磷脂介质诱导的三维培养物转化
Summary
本协议描述了未转化的乳腺上皮细胞系MCF10A的3D “顶部”培养物的建立,该培养物已被修改以研究血小板活化因子(PAF)诱导的转化。免疫荧光已被用于评估转化,并详细讨论。
Abstract
已经开发了几种模型来研究癌症,例如啮齿动物模型和已建立的细胞系。使用这些模型的研究提供了对致癌作用的宝贵见解。细胞系提供了与乳腺肿瘤发生相关的分子信号传导失调的理解,而啮齿动物模型广泛用于研究体内乳腺癌的细胞和分子特征。 乳腺上皮细胞和癌细胞的3D培养物的建立有助于通过模拟体内体外条件来弥合体内和体外模型之间的差距。该模型可用于了解复杂分子信号传导事件的失调和乳腺癌变过程中的细胞特征。在这里,修改了3D培养系统以研究磷脂介质诱导的(血小板活化因子,PAF)转化。免疫调节剂和其他分泌分子在乳房肿瘤的发生和进展中起主要作用。在本研究中,暴露于乳腺上皮细胞的3D腺泡培养物暴露于PAF表现出转化特征,例如极性丧失和细胞特征改变。该3D培养系统将有助于揭示由肿瘤微环境中各种小分子实体诱导的遗传和/或表观遗传扰动。此外,该系统还将为鉴定可能参与转化过程的新型和已知基因提供一个平台。
Introduction
有无数的模型可用于研究癌症的进展,每个模型都是独一无二的,代表了这种复杂疾病的一种亚型。每个模型都为癌症生物学提供了独特而有价值的见解,并改进了模拟实际疾病状况的方法。作为单层生长的已建立细胞系为体外重要过程提供了宝贵的见解,例如增殖,侵袭性,迁移和凋亡1。尽管二维(2D)细胞培养一直是研究哺乳动物细胞对几种环境扰动的反应的传统工具,但推断这些发现以预测组织水平的反应似乎不够令人信服。2D培养的主要局限性在于产生的微环境与乳腺组织本身的微环境有很大不同2。2D培养缺乏细胞与细胞外基质的相互作用,这对于任何组织的生长都至关重要。此外,细胞在单层培养物中经历的拉力会阻碍这些细胞的极性,从而改变细胞信号传导和行为3,4,5。三维(3D)培养系统具有模拟体外体内条件的能力,为癌症研究领域开辟了一条新途径。 在2D细胞培养中丢失的许多关键微环境线索可以使用富含层粘连蛋白的细胞外基质(lrECM)的3D培养物重新建立6。
各种研究已经确定了肿瘤微环境在致癌中的重要性7,8。炎症相关因素是微环境的主要部分。血小板活化因子(PAF)是由各种免疫细胞分泌的磷脂介质,介导多种免疫反应9,10。高水平的PAF由不同的乳腺癌细胞系分泌,并与增殖增强有关11。我们实验室的研究表明,腺泡培养物中PAF的长期存在导致乳腺上皮细胞的转化12。PAF激活PAF受体(PAFR),激活PI3K/Akt信号轴13。据报道,PAFR 也与 EMT、侵袭和转移有关14。
本协议展示了一个模型系统来研究PAF诱导的转化,使用乳腺上皮细胞的3D培养物,如Chakravarty等人之前所描述的那样12。在细胞外基质(3D培养物)上生长的乳腺上皮细胞倾向于形成极化生长停滞球状体。这些被称为腺泡,与乳腺组织的腺泡非常相似,乳腺是乳腺最小的功能单位,在 体内15。 这些球体(图1A,B)由单层紧密堆积的极化上皮细胞组成,围绕空心腔并附着在基底膜上(图1C)。这种形态发生过程已在文献16中得到了很好的描述。当接种在lrECM上时,细胞经历分裂和分化以形成细胞簇,然后从第4天开始极化。到第8天,腺泡由一组与细胞外基质直接接触的极化细胞和封闭在外极化细胞内的一簇非极化细胞组成,与基质没有接触。已知这些未极化的细胞在培养的第12天发生凋亡,形成空心腔。到第16天,形成生长停滞的结构16。
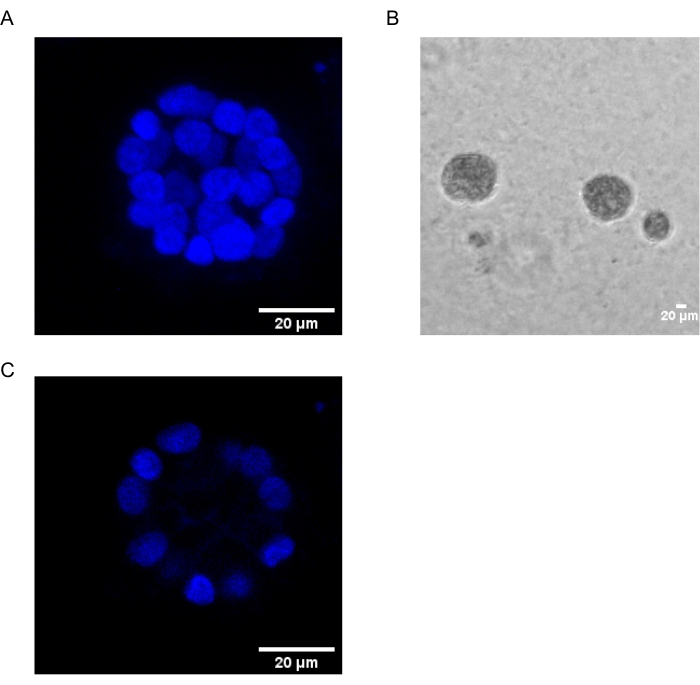
图1:用核染色的腺泡中的细胞核 。 (A)腺泡的3D构造。(B)在基质胶上生长20天的MCF10A腺泡的相衬图像。(C)最中间的部分显示了空心腔的存在。比例尺 = 20 μm。 请点击此处查看此图的大图。
与2D培养不同,腺泡培养有助于通过明显的形态变化区分正常细胞和转化细胞。未转化的乳腺上皮细胞形成具有空腔的腺泡,模仿正常人乳腺腺泡。这些球体在转化时显示出破坏的形态,其特征是极性严重丧失(癌症的标志之一),没有管腔或空腔的破坏(由于细胞凋亡的逃避),这可能是由于各种基因的失调引起的17,18,19,20.这些转化可以使用常用的技术(如免疫荧光)进行研究。因此,3D细胞培养模型可以作为研究乳腺腺泡形态发生和乳腺癌发生的过程的简单方法。建立3D培养系统以了解磷脂介质PAF的作用将有助于高通量临床前药物筛选。
这项工作采用了3D“顶部”培养协议16,21,以研究PAF22诱导的转化。使用免疫荧光研究了腺泡暴露于磷脂介质引起的表型变化。研究中使用了各种极性和上皮到间充质转化(EMT)标记12,16。表1提到了它们的正常定位和转化时的预期表型。
| 抗体 | 标志着 | 正常本地化 | 转化表型 |
| α6-整合素 | 基底外侧 | 基底侧染色较弱 | 强烈的横向/顶端染色 |
| β-连环蛋白 | 细胞-细胞连接 | 基底外侧 | 异常/核或细胞质定位 |
| 维门汀 | EMT | 不存在/弱存在 | 上调 |
表1:研究中使用的标记物。 在存在和不存在PAF治疗的情况下,使用不同的标记物进行定位。
该方法可以最好地用于研究/筛选各种乳腺癌亚型的合理药物和靶基因。这可以提供更接近 体内 情景的药物反应数据,有助于更快、更可靠的药物开发。此外,该系统可用于研究与药物反应和耐药性相关的分子信号传导。
Protocol
Representative Results
Discussion
已建立的基于细胞系的模型被广泛用于研究致癌过程。细胞的单层培养继续提供对介导癌细胞特征变化的各种分子信号通路的见解32。关于众所周知的癌基因(如Ras,Myc和突变p53)的作用的研究首先使用单层培养物作为模型系统33,34,35,36。然而,2D培养模型缺乏体内存在的?…
Declarações
The authors have nothing to disclose.
Acknowledgements
我们感谢IISER浦那显微镜设施提供设备和基础设施以及实验支持。这项研究得到了印度政府生物技术部(DBT)(BT / PR8699 / MED/30 / 1018 / 2013),印度政府科学与工程研究委员会(SERB)(EMR / 2016 / 001974)的资助,部分资金来自IISER,浦那核心资金。A.K.由CSIR-SRF奖学金资助,洛杉矶由DST-INSPIRE奖学金资助,V.C由DBT资助(BT / PR8699 / MED/30 / 1018 / 2013)。
Materials
| 0.05% Trypsin EDTA | Invitrogen | 25300062 | |
| 16% paraformaldehyde | Alfa Aesar | AA433689M | |
| Anti Mouse Alexa Flour 488 | Invitrogen | A11029 | |
| Anti Rabbit Alexa Flour 488 | Invitrogen | A-11008 | |
| BSA | Sigma | A7030 | |
| Chamber Coverglass | Nunc | 155409 | |
| Cholera Toxin | Sigma | C8052-1MG | 1 mg/mL in dH2O |
| Confocal Microscope | Leica | Leica SP8 | |
| DMEM | Gibco | 11965126 | |
| EDTA | Sigma | E6758 | |
| EGF | Sigma | E9644-0.2MG | 100 mg/mL in dH2O |
| F(ab’)2 fragment of antibody raised in goat against mouse antigen | Jackson Immunoresearch | 115-006-006 | |
| GM130 antibody | Abcam | ab52649 | |
| Goat Serum | Abcam | ab7481 | |
| Hoechst | Invitrogen | 33258 | |
| Horse Serum | Gibco | 16050122 | |
| Hydrocortisone | Sigma | H0888 | 1 mg/mL in ethanol |
| Image Processing Software | ImageJ | ||
| Insulin | Sigma | I1882 | 10 mg/mL stock dH2O |
| lrECM (Matrigel) | Corning | 356231 | |
| Mounting reagent (Slow fade Gold Anti-fade) | Invitrogen | S36937 | |
| Nuclear Stain (Hoechst) | Invitrogen | 33258 | |
| PAF | Cayman Chemicals | 91575-58-5 | Methylcarbamyl PAF C-16, procured as a 10 mg/mL in ethanol |
| Penicillin-Streptomycin | Lonza | 17-602E | |
| Sodium Azide | Sigma | S2002 | |
| Tris Base | Sigma | B9754 | |
| Triton X-100 | Sigma | T8787 | |
| Tween 20 | Sigma | P9416 | |
| Vimentin antibody | Abcam | ab92547 | |
| α6-integrin antibody | Millipore | MAB1378 |
Referências
- Lacroix, M., Leclercq, G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Research and Treatment. 83 (3), 249-289 (2004).
- Vargo-Gogola, T., Rosen, J. M. Modelling breast cancer: one size does not fit all. Nature Reviews Cancer. 7 (9), 659-672 (2007).
- Runswick, S. K., O’Hare, M. J., Jones, L., Streuli, C. H., Garrod, D. R. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nature Cell Biology. 3 (9), 823-830 (2001).
- Streuli, C. H., Bailey, N., Bissell, M. J. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. Journal of Cell Biology. 115 (5), 1383-1395 (1991).
- Streuli, C. H., et al. Laminin mediates tissue-specific gene expression in mammary epithelia. Journal of Cell Biology. 129 (3), 591-603 (1995).
- Bissell, M. J., Kenny, P. A., Radisky, D. C. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harbor Symposia on Quantitative Biology. 70, 343-356 (2005).
- Heinrich, E. L., et al. The inflammatory tumor microenvironment, epithelial mesenchymal transition and lung carcinogenesis. Cancer Microenvironment. 5 (1), 5-18 (2012).
- Gonda, T. A., Tu, S., Wang, T. C. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle. 8 (13), 2005-2013 (2009).
- Berdyshev, E. V., Schmid, P. C., Krebsbach, R. J., Schmid, H. H. Activation of PAF receptors results in enhanced synthesis of 2-arachidonoylglycerol (2-AG) in immune cells. The FASEB Journal. 15 (12), 2171-2178 (2001).
- Rola-Pleszczynski, M., Stankova, J. Cytokine gene regulation by PGE(2), LTB(4) and PAF. Mediators of Inflammation. 1 (2), 5-8 (1992).
- Bussolati, B., et al. PAF produced by human breast cancer cells promotes migration and proliferation of tumor cells and neo-angiogenesis. The American Journal of Pathology. 157 (5), 1713-1725 (2000).
- Chakravarty, V., et al. Prolonged exposure to platelet activating factor transforms breast epithelial cells. Frontiers in Genetics. 12, 634938 (2021).
- Chen, J., et al. Platelet-activating factor receptor-mediated PI3K/AKT activation contributes to the malignant development of esophageal squamous cell carcinoma. Oncogene. 34 (40), 5114-5127 (2015).
- Chen, J., et al. Feed-forward reciprocal activation of PAFR and STAT3 regulates epithelial-mesenchymal transition in non-small cell lung cancer. Pesquisa do Câncer. 75 (19), 4198-4210 (2015).
- Vidi, P. A., Bissell, M. J., Lelievre, S. A. Three-dimensional culture of human breast epithelial cells: the how and the why. Methods in Molecular Biology. 945, 193-219 (2013).
- Debnath, J., Muthuswamy, S. K., Brugge, J. S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 30 (3), 256-268 (2003).
- Barcellos-Hoff, M. H., Aggeler, J., Ram, T. G., Bissell, M. J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 105 (2), 223-235 (1989).
- Petersen, O. W., Ronnov-Jessen, L., Howlett, A. R., Bissell, M. J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proceedings of the National Academy of Sciences. 89 (19), 9064-9068 (1992).
- Shaw, K. R., Wrobel, C. N., Brugge, J. S. Use of three-dimensional basement membrane cultures to model oncogene-induced changes in mammary epithelial morphogenesis. Journal of Mammary Gland Biology and Neoplasia. 9 (4), 297-310 (2004).
- Weaver, V. M., Fischer, A. H., Peterson, O. W., Bissell, M. J. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochemistry and Cell Biology. 74 (6), 833-851 (1996).
- Lee, G. Y., Kenny, P. A., Lee, E. H., Bissell, M. J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nature Methods. 4 (4), 359-365 (2007).
- Bodakuntla, S., Libi, A. V., Sural, S., Trivedi, P., Lahiri, M. N-nitroso-N-ethylurea activates DNA damage surveillance pathways and induces transformation in mammalian cells. BMC Cancer. 14, 287 (2014).
- Banerjee, A., et al. A rhodamine derivative as a "lock" and SCN− as a "key": visible light excitable SCN− sensing in living cells. Chemical Communications. 49 (25), 2527-2529 (2013).
- Ren, G., et al. Reduced basal nitric oxide production induces precancerous mammary lesions via ERBB2 and TGFbeta. Scientific Reports. 9 (1), 6688 (2019).
- Anandi, L., Chakravarty, V., Ashiq, K. A., Bodakuntla, S., Lahiri, M. DNA-dependent protein kinase plays a central role in transformation of breast epithelial cells following alkylation damage. Journal of Cell Science. 130 (21), 3749-3763 (2017).
- Sonnenberg, A., et al. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. Journal of Cell Biology. 113 (4), 907-917 (1991).
- Pignatelli, M., Cardillo, M. R., Hanby, A., Stamp, G. W. Integrins and their accessory adhesion molecules in mammary carcinomas: loss of polarization in poorly differentiated tumors. Human Pathology. 23 (10), 1159-1166 (1992).
- Natali, P. G., et al. Changes in expression of alpha 6/beta 4 integrin heterodimer in primary and metastatic breast cancer. British Journal of Cancer. 66 (2), 318-322 (1992).
- Davis, T. L., Cress, A. E., Dalkin, B. L., Nagle, R. B. Unique expression pattern of the alpha6beta4 integrin and laminin-5 in human prostate carcinoma. Prostate. 46 (3), 240-248 (2001).
- Liu, J. S., Farlow, J. T., Paulson, A. K., Labarge, M. A., Gartner, Z. J. Programmed cell-to-cell variability in Ras activity triggers emergent behaviors during mammary epithelial morphogenesis. Cell Reports. 2 (5), 1461-1470 (2012).
- Liu, C. Y., Lin, H. H., Tang, M. J., Wang, Y. K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget. 6 (18), 15966-15983 (2015).
- Kapalczynska, M., et al. 2D and 3D cell cultures-a comparison of different types of cancer cell cultures. Archives of Medical Science. 14 (4), 910-919 (2018).
- Schulze, A., Lehmann, K., Jefferies, H. B., McMahon, M., Downward, J. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes & Development. 15 (8), 981-994 (2001).
- McCarthy, S. A., Samuels, M. L., Pritchard, C. A., Abraham, J. A., McMahon, M. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes & Development. 9 (16), 1953-1964 (1995).
- Willis, A., Jung, E. J., Wakefield, T., Chen, X. Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene. 23 (13), 2330-2338 (2004).
- Haupt, S., Raghu, D., Haupt, Y. Mutant p53 drives cancer by subverting multiple tumor suppression pathways. Frontiers in Oncology. 6, 12 (2016).
- Langhans, S. A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Frontiers in Pharmacology. 9, 6 (2018).
- Edmondson, R., Broglie, J. J., Adcock, A. F., Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. ASSAY and Drug Development Technologies. 12 (4), 207-218 (2014).
- Lv, D., Hu, Z., Lu, L., Lu, H., Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncology Letters. 14 (6), 6999-7010 (2017).
- Jensen, C., Teng, Y. Is it time to start transitioning from 2D to 3D cell culture. Frontiers in Molecular Biosciences. 7, 33 (2020).
- Saraiva, D. P., Matias, A. T., Braga, S., Jacinto, A., Cabral, M. G. Establishment of a 3D co-culture with MDA-MB-231 breast cancer cell line and patient-derived immune cells for application in the development of immunotherapies. Frontiers in Oncology. 10, 1543 (2020).

