current work, the RAFT agent groups at the surface provide a point for covalent attachment and polymer growth from the surface. In principle, a range of different surface resins may be used to functionalize the surfaces of the 3D printed objects to obtain the desired functionality. Indeed, as has been shown by our group previously15, the surface properties of an initially hydrophilic material can be switched to more hydrophobic via the use of hydrophobic monomers in the surface resin. Furthermore, the large monomer scope in radical and RAFT polymerization enables a broader range of available chemical functionalities for bulk and surface resins23.
From a hardware perspective, best results are obtained using a vat film completely free of imperfections; even slight imperfections in the surface film can create defects in the bulk materials and surface patterns, which is typical for digital light processing 3D printing. In addition, the resolution of the base material and the surface pattern is inherently limited by the 3D printer hardware; more highly resolved light will allow more finely detailed surface patterns with smaller characteristic lengths of the minor feature. As one would expect, 3D printer systems producing highly resolved features (higher resolution prints) are more expensive. It should be noted that the commercial 3D printers used in this work are comparatively cheap, with recent estimates placing the cost of these printers at only around USD 100. Critically, the robust chemistry in this procedure enables the use of the 3D printer without more specialized equipment such as gloveboxes to provide an inert atmosphere. This technique should thus allow more streamlined fabrication of materials with independently tunable bulk and interfacial properties for applications such as antifouling, antibacterial, conductive, and other smart materials.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge funding from the Australian Research Council and UNSW Australia via the Discovery Research program (DP210100094).
Materials
| 1-pyrenemethyl methacrylate | Sigma-Aldrich | 765120 | |
| 2-(n-butylthiocarbonothioylthio) propanoic acid | Boron Molecular | BM1640 | |
| 3D Printer | Photon | Mono S | light intensity at digital mask surface = 0.81 mW cm-2 |
| 3D Printing Slicing Software | Photon | Photon Workshop V2.1.19 | |
| 40 kHz Ultrasonic Bath | Thermoline | UB-410 | |
| Compressed Air | Coregas | 230142 | Tank operating at 130 kPa |
| Computer Assisted Design Program | SpaceClaim | SpaceClaim Design Manager V19.1 | |
| Diphenyl (2,4,6-trimethylbenzoyl) phosphine oxide | Sigma-Aldrich | 415952 | |
| Ethanol Undenatured 100% AR | ChemSupply | EL043-2.5L-P | |
| Ethanol Wash bottle | Rowe Scientific | AZLWGF541P | |
| Fluorescence Imager | Bio-Rad | Gel Doc XR+ | Uses a 302 nm gas discharge lamp as emission source |
| Light intensity power meter | Newport | 843-R | |
| Mechanical Tester | Mark–10 | ESM303 | 1 kN force gauge M5–200 |
| Moldable plastic film | Parafilm | PM992 | |
| N,N-dimethlacrylamide | Sigma-Aldrich | 274135 | |
| N,N-Dimethylformamide HPLC | ChemSupply | LC1051-G4L | |
| Poly(ethylene glycol) diacrylate average Mn 250 | Sigma-Aldrich | 475629 | |
| Post Cure Lamp | Leoway | B0869BY79P | 60 W 405 nm |
| Standards document | ASTM | ASTM Standard D638-14 | |
| Tensile testing machine | Mark-10 | ||
| UV Light | Fisher Scientific | 11-982-30 | 6 W Spectroline E-Series, Gas discharge lamp |
| Vortex Mixer IKA Vortex 3 | LabTek | 3340000I |
Referências
- Ligon, S. C., Liska, R., Stampfl, J., Gurr, M., Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chemical Reviews. 117 (15), 10212-10290 (2017).
- Lewis, J. A. Direct ink writing of 3D functional materials. Advanced Functional Materials. 16 (17), 2193-2204 (2006).
- Kumar, S. Selective laser sintering: A qualitative and objective approach. JOM. 55 (10), 43-47 (2003).
- Jung, K., et al. Designing with light: Advanced 2D, 3D, and 4D materials. Advanced Materials. 32 (18), 1903850 (2020).
- Lim, K. S., et al. Fundamentals and applications of photo-cross-linking in bioprinting. Chemical Reviews. 120 (19), 10662-10694 (2020).
- Chen, H., et al. Photoinitiators derived from natural product scaffolds: Monochalcones in three-component photoinitiating systems and their applications in 3D printing. Polymer Chemistry. 11 (28), 4647-4659 (2020).
- Chen, H., et al. Novel D-π-A and A-π-D-π-A three-component photoinitiating systems based on carbazole/triphenylamino based chalcones and application in 3D and 4D printing. Polymer Chemistry. 11 (40), 6512-6528 (2020).
- Zhang, J., Xiao, P. 3D printing of photopolymers. Polymer Chemistry. 9 (13), 1530-1540 (2018).
- Zhu, Y., Ramadani, E., Egap, E. Thiol ligand capped quantum dot as an efficient and oxygen tolerance photoinitiator for aqueous phase radical polymerization and 3D printing under visible light. Polymer Chemistry. 12 (35), 5106-5116 (2021).
- Jiang, P., Ji, Z., Wang, X., Zhou, F. Surface functionalization – a new functional dimension added to 3D printing. Journal of Materials Chemistry C. 8 (36), 12380-12411 (2020).
- Gonzalez, G., Chiappone, A., Dietliker, K., Pirri, C. F., Roppolo, I. Fabrication and functionalization of 3D printed polydimethylsiloxane-based microfluidic devices obtained through digital light processing. Advanced Materials Technologies. 5 (9), 2000374 (2020).
- Yao, X., Song, Y., Jiang, L. Applications of bio-inspired special wettable surfaces. Advanced Materials. 23 (6), 719-734 (2011).
- Bose, S., Robertson, S. F., Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomaterialia. 66, 6-22 (2018).
- Wang, X., et al. i3DP, a robust 3D printing approach enabling genetic post-printing surface modification. Chemical Communications. 49 (86), 10064-10066 (2013).
- Lee, K., Corrigan, N., Boyer, C. Rapid high-resolution 3D printing and surface functionalization via type I photoinitiated raft polymerization. Angewandte Chemie International Edition. 60 (16), 8839-8850 (2021).
- Zhang, Z., Corrigan, N., Bagheri, A., Jin, J., Boyer, C. A versatile 3D and 4D printing system through photocontrolled raft polymerization. Angewandte Chemie International Edition. 58 (50), 17954-17963 (2019).
- Corrigan, N., et al. Reversible-deactivation radical polymerization (controlled/living radical polymerization): From discovery to materials design and applications. Progress in Polymer Science. 111, 101311 (2020).
- Moad, G., Rizzardo, E., Thang, S. H. Living radical polymerization by the raft process – A third update. Australian Journal of Chemistry. 65 (8), 985-1076 (2012).
- Chiefari, J., et al. Living free-radical polymerization by reversible addition−Fragmentation chain transfer: The RAFT process. Macromolecules. 31 (16), 5559-5562 (1998).
- Fromel, M., et al. User-friendly chemical patterning with digital light projection polymer brush photolithography. European Polymer Journal. 158, 110652 (2021).
- Fromel, M., Li, M., Pester, C. W. Surface engineering with polymer brush photolithography. Macromolecular Rapid Communications. 41 (18), 2000177 (2020).
- Wang, C. -. G., Chen, C., Sakakibara, K., Tsujii, Y., Goto, A. Facile fabrication of concentrated polymer brushes with complex patterning by photocontrolled organocatalyzed living radical polymerization. Angewandte Chemie International Edition. 57 (41), 13504-13508 (2018).
- Zoppe, J. O., et al. Surface-initiated controlled radical polymerization: state-of-the-art, opportunities, and challenges in surface and interface engineering with polymer brushes. Chemical Reviews. 117 (3), 1105 (2017).
- Pester, C. W., et al. Ambiguous antifouling surfaces: Facile synthesis by light-mediated radical polymerization. Journal of Polymer Science Part A: Polymer Chemistry. 54 (2), 253-262 (2016).
- Poelma, J. E., Fors, B. P., Meyers, G. F., Kramer, J. W., Hawker, C. J. Fabrication of complex three-dimensional polymer brush nanostructures through light-mediated living radical polymerization. Angewandte Chemie International Edition. 52 (27), 6844-6848 (2013).
- Zhu, Y., Egap, E. PET-RAFT polymerization catalyzed by cadmium selenide quantum dots (QDs): Grafting-from QDs photocatalysts to make polymer nanocomposites. Polymer Chemistry. 11 (5), 1018-1024 (2020).
- ASTM International. ASTM Standard D638-14: Standard Test method for tensile properties of plastics. ASTM International. , (2014).
- Moad, G. RAFT (Reversible addition-fragmentation chain transfer) crosslinking (co)polymerization of multi-olefinic monomers to form polymer networks. Polymer International. 64 (1), 15-24 (2015).
- Li, M., et al. SI-PET-RAFT: Surface-initiated photoinduced electron transfer-reversible addition-fragmentation chain transfer polymerization. ACS Macro Letters. 8 (4), 374-380 (2019).

