Mass-Rearing and Molecular Studies in Tortricidae Pest Insects
Summary
The present protocol describes the rearing method of tortricid pest insects in the laboratories. The procedures to distinguish insects’ sex and extract nucleic acids for high throughput sequencing are established using two tortricid pests.
Abstract
Tortricidae (Lepidoptera), commonly known as tortrix or leafroller moths, comprises many agricultural and forestry pests, which cause serious agricultural losses. To understand the biology of such pest moths, fundamental techniques have been in high demand. Here, methods for mass-rearing, observations, and molecular studies are developed using two tea tortrix, Homona magnanima and Adoxophyes honmai (Lepidoptera: Tortricidae). Insects were mass-reared with sliced artificial diet and maintained by inbreeding for over 100 generations by considering their biological characteristics. Insects have various sex dimorphisms; hence it is difficult to distinguish the sex during the developing stages, which have prevented subsequent assays. The present work highlighted that the sex of tortricids larvae could be determined by observing testes or lactic-acetic orcein staining to visualize the female-specific W chromosome. Moreover, using the sex determination methods, the present study enabled nucleic acid extractions from sex determined embryos and application toward high throughput sequencing. These tips are applicable for other pest insects and will facilitate further morphological and genetic studies.
Introduction
Lepidopteran insects represent more than 10% of all described living species1, and certain taxa species cause severe damage to plants and serious agricultural losses2,3. Although molecular and genetic studies have been developed using model insects such as the silkworm Bombyx mori4,5, pest insects remain uninvestigated, partly because of the difficulties for rearing and handling6,7. Therefore, fundamental studies and techniques are necessary to understand the biology of such non-model pest insects.
The Tortricidae (Lepidoptera), commonly known as tortrix or leafroller moths, comprises many agricultural and forestry pests8. Of the insect taxa, the oriental tea tortrix Homona magnanima Diakonoff and the summer fruit tortrix Adoxophyes honmai Yasuda are serious polyphagous pests known to damage tea trees in East Asia7. The two species lay flat and oval scale-like egg clusters (or egg masses) consisting of thin, soft, and fragile eggs covered by maternal secretions. Although embryogenesis stages are crucial for insect development and sex determinations9, structures of the eggs prevent further analysis from understanding the biology of the insects. It is important to overcome the difficulties for further study on pests ovipositing such complex egg mass.
Here, to understand the biology of tortricids, methods for mass rearing, observations, and molecular studies have been developed using A. honmai and H. magnanima. First, mass rearing methods maintain both tortricids over 100 generations by inbred. The separation of eggs from the concatenated scale-like egg mass facilitated embryogenesis observation of the tortricids using alkaline and organic solvents previously developed from techniques used in flies10. In addition, the present study established sex discrimination of small embryos by developing staining methods of the sex chromatin of lepidopteran females using lactic-acetic orcein11. By combining these methods, high quality and quantity nucleic acids were extracted from sex determined embryos, which was otherwise difficult to establish6. The extracted RNA was utilized for next-generation sequencing. Collectively, the methods presented here apply to other lepidopteran insects and other insect taxa.
Protocol
1. Insect collection and mass rearing
- Collect tortricid insects from fields following previously published References8,12.
NOTE: H. magnanima and A. honmai larvae are collected from damaged tea leaves (Figure 1A); adults are attracted using 4 W portable UV light (365 nm wavelength, see Table of Materials, Figure 1B). - Rear the collected larvae (Figure 1C,D) individually on a piece of artificial diet in a 1/2-oz cup for 2-3 weeks until adult eclosion (Figure 1E,F). Confirm the sex of pupae and adults by morphological traits (Figure 1G,I).
- Mate the males and females in a plastic box (30 cm x 20 cm x 5 cm) to oviposit egg masses on a paraffin paper (Figure 1J-L). Place a female and two males in a 120 mL plastic cup with a piece of paraffin paper to establish a matriline.
NOTE: It is important to make creases on the paraffin paper. For H. magnanima, paraffin paper needs to be on the bottom of the case. Meanwhile, for A. honmai, put a paper on the case ceiling to collect eggs (Figure 1L) since H. magnanima oviposit egg mass on the upper side of tea leaves, but A. honmai lays eggs on the underside of the leaves8. The procedures were also verified in other tortricid species, and it is better to place papers on both sides if the ecology and behavior of the target species are unclarified. - Cut out the egg masses (approximately 100-200 eggs per egg mass12, Figure 1M) on the paraffin paper with scissors. Place the eggs in a 1/2 oz cup with dumped paper for 5-7 days.
NOTE: The matured embryo exhibits a blackhead capsule (Figure 1N). The periods of embryogenesis are diversely attributed to tortricid species, but generally 5 days post oviposition (dpo) for H. magnanima and 4 dpo for A. honmai at 25 °C with 60% relative humidity, 16 h light/8 h dark cycles7. - Store the egg masses showing black head capsules at 4-8 °C for 7 days.
- Slice ~60 g artificial diets using a grater for mass rearing. Place the egg mass filled with matured embryos on the sliced artificial diet in a plastic container (23 cm x 16 cm x 8 cm). Place paraffin papers on the egg mass with the sliced diets (Figure 10).
NOTE: Below 30% relative humidity is considered over dry, while over 70% is too humid. Creating holes on the plastic container's lid is better to enable better ventilation. Fill the holes with cotton to prevent the escapes of small larvae. - To eliminate surface contaminants from the eggs, soak the egg mass into 3% formalin and 0.2% Benzalkonium chloride solution for 5 min, respectively12. To eradicate gut or intracellular bacteria, utilize an artificial diet supplemented with 0.05% (w/w) Tetracycline hydrochloride or 0.06% (w/w) rifampicin instead of a normal artificial diet (see Table of Materials).
NOTE: This step is optional. It is important to knead Silk Mate 2S and 0.05% (w/w) Tetracycline hydrochloride or 0.06% (w/w) rifampicin equably for consistency. - Collect the pupae from the plastic container and distinguish the sex based on morphological12 characteristics (Figure 1G-I).
NOTE: Generally, tortricids present 5-6 instars until pupation12. The H. magnanima and A. honmai larvae take 3 weeks and 2 weeks, respectively, after hatching until pupation. - Place 15 males and 10 females in a plastic box (30 cm x 20 cm x 5 cm, Figure 1K) for mating with 25 °C, 16 L/8 D. Collect eggs per 5-7 days and repeat steps 1.4-1.9. for each generation.
NOTE: If collecting newly oviposited egg masses for subsequent analysis is needed, set the dark period's start and end, e.g., from 9 AM to 5 PM. In this condition, H. magnanima and A. honami usually oviposit eggs after 5 h (2 PM).
2. Separation of eggs and pharate larvae from egg masses for fixation, permeabilization, and staining
- Soak the egg mass into 1,000 µL of 1.2% sodium hypochlorite aqueous solution for 10 min or into 1,000 µL of 5 M potassium hydroxide aqueous solution for 30 min to separate the eggs.
- Wash the separated eggs in 1,000 µL of PBSt (137 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4, 0.05% of Polyoxyethylene (20) Sorbitan Monolaurate [Tween-20] pH 7.4, see Table of Materials) following the previously published report7.
- Soak eggs in a mixture of 500 µL of 100% heptane and 500 µL of 4% paraformaldehyde-PBSt solution (w/v). Mix for 10 min at 1,500 rpm using a vortex mixer.
- Soak the eggs into a mixture of 500 µL of 100% heptane and 500 µL of 100% methanol. Mix for 10 min at 1,500 rpm.
- Wash the eggs twice with 1,000 µL of 100% methanol and store at 4 °C in 100% methanol until further experiments (Figure 2A).
NOTE: The eggs can be stored for at least 1 year at 4 °C. - Soak the eggs sequentially in 99%, 70%, 50% ethanol, and PBSt for 5 min each for hydrophilization following the previously published report7.
- Wash the eggs with PBSt, immerse the eggs in 1 µg/mL of DAPI solution for 5 min, and then wash the eggs with 1x PBS twice. Soak the eggs in 20 µL of 1.25% (w/w) lactic-acetic orcein solution (see Table of Materials)11 to visualize heterochromatin until the nuclei exhibit a brilliant red color (this varies from 5-60 min) (Figure 2B,C).
NOTE: The lactic-acetic orcein staining periods depend on temperature and humidity. It is better to check for proper staining using a microscope with 4x-10x magnification. - Transfer the stained eggs using a pipette to a glass slide. Enclose the stained eggs with antifade reagent (see Table of Materials) and a cover glass7.
- Extract pharate H. magnanima and A. honmai larvae (4-5 days post oviposition (dpo)) from the egg masses using forceps (Figure 2D). Bisect larvae on a glass slide (Figure 2E).
- Fix any tissues (e.g., suboesophageal ganglion, thoracic ganglia, malpighian tubule, etc.) with a mixture of 1:3 (v/v) 99.7% acetic acid/100% methanol for 5 min. Stain those with 1.25% (w/w) lactic-acetic orcein solution11 until the nuclei are stained (this can take 5-60 min, depending on the temperature and humidity).
- Determine the sex of each specimen by observing the presence (female) or absence (male) of heterochromatin (the W chromosome, Figure 2F,G) under a microscope.
NOTE: The sex is determined by visualizing the sex chromatin. Each cell represents the sex chromatin as a dot in females11,12, while the remaining portion of pharate larval tissues (not fixed) should be immediately immersed in cell lysis buffer12 (10 mM Tris-HCl, 100 mM EDTA, and 1% SDS, pH 8.0) or phenol-containing RNA extraction reagents for either DNA or RNA extraction. Before the dissections, dispense 20 μL of the reagents into 0.2 mL PCR tubes in advance (Figure 3A). Immerse and store samples at -80 °C until further extraction. The samples can be stored for at least 3 months, but it is better to proceed with the downstream experiments to prevent the degradation of nucleic acids.
3. DNA and RNA extractions from sex determined pharate larvae
- Pool 12 sex-determined male or female pharate larvae (5 dpo embryo) and add either cell lysis buffer or RNA extraction reagents (see step 2.11 and Table of Materials) into one 1.5 mL tube.
NOTE: Follow steps 3.2-3.7 for DNA extraction and steps 3.8-3.11 for RNA extraction. - Homogenize tissues in 600 μL of the cell lysis buffer (10 mM Tris-HCl, 100 mM EDTA, and 1% SDS, pH 8.0), and centrifuge the samples at 10,000 x g for 5 min at 4 °C.
- Collect the supernatant (500 μL) using a pipette and incubate at 50 °C with 1.5 µL of Proteinase K (20 mg/mL, see Table of Materials) for 5 h on a heat block.
- Treat the samples with 1.0 μL of 10 mg/mL of RNase solution (see Table of Materials) at 37 °C for 30 min.
- Add 200 μL of protein precipitation solution (see Table of Materials) to the tubes7, followed by a centrifuge at 17,000 x g for 10 min at 4 °C.
- Mix the supernatant (500 μL) with 500 μL of 100% isopropanol, and then centrifuge at 20,400 x g for 10 min at 4 °C.
- Wash the pelleted DNA twice with 1,000 μL of 70% ethanol. Then, air-dry (5-10 min at room temperature), dissolve the DNA in 30 μL of 10 mM Tris-Cl buffer (pH 8.5).
- Homogenize the tissues in 600 μL of RNA extraction reagents and add 240 μL of ultra-pure distilled water to the tube. Centrifuge the tubes at 12,000 x g for 15 min at 4 °C.
- Mix the supernatant (600 μL) with 600 μL of 100% isopropanol and transfer the mixture to a silica spin column (see Table of Materials). Centrifuge the tubes at 17,900 x g for 1 min at 4 °C.
- Wash the column with 750 μL of 70% ethanol, and centrifuge the column twice at 17,900 x g for 1 min each at 4 °C.
- Load 15 μL of Ultra-pure distilled water to the column. Centrifuge the tubes at 17,900 x g for 1 min at 4 °C to elute the RNA.
- Calculate and verify the quality and quantity of DNA and RNA using a UV-based spectrophotometer. Assess the purity of nucleic acids using the A260/A280 (Nucleic acids/protein) and A260/A230 (Nucleic acids/salts and other contaminants) ratios13.
NOTE: For RNA extraction, the previously published procedure12 was followed, which resulted in phenol contamination (Table 1). The extracted DNA and RNA from a single embryo or pharate larvae yields low-amount and low-quality samples. Typically, DNA extraction from 12 pharate larvae yields 100-600 ng, while RNA extraction using the current method generates 900-1,500 ng, as shown Table 1.
NOTE: Steps 3.13-3.15 are optional for further assays of high throughput sequencings. - Calculate the amounts of DNA and RNA using a fluorescence-based spectrophotometer.
NOTE: The ratio of RNA quantities (UV-based concentration (ng/μL)/fluorescence-based concentration (ng/μL)) was calculated to assess the quality for further experimental application. For example, when UV and fluorescence-based concentrations are 80 ng/uL and 60 ng/uL, respectively, the ratio will be 1.5 (80/60). Usually, ratios under 1.5 indicate sufficient purity for downstream applications using high throughput sequencing13. - Analyze the quality of RNA by using microchip electrophoresis14.
- Utilize the qualified RNAs to prepare the RNA library using an RNA library preparation kit (see Table of Materials). Sequence the libraries using a platform suitable for the prepared libraries.
Representative Results
Establishment of host lines and their maintenance
The viability of field-collected larvae is differently attributed to field location, seasons, and rearing conditions (e.g., 90% of viability in Taiwan, Taoyuan, as shown in Arai et al.12). Approximately 30%-50% of pairs will generate the next generation as usual. For H. magnanima and A. honmai, matrilines have been maintained by inbreeding for over 100 generations.
Morphological observations and sex determinations
The treatment with either potassium hydroxide (3 or 5 M KOH) or sodium hypochlorite (1.2% Cl2 or NaClO) separates eggs from their concatenated egg masses (Figure 2A). The lower concentration of the reagents could not achieve separation. All the fixation, permeabilization, and staining steps enabled the visualization of nuclei with DAPI solution. The eggs treated with only KOH or NaClO (separated) without the fixation and permeabilization steps were not stained with these dyes7. Non-treated H. magnanima egg masses were not stained. The lactic-acetic orcein stains heterochromatin (W chromosome) with a dark red color and nuclei with a bright red color as usual (Figure 3A). This staining enables easy and fast sex determination from 4 dpo embryo to sixth instar larvae. Although nuclei were visualized in 0-3 dpo embryos, it was hard to observe the W chromosome with 400x magnification.
High-quality DNA and RNA were obtained from sex-determined and pooled embryos
From 12 pharate larvae, high quality DNA (A260/A280 = 1.7-2.0; A260/A230 = 1.7-2.4) were extracted with 100-600 ng in total amount (Table 1). Although the RNA extracted following the previously published method12 yielded 500-1,000 ng product with low quality: A260/A280 = 1.7-2.1; A260/A230 = 0.1-0.5 (Table 1), the modified protocols using a spin column improved the quality of the RNA, producing 900-1,500 ng product with A260/A280 = 1.9-2.1 and A260/A230 = 1.9-2.3 (Table 1). Furthermore, the RNA concentration ratio (UV-based/Qbit fluorescence-based values) for the modified protocol was below 1.2, meeting the quality requirements for next-generation sequencing13. Conversely, the quality and quantity of nucleic acids extracted from a single embryo were too low to be calculated. The intactness of the RNA extracted from sex-determined pharate larvae using the modified protocol was also confirmed using microchip electrophoresis (Figure 3B). The prepared libraries were confirmed to yield high Q30 score reads (Table 2).
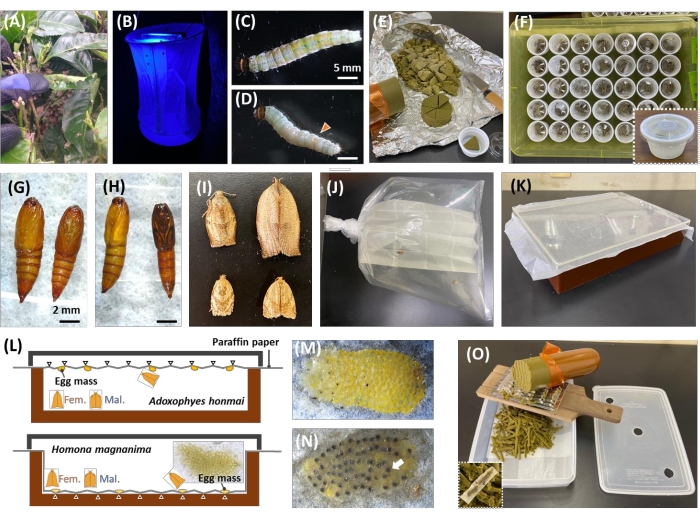
Figure 1: Overview of insect collection and mass rearing of H. magnanima and A. honmai. (A) Tea leaves and nests damaged by a larva. (B) Light trap with UV light. (C,D) A female (C) and a male (D) 6th instar larva of H. magnanima. The Orange triangle indicates the testis. (E,F) Collected larvae were reared individually in a 1/2 oz cup with a piece of artificial diet. (G,H) Pupae of H. magnanima (G) and A. honmai (H). The female is shown on the left, while the male is shown on the right. (I) Adults of H. magnanima (above) and A. honmai (below). Both males and females are shown on the right and left, respectively. (J,K) A plastic bag (J) or plastic case (K) for egg mass collection. (L) A. honmai oviposit eggs on paraffin paper placed on the lid, and H. magnanima lay eggs on paraffin paper placed on the bottom of the case. (M,N) Egg mass of H. magnanima. The matured embryos exhibit black head capsules indicated with white arrows (N). (O) Sliced artificial diets with a grater for mass rearing. The larvae hatched from eggs, placed on the sliced diet, will form pupae 3-4 weeks after hatching at 25 °C (under 16 h light/8 h dark cycle). Please click here to view a larger version of this figure.
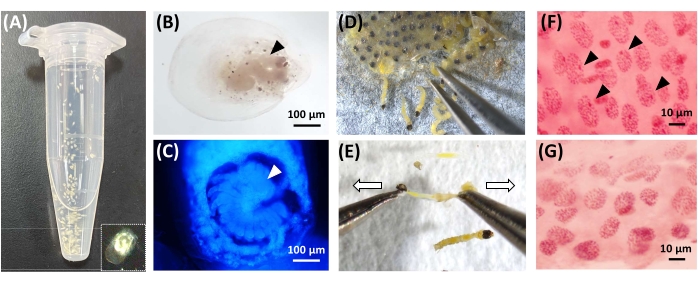
Figure 2: Observations of embryos. (A) The separated, fixed, and permeabilized eggs of H. magnanima using 5 N KOH, 4% PFA / heptane, methanol/heptane, and methanol. A separated egg is highlighted with broken lines. (B) The 3 dpo embryo was stained with lactic-acetic orcein. The black arrowhead indicates the embryo. (C) The 4 dpo embryo was stained with DAPI. The white arrowhead indicates the embryo. (D,E) Dissection of pharate larvae (5 dpo) using forceps. The pharate larvae are extracted from egg-mass (D), bisected on a glass slide (E). (F,G) Lactic-acetic orcein staining using the dissected pharate larvae. Females exhibit heterochromatin as a dot (indicated with black arrowhead, (F), but males lack the heterochromatin (G). Please click here to view a larger version of this figure.
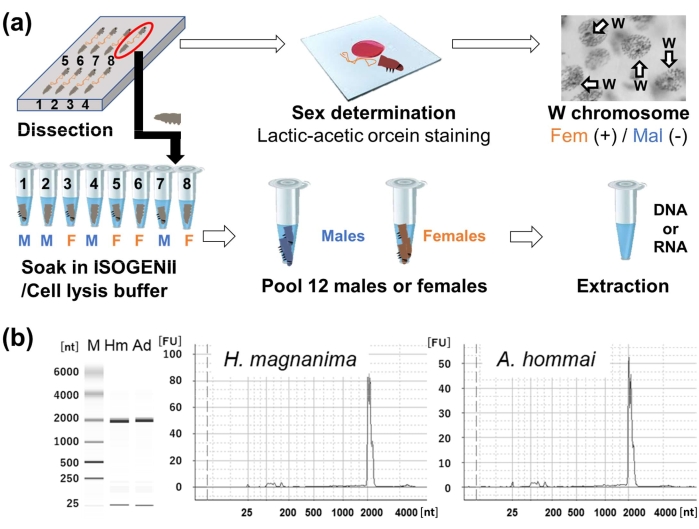
Figure 3: Graphical summary of nucleic acid extraction using sex-determined pharate larvae and RNA qualities of H. magnanima and A. honmai. (A) Tissues of dissected pharate larvae on glass slides were stained with lactic-acetic orcein to determine their sex. The remaining tissues were soaked in reagents, pooled into one tube sorted by the sex of the individuals, and then subjected to DNA/RNA extraction. (B) The quality of RNA extracted from 12 male H. magnanima or A. honmai was assessed using a microchip electrophoresis system. Abbreviations: [nt], nucleotide size; [FU], fluorescence unit; M, molecular marker; Hm, H. magnanima; Ad, A. honmai. Please click here to view a larger version of this figure.
Table 1: Concentration and quality of nucleic acids extracted from sex determined pharate larvae of H. magnanima. Please click here to download this Table.
Table 2: Qualities of RNA-sequencing raw data. 1Q20 (%) indicates the probability of reads with 99% read accuracy. 2Q30 (%) indicates the probability of reads with 99.9% read accuracy. Please click here to download this Table.
Discussion
Tortricid comprises several agricultural and forestry pests; the present study presented methods to rear tortrix over generations, observe embryogenesis and sex of the insects, and conduct molecular analysis using matured embryos.
One of the obstacles for pest insect study is to establish rearing methods. Especially, inbreeding sometimes affect the fitness of the species negatively. The fitness reduction by the inbred, called inbreeding depression, has widely been observed in various plants and animals, including insects15,16,17,18. As noted, both H. magnanima and A. honmai have been maintained for over 100 generations (more than 10 years) with no apparent fitness costs. Although it is not enough to assess whether other tortricids also have a high tolerance to inbreeding in general, the two species may have developed the characteristics by adapting monotonous environmental conditions (i.e., tea plantations) and against several endosymbiotic microorganisms (e.g., Wolbachia11,12). The eggs of the two species are easily obtained, but their oviposition behaviors are differently attributed to their ecology. Indeed, humidity and crease direction seem to directly affect the number of oviposited eggs, which might reflect the natural histories of the insects in fields. It is important to adjust rearing methods to species regarding their biological characteristics in nature.
The protocols established in this study enabled observation of embryogenesis and molecular studies using sex-determined matured embryos and larvae. The insect eggs are generally coated with several shell layers10,19. Moreover, the eggs of H. magnanima and A. honmai are covered with maternal secretions. To stain the eggs with such complexed structure, separation, fixation, and permeabilization seems to be all necessary. In Ostrinia furnacalis (Crambidae), which oviposits scale-like egg mass such as H. magnanima and A. honmai, PCR-based sex determination protocols using single embryos have been proposed, but the low quality and quantity of nucleic acids have been a problem for subsequent analysis6,20. In contrast, the present study suggested extracting nucleic acids from pooled sex-determined embryos after observing female-specific sex chromatin. RNAs are easily degraded, and the extraction procedure shown here did not affect the quality of RNA, which has also been confirmed by the transcriptome analysis (RNA-seq).The techniques shown here are applicable for studies of embryogenesis of various insect species. In addition, the protocols have the potentials to be used to assess the effects of chemical pesticides or intracellular microbes such as Wolbachia, which causes sex-specific defects during embryogenesis6,11,12.
The present study has several limitations. First, the sex of immature embryos (0-3 dpo) was difficult to determine using lactic-acetic orcein staining in H. magnanima and A. honmai. This is because the number and size of the nuclei are generally small during early embryogenesis, making it difficult to observe the W chromosome. To clarify the sex of the tortricids during early embryogenesis, detection and quantification of markers on sex chromosomes6,20 could be an alternative approach. Second, it was difficult to extract nucleic acids with high purity from a single individual after sex determination, possibly due to the small number of cells. However, the RNA or DNA extracted from a single embryo may apply to subsequent analyses such as PCR assays and single-cell sequencings.
In summary, the present protocol describes mass rearing, morphological observations, and genetic analyses of the eggs of two non-model lepidopteran pest insects, H. magnanima and A. honmai. These simple techniques are expected to be applicable for further research on tortricid, other lepidopterans insects, and other taxa.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors wish to acknowledge support from the Japan Society for the Promotion of Science (JSPS) Research Fellowships for Young Scientists [Grant Number 19J13123 and 21J00895].
Materials
| 1/2 ounce cup | FP CHUPA | CP070009 | insect rearing; https://www.askul.co.jp/p/6010417/ |
| 1/2 ounce cup lid | FP CHUPA | CP070011 | insect rearing; https://www.askul.co.jp/p/6010434/?int_id=recom_DtTogether |
| 99.7% acetic acid | FUJIFILM Wako Chemicals Co., Osaka, Japan | 36289 | fixation; https://labchem-wako.fujifilm.com/jp/product/detail/W01ALF036289.html |
| Agilent 2100 Bioanalyzer | Agilent Technologies | not shown | Nucleic acids quantification; https://www.agilent.com/en/product/automated-electrophoresis/bioanalyzer-systems/bioanalyzer-instrument |
| Agilent RNA6000 nano kit | Agilent Technologies | 5067-1511 | Nucleic acids quantification; https://www.agilent.com/cs/library/usermanuals/Public/G2938-90034_RNA6000Nano_KG |
| benzalkonium chloride solution | Nihon Pharmaceutical Co., Ltd | No.4987123116046 | Sterilization; https://www.nihon-pharm.co.jp/consumer/products/disinfection.html |
| Cotton | AOUME | 8-1611-02 | insect rearing; https://item.rakuten.co.jp/athlete-med/10006937/?scid=af_pc_etc&sc2id=af_113_0_1 |
| DAPI solution | Dojindo, Tokyo, Japan | 340-07971 | stainings; https://www.dojindo.co.jp/products/D523/ |
| Disodium Hydrogenphosphate | FUJIFILM Wako Chemicals Co. | 4.98748E+12 | Na2HPO4; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0119-0286.html |
| dsDNA HS quantification kit | Invitrogen | Q33231 | Nucleic acids quantification; https://www.thermofisher.com/order/catalog/product/Q33230?SID=srch-srp-Q33230 |
| Econospin RNA II column | Epoch Life Science Inc. | EP-11201 | RNA extraction; http://www.epochlifescience.com/Product/SpinColumn/minispin.aspx |
| Ethanol | FUJIFILM Wako Chemicals Co., Osaka, Japan | 4.98748E+12 | fixation; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0105-0045.html |
| Ethylenediamine-N,N,N',N'-tetraacetic Acid Tetrasodium Salt Tetrahydrate (4NA) | FUJIFILM Wako Chemicals Co. | 4.98748E+12 | Cell lysis buffer (EDTA); https://labchem-wako.fujifilm.com/jp/product/detail/W01T02N003.html |
| Glassine paper | HEIKO | 2100010 | insect rearing; https://www.monotaro.com/p/8927/0964/?utm_id=g_pla& utm_medium=cpc&utm_source= Adw |
| heat block WSC-2620 PowerBLOCK | ATTO, Tokyo, Japan | 4002620 | incubation; https://www.attoeng.site/ |
| heptane | FUJIFILM Wako Chemicals Co., Osaka, Japan | 4.98748E+12 | fixation; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0108-0015.html |
| INSECTA LF | Nosan Co., Ltd | not shown | Artificial diet; https://www.nosan.co.jp/business/fodder/ist.htm |
| ISOGENII | Nippon Gene | 311-07361 | RNA extraction; https://www.nippongene.com/siyaku/product/extraction/isogen2/isogen2.html |
| isopropanol | FUJIFILM Wako Chemicals Co. | 4.98748E+12 | nucleic acids extraction; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0232-0004.html |
| Lactic acid | FUJIFILM Wako Chemicals Co. | 4.98748E+12 | Stainings; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0112-0005.html |
| methanol | FUJIFILM Wako Chemicals Co., Osaka, Japan | 4.98748E+12 | fixation; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0113-0182.html |
| MSV-3500 vortex | Biosan | BS-010210-TAK | Voltex mixer; https://biosan.lv/products/-msv-3500-multi-speed-vortex/ |
| Nano Photometer NP 80 | Implen | not shown | Nucleic acids quantification; https://www.implen.de/product-page/implen-nanophotometer-np80-microvolume-cuvette-spectrophotometer/tech-specs/ |
| Natural pack wide | Inomata chemical | 1859 | insect rearing; https://www.monotaro.com/g/03035766/?t.q=%E3%83%8A%E3%83%81%E3%83%A5%E3% 83%A9%E3%83%AB%E3%83%91% E3%83%83%E3%82%AF%E3%83% AF%E3%82%A4%E3%83%89 |
| NEBNext Ultra II RNA Library Prep Kit for Illumina | New England BioLabs | E7770S | Library preparation; https://www.nebj.jp/products/detail/2039 |
| orcein | FUJIFILM Wako Chemicals Co. | 4.98748E+12 | Stainings; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0115-0094.html |
| Paraformaldehyde | FUJIFILM Wako Chemicals Co. | 160-16061 | fixation; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0116-1606.html |
| Polyoxyethylene(20) Sorbitan Monolaurate | FUJIFILM Wako Chemicals Co. | 4.98748E+12 | Tween-20; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0116-2121.html |
| Portable UV Black Light (4W, 365nm wavelength) | Southwalker Co., Ltd., Kanagawa, Japan | not shown | Insect collection; http://www.southwalker.com/shopping/?pid=1364614057-467328 |
| Potassium Chloride | FUJIFILM Wako Chemicals Co. | 4.98748E+12 | KCl; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0116-0354.html |
| Potassium Dihydrogen Phosphate | FUJIFILM Wako Chemicals Co. | 4.98748E+12 | KH2PO4; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0116-0424.html |
| ProLong Diamond Antifade Mountant | Invitrogen, MA, USA | P36965 | antifade; https://www.thermofisher.com/order/catalog/product/P36965 |
| Proteinase K Solution | Merck | 71049-4CN | DNA extraction; https://www.merckmillipore.com/JP/ja/product/Proteinase-K-Solution-600-mAU-ml,EMD_BIO-71049 |
| protein precipitation solution | Qiagen | 158912 | DNA extraction; https://www.qiagen.com/us/products/discovery-and-translational-research/lab-essentials/buffers-reagents/puregene-accessories/?cmpid=PC_DA_NON_ BIOCOMPARE_ProductListing_ 0121_RD_MarketPlace_ProductC |
| Qubit V4 | Invitrogen | Q33238 | Nucleic acids quantification; https://www.thermofisher.com/order/catalog/product/Q33238 |
| rifampicin | FUJIFILM Wako Chemicals Co., Osaka, Japan | 4.98748E+12 | Sterilization; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0118-0100.html |
| RNA HS quantification kit | Invitrogen | Q32855 | Nucleic acids quantification; https://www.thermofisher.com/order/catalog/product/Q32852 |
| RNase solution | Nippon Gene | 313-01461 | RNA extraction; https://www.nippongene.com/siyaku/product/modifying-enzymes/rnase-a/rnase-s.html |
| Silk Mate 2S | Nosan Co., Ltd | not shown | Artificial diet; https://www.nosan.co.jp/business/fodder/ist.htm |
| Sodium Chloride | FUJIFILM Wako Chemicals Co. | 4.98748E+12 | NaCl; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0119-0166.html |
| Sodium Dodecyl Sulfate | FUJIFILM Wako Chemicals Co. | 4.98748E+12 | Cell lysis buffer (SDS); https://labchem-wako.fujifilm.com/jp/product/detail/W01W0119-1398.html |
| sodium hypochlorite aqueous solution | FUJIFILM Wako Chemicals Co., Osaka, Japan | 4.98748E+12 | egg separation; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0119-0220.html |
| Tetracycline Hydrochloride | FUJIFILM Wako Chemicals Co., Osaka, Japan | 4.98748E+12 | Sterilization; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0120-1656.html |
| Tris-HCl | FUJIFILM Wako Chemicals Co. | 4.98748E+12 | Cell lysis buffer; https://labchem-wako.fujifilm.com/jp/product/detail/W01W0120-1536.html |
| ultra-pure distilled water | Invitrogen | 10977023 | RNA extraction; https://www.thermofisher.com/order/catalog/product/10977015 |
Referências
- Gaston, K. J. The magnitude of global insect species richness. Conservation Biology. 5 (3), 283-296 (1991).
- Pogue, M. A world revision of the genus Spodoptera Guenée (Lepidoptera: Noctuidae). Memoirs of the American Entomological Society. 43, 1 (2002).
- Matsuura, H., Naito, A. Studies on the cold-hardiness and overwintering of Spodoptera litura F. (Lepidoptera: Noctuidae): VI. Possible overwintering areas predicted from meteorological data in Japan. Applied Entomology and Zoology. 32 (1), 167-177 (1997).
- Mita, K., et al. The construction of an EST database for Bombyx mori and its application. Proceedings of the National Academy of Sciences of the United States of America. 100 (24), 14121-14126 (2003).
- Kawamoto, M., et al. High-quality genome assembly of the silkworm, Bombyx mori. Insect Biochemistry and Molecular Biology. 107, 53-62 (2019).
- Fukui, T., et al. In vivo masculinizing function of the Ostrinia furnacalis Masculinizer gene. Biochemical and Biophysical Research Communications. 503 (3), 1768-1772 (2018).
- Arai, H., Ishitsubo, Y., Nakai, M., Inoue, M. N. A simple method to disperse eggs from lepidopteran scale-like egg masses and to observe embryogenesis. Entomological Science. 25 (1), 12497 (2022).
- vander Geest, L. P., Evenhuis, H. H. . Tortricid pests: their biology, natural enemies and control. , (1991).
- Kiuchi, T., et al. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature. 509 (7502), 633-636 (2014).
- Rand, M. D., Kearney, A. L., Dao, J., Clason, T. Permeabilization of Drosophila embryos for introduction of small molecules. Insect Biochemistry and Molecular Biology. 40 (11), 792-804 (2010).
- Kageyama, D., Traut, W. Opposite sex-specific effects of Wolbachia and interference with the sex determination of its host Ostrinia scapulalis. Proceedings of the Royal Society B. 271 (1536), 251-258 (2004).
- Arai, H., Lin, S. R., Nakai, M., Kunimi, Y., Inoue, M. N. Closely related male-killing and nonmale-killing Wolbachia strains in the oriental tea tortrix Homona magnanima. Microbial Ecology. 79 (4), 1011-1020 (2020).
- Schalamun, M., et al. Harnessing the MinION: An example of how to establish long-read sequencing in a laboratory using challenging plant tissue from Eucalyptus pauciflora. Molecular Ecology Resources. 19 (1), 77-89 (2019).
- Winnebeck, E. C., Millar, C. D., Warman, G. R. Why does insect RNA look degraded. Journal of Insect Science. 10 (1), 159 (2010).
- Ivey, C. T., Carr, D. E., Eubanks, M. D. Effects of inbreeding in Mimulus guttatus on tolerance to herbivory in natural environments. Ecology. 85 (2), 567-574 (2004).
- Saccheri, I., et al. Inbreeding and extinction in a butterfly metapopulation. Nature. 392 (6675), 491-494 (1998).
- Crnokrak, P., Roff, D. A. Inbreeding depression in the wild. Heredity. 83 (3), 260-270 (1999).
- Keller, L. F., Waller, D. M. Inbreeding effects in wild populations. Trends in Ecology & Evolution. 17 (5), 230-241 (2002).
- Margaritis, L. H., Kafatos, F. C., Petri, W. H. The eggshell of Drosophila melanogaster. I. Fine structure of the layers and regions of the wild-type eggshell. Journal of Cell Science. 43 (1), 1-35 (1980).
- Sugimoto, T. N., Ishikawa, Y. A male-killing Wolbachia carries a feminizing factor and is associated with degradation of the sex-determining system of its host. Biology Letters. 8 (3), 412-415 (2012).

