A Flow Cytometry-Based High-Throughput Technique for Screening Integrin-Inhibitory Drugs
Summary
This protocol describes a flow cytometry-based, high-throughput screening method to identify small-molecule drugs that inhibit β2 integrin activation on human neutrophils.
Abstract
This protocol aims to establish a method for identifying small molecular antagonists of β2 integrin activation, utilizing conformational-change-reporting antibodies and high-throughput flow cytometry. The method can also serve as a guide for other antibody-based high-throughput screening methods. β2 integrins are leukocyte-specific adhesion molecules that are crucial in immune responses. Neutrophils rely on integrin activation to exit the bloodstream, not only to fight infections but also to be involved in multiple inflammatory diseases. Controlling β2 integrin activation presents a viable approach for treating neutrophil-associated inflammatory diseases. In this protocol, a monoclonal antibody, mAb24, which specifically binds to the high-affinity headpiece of β2 integrins, is utilized to quantify β2 integrin activation on isolated primary human neutrophils. N-formylmethionyl-leucyl-phenylalanine (fMLP) is used as a stimulus to activate neutrophil β2 integrins. A high-throughput flow cytometer capable of automatically running 384-well plate samples was used in this study. The effects of 320 chemicals on β2 integrin inhibition are assessed within 3 h. Molecules that directly target β2 integrins or target molecules in the G protein-coupled receptor-initiated integrin inside-out activation signaling pathway can be identified through this approach.
Introduction
Many inflammatory diseases are characterized by the infiltration of neutrophils at the site of swelling or injury1. To infiltrate these tissues, neutrophils must complete the neutrophil recruitment cascade, which involves arrest to the endothelium, extravasation across the vessel wall, and recruitment into the tissue2. Circulating neutrophils need β2 integrin activation to complete this cascade, especially for the arrest phase. Thus, integrin-inhibiting drugs that reduce neutrophil adhesion, extravasation, and recruitment may effectively treat inflammatory diseases3,4.
β2 integrins have been targeted for inflammatory diseases before. Efalizumab, a monoclonal antibody directly targeting integrin αLβ2, was developed to treat psoriasis5. However, efalizumab was withdrawn due to its lethal side effect – progressive multifocal leukoencephalopathy resulting from JC virus reactivation6,7. New anti-inflammatory integrin-based therapies should consider maintaining the anti-infection functions of leukocytes to minimize side effects. The side effects of efalizumab might be due to the prolonged circulation of monoclonal antibodies in the bloodstream, which could inhibit immune functions in the long term8. A recent study shows that efalizumab mediates αLβ2 crosslinking and the unwanted internalization of α4 integrins, providing an alternative explanation for the side effects9. Thus, short-lived, small-molecule antagonists might avoid this problem.
A high-throughput method to screen small-molecule β2 integrin antagonists using human neutrophils is presented here. β2 integrin activation requires conformational changes of the integrin ectodomain to gain access to and increase its binding affinity to its ligand. In the canonical switchblade model, the bent-closed integrin ectodomain first extends to an extended-closed conformation and then opens its headpiece to a fully activated extended-open conformation10,11,12,13. There is also an alternative pathway that starts from the bent-closed to bent-open and extended-open, eventually14,15,16,17,18,19. The conformation-specific antibody mAb24 binds to an epitope in the human β2-I-like domain when the headpiece of the ectodomain is open20,21,22,23.
Here, mAb24-APC is used to determine whether the β2 integrins are activated. To activate neutrophils and integrin, N-formylmethionyl-leucyl-phenylalanine (fMLP), a bacterial-derived short chemotactic peptide that can activate neutrophil β2 integrins24, is used as a stimulus in this protocol. When fMLP binds to the Fpr1 on neutrophils, downstream signaling cascades involving G-proteins, phospholipase Cβ, and phosphoinositide 3-kinase γ are activated. These signaling events ultimately result in integrin activation via the inside-out signaling pathway18,25. Besides small molecule antagonists that directly bind to β2 integrins and prevent conformational changes of integrin activation26, compounds that can inhibit components in the β2 integrin inside-out activation signaling pathway would also be detected with this method. Automated flow cytometers enable high-throughput screening. Identifying new antagonists may not only deepen our understanding of integrin physiology but also provide translational insight into integrin-based anti-inflammation therapy.
Protocol
Heparinized whole-blood samples were obtained from de-identified healthy human donors after obtaining informed consent, as approved by the Institutional Review Board of UConn Health, following the principles of the Declaration of Helsinki. Informed consent was obtained from all donors. The inclusion/exclusion criteria for this study were carefully developed to ensure the suitability of participants and to minimize potential risks. Eligible participants were aged between 18 and 65 years, of any ethnicity, fluent in English, and capable of providing informed consent. Excluded participants included those unable to provide informed consent for themselves, such as those requiring a legally authorized representative, individuals under 18 or over 65 years, incarcerated individuals, and pregnant women. Additionally, participants had to be free from anti-inflammatory medication usage and inflammatory conditions. Current infections or ongoing chronic or acute inflammatory conditions were also exclusion criteria. Finally, individuals with a current or recent history of COVID-19 infection were ineligible for the study. These criteria were designed to ensure participant safety and suitability while minimizing potential confounding factors that could impact the study results.
1. Preparation of reagents
- Neutrophil medium: Prepare the neutrophil medium by adding 2% human serum albumin to RPMI-1640 without phenol red (see Table of Materials).
- fMLP solution: Prepare the fMLP solution by diluting it 100 times from the 10 mM fMLP dimethyl sulfoxide (DMSO) storage solution (see Table of Materials) with RPMI 1640 without phenol red, resulting in a 100 µM fMLP solution.
- Antibody solution: Dilute 120 µL of allophycocyanin (APC)-conjugated monoclonal antibody mAb24 (mAb24-APC) (see Table of Materials), which reports the high-affinity conformation of β2 integrin, in 10 mL of neutrophil medium, creating a 1.2 µg/mL mAb24-APC solution.
- Compound library: Dilute the initial compound library with a concentration of 10 mM to 1 mM by adding 5 µL of the 10 mM library (see Table of Materials) to 45 µL of DMSO in 384-well plates using the liquid handler. Subsequently, transfer 5 µL per well of the 1 mM compound library to an empty 384-well plate using the liquid handler.
- As illustrated in Figure 1, four columns (1, 2, 23, 24) contained only DMSO but no compounds, serving as positive and negative controls in the screening. Further dilute each well by adding 45 µL of RPMI-1640 without phenol red, resulting in a final concentration of 100 µM for all compounds.
2. Neutrophil isolation from human blood
- Using a serological pipette, carefully layer 4 mL of blood over 8 mL of a commercially available density gradient medium (see Table of Materials) in a 15 mL centrifuge tube (Figure 2A).
- Centrifuge the blood at 550 × g for 30-50 min at 20 °C. Decelerate the rotor gradually (deceleration factor of 1).
NOTE: The successful separation of neutrophils (band 2 in Figure 2B) from red blood cells may vary among donors. For most donors, a 30 min centrifugation is sufficient. However, for some donors, an additional 10-30 min of centrifugation may be required if separation is not successful (Figure 2C). - Carefully remove the plasma (the yellow liquid on top) and mononuclear cells (the upper cloudy band; PBMC band in Figure 2B) using a 1 mL pipette.
- Collect neutrophils from the lower cloudy band (neutrophil band in Figure 2B) and approximately 3-4 mL below the clear liquid into a 15 mL centrifuge tube containing 10 mL of phosphate-buffered saline (PBS). Gently mix the neutrophil suspension by inverting it 2-3 times.
- Centrifuge the suspension at 400 × g for 10 min at 20 °C, and then carefully remove the supernatant by decanting.
- Gently resuspend the pellet with 5 mL of PBS and centrifuge it at 300 × g for 5 min at 20 °C.
- Remove the supernatant by decanting, and carefully eliminate any residual supernatant around the tube mouth and on the tube wall using vacuum suction. Resuspend the pellet in 1 mL of neutrophil medium.
- Count the cell numbers using a hemocytometer. Typically, 1 to 4 × 107 neutrophils were obtained from 8 mL of human blood.
NOTE: There may be red blood cell contamination in the neutrophil suspension. Red blood cells do not significantly affect most assays and can prevent neutrophil activation/priming27. Red blood cells need to be lysed before counting to obtain an accurate neutrophil concentration. Add 10 µL of the cell suspension to 891 µL of deionized water for 10-30 s to lyse the red blood cells, then add 99 µL of 10× PBS to balance the osmotic pressure, preventing lysis of the neutrophils. - Adjust the cell density to 6.25 × 105 cells/mL by adding neutrophil medium.
3. Preparation of the 384-well plate
- In a 1.5 mL centrifuge tube, combine 1 mL of the antibody solution (1.2 µg/mL mAb24-APC) with 0.2 mL of neutrophil medium to create a 1 µg/mL mAb24-APC solution. Then, add 25 µL of this mixture to the negative control wells in a 384-well plate (the blue wells in Figure 1).
- In a 15 mL centrifuge tube, mix 9 mL of the antibody solution with 21.6 µL of the fMLP solution (100 µM) and 1.7784 mL of neutrophil medium to create a solution containing 1 µg/mL mAb24-APC and 200 nM fMLP. Next, add 25 µL of this mixture to the positive control and testing wells in a 384-well plate (the red and blue wells in Figure 1).
- Transfer 5 µL of the 100 µM compound solutions from the compound library plate to the 384-well plate using a multi-channel pipette.
- Centrifuge the liquid for 1 min at 500 × g, at room temperature.
NOTE: To centrifuge plates, a swing-bucket rotor and plate buckets are required.
4. Treatment of cells
- Add 20 µL of the neutrophil suspension to each well using a 16-channel pipette (5-50 µL range, for each column of the 384-well plate). Gently mix 5-10 times using the pipette, maintaining a consistent time interval between each pipetting.
NOTE: The final concentrations in the wells are as follows: neutrophils 2.5 × 105 cells/mL (all wells); mAb24-APC 0.5 µg/mL (all wells); fMLP 100 nM (positive control and testing wells); compounds 10 µM (testing wells). - Incubate the plate on a shaker at 300 rpm, at room temperature (RT), for 10 min.
- Fix the cells by adding 3 µL of 16% paraformaldehyde (PFA) to each well using a 16-channel pipette (final PFA concentration ~0.91%). Then, incubate on ice for 10 min.
NOTE: It is recommended to maintain a consistent time interval between different columns when adding neutrophils and PFA. This ensures that neutrophils in each well have the same incubation time with fMLP and mAb24-APC.
5. Flow cytometry
- Turn on the flow cytometer (see Table of Materials), and ensure that the computer connected to the instrument is also turned on.
- Ensure that the sheath fluid container is filled, and the waste container is empty and properly connected to the instrument.
- Load the 384-well plate onto the sample loader of the flow cytometer. Ensure that the A1 well of the plate aligns with the A1 mark on the loader.
- Open the software and create a new experiment. Select 384 well and choose the desired fluorophores. Next, click on Plate setup, drag to select all wells, and then click on Sample. Turn on the "High throughput" switch, and select High under the rate panel.
- In the volume box, enter 50. Select samples in odd-numbered columns and then activate the "Agitation" switch. Finally, name the samples in the right panel.
- Click on Plot and gate. Then, click on the Apply button (two arrows on a green-filled circle), and subsequently, click on the play button (triangle shape). Wait for a few seconds to observe some cells from the first well, and then click on the pause button.
- Adjust the FSC and SSC voltages to ensure proper visualization of cells on the plot. Click on Acquisition, and then click on the play button (triangle shape) to start the assay.
- The flow cytometer will sequentially sample ~50 µL of 1000-3000 neutrophils from each well. It will take approximately 3 h to complete the entire 384-well plate.
- After completing all samples, export the .fcs files for the next step.
6. Data analysis
- Open the flow cytometry data analysis software (see Table of Materials). Select and drag the folder containing all .fcs files into the software window, then release to import the data into the software.
- Double-click on one sample, gate single neutrophils based on FSC and SSC28,29,30 in the popped-up window, as shown in Figure 3. Select the gated rows and drag them to the folder, then release to apply the gating to all samples in that folder.
- Calculate and export the APC median fluorescence intensity (MFI) of neutrophils from each well using the software's table editor function. Click on Add Column under the Edit tab. Select Median in the Statistic tab, choose the gated population neutrophils, and select APC as the parameter. Click on the OK button.
- Return to the "Table editor" tab, select All Samples in the "Group" tab, and press the Create table button. A new window will appear displaying the created table. Save it as a text, CSV, or Excel file.
- Open the exported table, calculate the mean value and standard deviation of the APC MFI for the positive and negative control samples (Figure 4).
- Calculate the Z'-factor (Z') of the plate using the equation:
Z' = 1 – 3(σp + σn)/(µp – µn),
where σp and σn are the standard deviations of the positive and negative controls, respectively, and µp and µn are the means of the positive and negative controls, respectively31.
NOTE: The Z' factor indicates the separation of the positive and negative control distributions. A Z' of 0 means no separation, while a Z' of 0.5 indicates equal separation. As mentioned in a previous study31, Z' > 0.5 indicates an excellent assay for compound screening. A Z' in the range of 0.5 to 0 is acceptable, but it may only identify strong hits in the assay, which will require further validation in secondary assays. - Consider compounds as "hits" for screening when the APC MFI of compound-treated neutrophils is lower than three times the standard deviation of the positive control MFI subtracted from the positive control MFI (P = 0.0013, represented by the dotted line in Figure 4).
Representative Results
Data from a representative 384-well plate screening (Figure 4) revealed that negative controls had an MFI of mAb24-APC of 3236 ± 110, while positive controls had an MFI of mAb24-APC of 7588 ± 858. The Z' factor for this plate is approximately 0.33, which is within an acceptable range31. However, Z' requires further validation in secondary assays.
To normalize the data, all values were scaled to assign a maximum value of 1 to the positive mean and a minimum value of 0 to the negative mean. The Z' factor will undergo more rigorous validation in secondary assays. The cutoff for this plate is set at 0.41, which means that samples with a relative MFI lower than 0.41 will be considered as hits inhibiting fMLP-induced β2 integrin activation in human neutrophils. No hits were identified from this plate.
To confirm the protocol's effectiveness, Nexinhib20, which inhibits β2 integrin activation by antagonizing Rac-1 function, and lifitegrast, which antagonizes integrin αLβ2 directly32,33, were used. However, incubation times were adjusted to one hour for Nexinhib20 and half an hour for lifitegrast. The resulting data from these experiments were normalized using the same scaling method described above. These data points were then combined with the plate results and analyzed collectively (Figure 4).
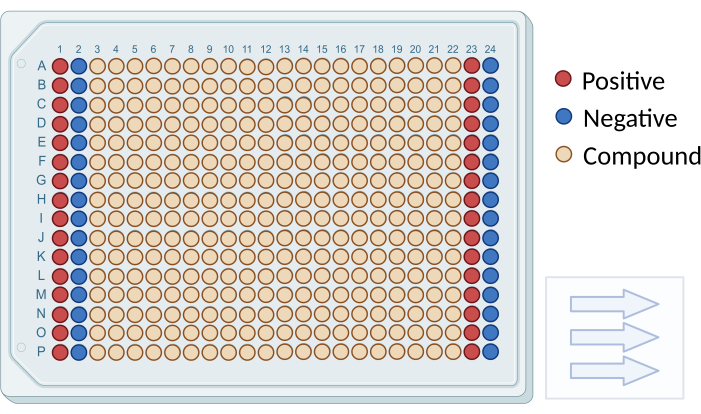
Figure 1: Plate layout for compound screening. A schematic diagram illustrating the arrangement of screening compounds and controls in a 384-well plate. Negative control wells are depicted in blue (columns 1 and 23), and positive control wells are shown in red (columns 2 and 24). Testing wells are represented in beige (columns 3 to 22). Arrows indicate the sequence for reading the plate. Please click here to view a larger version of this figure.
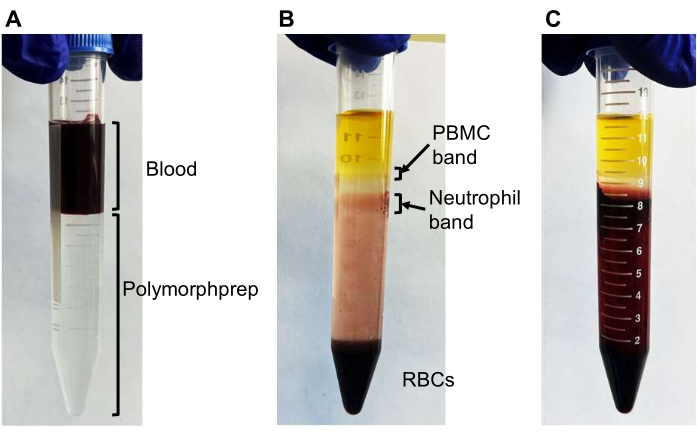
Figure 2: Neutrophil separation using density gradient medium. Representative photos demonstrating the successful and unsuccessful separation of neutrophils using density gradient medium. (A) Initially, 4 mL of blood is layered onto 8 mL of density gradient medium. (B) After centrifugation, two cloudy bands should be visible: the upper band containing primarily peripheral blood mononuclear cells (PBMCs) and the lower band containing mostly neutrophils with some red blood cells (RBCs). Most RBCs are pelleted at the bottom. (C) An unsuccessful separation where RBCs are not pelleted, and the neutrophil band is not observed. Additional centrifugation (10-30 min) would be required to separate the neutrophil band. Please click here to view a larger version of this figure.
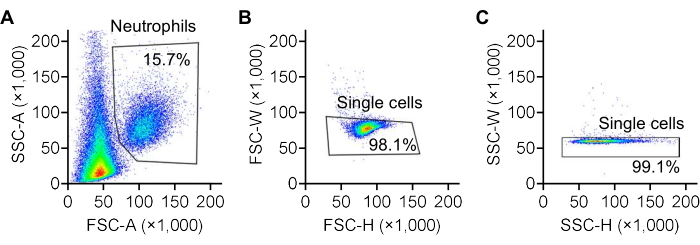
Figure 3: Gating of neutrophils using FSC/SSC plots. Representative forward scatter (FSC) and side scatter (SSC) plots illustrating the gating strategy for identifying neutrophils. (A) Neutrophils are gated based on the area of forward scatter (FSC-A) and side scatter (SSC-A) recorded by the flow cytometer. (B) Single cells are further gated based on the width (FSC-W) and height (FSC-H) of forward scatter, and (C) the width (SSC-W) and height (SSC-H) of side scatter. The color scale represents cell density, transitioning from red to yellow, green, and blue as density decreases. Please click here to view a larger version of this figure.
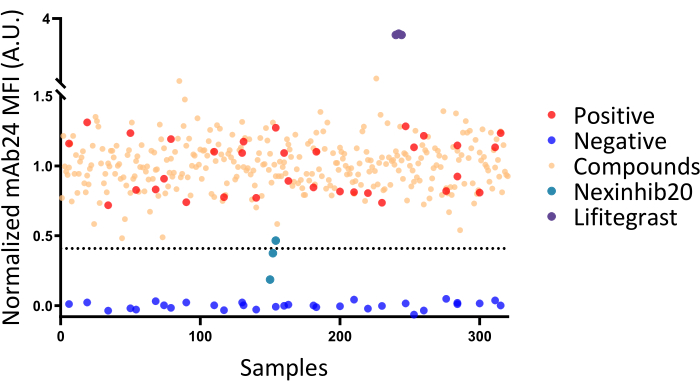
Figure 4: Screening results in a representative 384-well plate. The screening results from a representative 384-well plate, demonstrating a Z' factor of 0.3. Negative and positive controls are indicated by blue and red dots, respectively. Testing samples treated with various compounds are represented as beige dots. The dashed line represents the mean fluorescence intensity (MFI) cut-off for identifying hits. None of the tested compounds were identified as β2 integrin antagonists, as all tested compounds displayed MFI values above the cut-off line. Results from independent experiments testing known β2 integrin antagonists with varying incubation times (Nexinhib20 for 1 h, lifitegrast for half an hour) are pooled for presentation in this figure. Please click here to view a larger version of this figure.
Discussion
The initiation and termination of neutrophil stimulation and staining are determined by the addition of neutrophils and the fixative PFA. Therefore, ensuring the same time interval between pipetting neutrophils or PFA into each column is critical. This ensures that the stimulation and staining time of neutrophils from each well remains consistent. Due to the short lifespan of neutrophils, the entire experiment, from collecting blood from donors to completing flow cytometry, must be carried out on the same day. Neutrophils are highly sensitive to temperature changes and can become activated when exposed to rapid temperature increases, such as transitioning from 4 °C to room temperature or from room temperature to 37 °C. Additionally, based on our previous experience, fMLP-induced neutrophil β2 integrin activation does not occur when cells are stored on ice or at 4 °C (data not shown). Therefore, before fixation, whole blood and neutrophils should be kept at room temperature or 20 °C (during the isolation centrifugation step). Do not place whole blood and neutrophils on ice.
The staining of mAb24 in negative controls should yield very low results. If a high mAb24 staining level is observed in an experiment, please check the following: (1) whether there was a significant temperature change during the experiment before fixation; (2) whether there was fMLP or endotoxin contamination in the neutrophil medium; (3) whether sample handling was too aggressive, such as generating bubbles during pipetting and mixing.
The current protocol employs mAb24 to report the opening of the β2 integrin headpiece. Theoretically, KIM127, a monoclonal antibody reporting the extension of β2 integrins34,35, can be used in conjunction with mAb24 to comprehensively assess β2 integrin conformation. However, the signal-to-noise ratio of KIM127 staining (1.5 to 2-fold) is not as favorable as that of mAb24 (5 to 10-fold), which typically does not provide a satisfactory Z' factor in the 384-well plate assay. In the 96-well plate assay, samples can be washed before performing flow cytometry, reducing soluble antibody-derived background signals. Therefore, the KIM127-based assay can be conducted in the 96-well plate assay, which has lower throughput compared to the 384-well plate assay.
Since this method uses fluorescence intensity as a readout to assess the integrin inhibitory effect of drugs, some fluorescent drugs may interfere with the results. Additionally, toxic drugs that induce neutrophil death within the 10 min stimulation period will also appear to report integrin inhibition. Drugs that inhibit neutrophil degranulation will suppress overall β2 integrin expression. These degranulation inhibitors will also be identified in our screening. Therefore, secondary screening with other controls is needed to confirm the inhibitory effects of the hits. Secondary screens are referenced as a means to both validate and determine the mechanism of action of hits. In addition to repeating the mAb24 tests, the total CD18 expression on the cell surface will be assessed using a pan anti-human CD18 antibody. The level of activated β2 integrin, as measured by mAb24, will be normalized by the total CD18 expression. This will enable us to determine whether the agent's mechanism of action involves antagonizing integrin activation and/or inhibiting the expression of CD18 on the cell surface. A viability assay should also be performed in the secondary screen to exclude any toxic effects of the hits.
This protocol has limitations. First, mAb24 is capable of detecting the β2 I-like domain only in cases where the integrin is in a high-affinity state. Therefore, it cannot identify α/β I-like allosteric antagonists such as lifitegrast through decreasing mAb24 binding. Lifitegrast induces more mAb24 binding32 and shows an abnormally increased MFI value with mAb24 (Figure 4). For such abnormal values, other assays may be needed to verify whether these hits are α/β I-like allosteric antagonists like lifitegrast. Second, the Z' factor for this assay is suboptimal and could potentially be improved through the use of automatic pipetting and agitation. Unfortunately, our laboratory lacks the necessary equipment to test this hypothesis. Duplicating or triplicating assays will be helpful in identifying false positives and negatives in case the Z' factor cannot be further improved with the methods above. Furthermore, it may be beneficial to extend the incubation time to identify more hits, as observed with the known β2 integrin activation inhibitor Nexinhib20, which requires an hour of incubation to produce inhibitory effects. This study focused on identifying fast-acting agents. Researchers should note that they can modify the incubation time to suit their specific needs.
To our knowledge, this is the first high-throughput screening method for β2 integrin antagonists. This approach could be used to identify small molecule compounds that directly bind to β2 integrins and prevent conformational changes leading to intermediate/high-affinity integrin states, similar to antagonists without "agonistic" properties recently described for integrins αIIbβ3 and α4β126. Neutrophils are critical in many inflammatory diseases, such as myocardial ischemia-reperfusion injury36, sepsis37, and autoimmune diseases38,39. Small molecule drugs may offer more flexibility in treating these diseases compared to antibody-based drugs. Hits from our screen could provide potential treatments for inflammatory diseases.
The current method is a fluorescent antibody-based, high-throughput screen. Since activation reporter antibodies are also available for β140,41,42,43,44, αIIbβ345,46, and αL integrins47,48,49,50, this method can be extended to identifying antagonists for other integrins. A conformationally sensitive antibody like HUTS-21, which binds to the β1 hybrid domain51,52,53, has been used in a high-throughput screen to identify very late antigen-4 (VLA-4, integrin α4β1) allosteric antagonists54. The present screening method can also be modified and extended to find drugs that inhibit or promote the expression of other surface receptors, such as compounds that increase cystic fibrosis transmembrane conductance regulator (CFTR) surface expression on cystic fibrosis (CF) cells. In CF, multiple mutations lead to CFTR misfolding, resulting in absent expression of CFTR on the cell membrane55. Small-molecule drugs have been shown to restore the CFTR expression56. For protocol modifications, it is necessary to increase the incubation time of drugs to several hours to allow protein expression changes to occur.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Evan Jellison and Ms. Li Zhu in the flow cytometry core at UConn Health for their assistance with flow cytometry, Dr. Lynn Puddington in the Department of Immunology at UConn Health for her support of the instruments, Ms. Slawa Gajewska and Dr. Paul Appleton in the clinical research core at UConn Health for their help in obtaining blood samples. We acknowledge Dr. Christopher "Kit" Bonin and Dr. Geneva Hargis from UConn School of Medicine for their help with scientific writing and editing of this manuscript. This research was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL145454), National Institute of General Medical Sciences (P20GM121176), USA, a Career Development Award from the American Heart Association (18CDA34110426), and a startup fund from UConn Health. Figure 1 was created with BioRender.com.
Materials
| 16-channel pipettes | Thermo | 4661090N | Instrument |
| 384-well plate | Greiner | 784201 | Materials |
| APC anti-human CD11a/CD18 (LFA-1) Antibody Clone: m24 | BioLegend | 363410 | Reagents |
| Bravo Automated Liquid Handling Platform | Agilent | 16050-102 | 384 multi-channel liquid handler |
| Centrifuge | Eppendorf | Model 5810R | Instrument |
| FlowJo | Becton, Dickinson & Company | NA | Software |
| Human Serum Albumin Solution (25%) | GeminiBio | 800-120 | Reagents |
| Lifitegrast | Thermofisher | 50-208-2121 | Reagents |
| Nexinhib20 | Tocris | 6089 | Reagents |
| N-Formyl-Met-Leu-Phe (fMLP) | Sigma | F3506 | Reagents |
| Paraformaldehyde 16% solution | Electron Microscopy Sciences | 15710 | Reagents |
| Plate buckets | Eppendorf | UL155 | Accessory |
| Plate shaker | Fisher | 88-861-023 | Instrument |
| PolymorphPrep | PROGEN | 1895 (previous 1114683) | Reagents |
| Prestwick Chemical Library Compound Plates (10 mM) | Prestwick Chemical Libraries | Ver19_384 | 1520 small molecules, 98% marketed approved drugs (FDA, EMA, JAN, and other agencies approved) |
| RPMI 1640 Medium, no phenol red | Gibco | 11-835-030 | Reagents |
| Swing-bucket rotor | Eppendorf | A-4-62 | Rotor |
| ZE5 Cell Analyzer | Bio-Rad Laboratories | Model ZE5 | Instrument |
Referências
- Herrero-Cervera, A., Soehnlein, O., Kenne, E. Neutrophils in chronic inflammatory diseases. Cellular & Molecular Immunology. 19 (2), 177-191 (2022).
- Sadik, C. D., Kim, N. D., Luster, A. D. Neutrophils cascading their way to inflammation. Trends in immunology. 32 (10), 452-460 (2011).
- Mitroulis, I. et al. Leukocyte integrins: Role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacology & Therapeutics. 147, 123-135 (2015).
- Slack, R. J., Macdonald, S. J. F., Roper, J. A., Jenkins, R. G., Hatley, R. J. D. Emerging therapeutic opportunities for integrin inhibitors. Nature Reviews Drug Discovery. 21 (1), 60-78 (2022).
- Frampton, J. E., Plosker, G. L. Efalizumab. American Journal of Clinical Dermatology. 10 (1), 51-72 (2009).
- Talamonti, M. et al. Efalizumab. Expert Opinion on Drug Safety. 10 (2), 239-251 (2011).
- Saribaş, A. S., Özdemir, A., Lam, C., Safak, M. JC virus-induced progressive multifocal leukoencephalopathy. Future Virology. 5 (3), 313-323 (2010).
- Chames, P., Van Regenmortel, M., Weiss, E., Baty, D. Therapeutic antibodies: successes, limitations and hopes for the future. British Journal of Pharmacology. 157 (2), 220-233 (2009).
- Mancuso, R. V., Casper, J., Schmidt, A. G., Krähenbühl, S., Weitz-Schmidt, G. Anti-αLβ2 antibodies reveal novel endocytotic cross-modulatory functionality. British Journal of Pharmacology. 177 (12), 2696-2711 (2020).
- Anderson, J. M., Li, J., Springer, T. A. Regulation of integrin α5β1 conformational states and intrinsic affinities by metal ions and the ADMIDAS. Molecular Biology of the Cell. 33 (6), ar56 (2022).
- Jensen, R. K. et al. Complement receptor 3 forms a compact high-affinity complex with iC3b. The Journal of Immunology. 206 (12), 3032-3042 (2021).
- Li, J., Yan, J., Springer, T. A. Low affinity integrin states have faster ligand binding kinetics than the high affinity state. Elife. 10, e73359 (2021).
- Luo, B. H., Carman, C. V., Springer, T. A. Structural basis of integrin regulation and signaling. Annual Review of Immunology. 25, 619-647 (2007).
- Fan, Z. et al. Neutrophil recruitment limited by high-affinity bent β2 integrin binding ligand in cis. Nature communications. 7 (1), 1-14 (2016).
- Fan, Z. et al. High-affinity bent β2-integrin molecules in arresting neutrophils face each other through binding to ICAMs in cis. Cell reports. 26 (1), 119-130 (2019).
- Gupta, V. et al. The β-tail domain (βTD) regulates physiologic ligand binding to integrin CD11b/CD18. Blood. 109 (8), 3513-3520 (2006).
- Sen, M., Yuki, K., Springer, T. A. An internal ligand-bound, metastable state of a leukocyte integrin, αXβ2. Journal of Cell Biology. 203 (4), 629-642 (2013).
- Sun, H., Hu, L., Fan, Z. β2 integrin activation and signal transduction in leukocyte recruitment. American Journal of Physiology-Cell Physiology. 321 (2), C308-C316 (2021).
- Sun, H., Zhi, K., Hu, L., Fan, Z. The activation and regulation of β2 integrins in phagocytes. Frontiers in Immunology. 12, 978 (2021).
- Kamata, T. et al. The role of the CPNKEKEC sequence in the β2 subunit I domain in regulation of integrin αLβ2 (LFA-1). The Journal of Immunology. 168 (5), 2296-2301 (2002).
- Lu, C., Shimaoka, M., Zang, Q., Takagi, J., Springer, T. A. Locking in alternate conformations of the integrin αLβ2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proceedings of the National Academy of Sciences. 98 (5), 2393-2398 (2001).
- Yang, W., Shimaoka, M., Chen, J., Springer, T. A. Activation of integrin β-subunit I-like domains by one-turn C-terminal α-helix deletions. Proceedings of the National Academy of Sciences. 101 (8), 2333-2338 (2004).
- Dransfield, I., Hogg, N. Regulated expression of Mg2+ binding epitope on leukocyte integrin alpha subunits. The EMBO Journal. 8 (12), 3759-3765 (1989).
- Torres, M., Hall, F., O'neill, K. Stimulation of human neutrophils with formyl-methionyl-leucyl-phenylalanine induces tyrosine phosphorylation and activation of two distinct mitogen-activated protein-kinases. The Journal of Immunology. 150 (4), 1563-1577 (1993).
- Dorward, D. A. et al. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. The American Journal of Pathology. 185 (5), 1172-1184 (2015).
- Lin, F. Y. et al. A general chemical principle for creating closure-stabilizing integrin inhibitors. Cell. 185 (19), 3533-3550 (2022).
- Lizcano, A. et al. Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation. Blood, The Journal of the American Society of Hematology. 129 (23), 3100-3110 (2017).
- Tadema, H., Abdulahad, W. H., Stegeman, C. A., Kallenberg, C. G., Heeringa, P. Increased expression of Toll-like receptors by monocytes and natural killer cells in ANCA-associated vasculitis. PloS One. 6 (9), e24315 (2011).
- Nagelkerke, S. Q., aan de Kerk, D. J., Jansen, M. H., van den Berg, T. K., Kuijpers, T. W. Failure to detect functional neutrophil B helper cells in the human spleen. PloS one. 9 (2), e88377 (2014).
- Blanco-Camarillo, C., Alemán, O. R., Rosales, C. Low-density neutrophils in healthy individuals display a mature primed phenotype. Frontiers in Immunology. 12, 672520 (2021).
- Zhang, J. H., Chung, T. D., Oldenburg, K. R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. Journal of biomolecular screening. 4 (2), 67-73 (1999).
- Shimaoka, M., Salas, A., Yang, W., Weitz-Schmidt, G., Springer, T.A. Small molecule integrin antagonists that bind to the β2 subunit I-like domain and activate signals in one direction and block them in the other. Immunity. 19 (3), 391-402 (2003).
- Liu, W. et al. Nexinhib20 Inhibits neutrophil adhesion and β2 integrin activation by antagonizing Rac-1-Guanosine 5′-Triphosphate interaction. The Journal of Immunology. 209 (8), 1574-1585 (2022).
- Robinson, M. et al. Antibody against the Leu-CAM beta-chain (CD18) promotes both LFA-1-and CR3-dependent adhesion events. The Journal of Immunology. 148 (4), 1080-1085 (1992).
- Lu, C., Ferzly, M., Takagi, J., Springer, T. A. Epitope mapping of antibodies to the C-terminal region of the integrin β2 subunit reveals regions that become exposed upon receptor activation. The Journal of Immunology. 166 (9), 5629-5637 (2001).
- Mauler, M. et al. Platelet serotonin aggravates myocardial ischemia/reperfusion injury via neutrophil degranulation. circulation. 139 (7), 918-931 (2019).
- Shen, X. F., Cao, K., Jiang, J., Guan, W. X., Du, J. F. Neutrophil dysregulation during sepsis: an overview and update. Journal of Cellular and Molecular Medicine. 21 (9), 1687-1697 (2017).
- Chiang, C. C., Cheng, W. J., Korinek, M., Lin, C. Y., Hwang, T. L. Neutrophils in Psoriasis. Frontiers in Immunology. 10, 02376 (2019).
- Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nature medicine. 22 (2), 146-153 (2016).
- Bazzoni, G., Shih, D. T., Buck, C. A., Hemler, M. E. Monoclonal antibody 9EG7 defines a novel β1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. Journal of Biological Chemistry. 270 (43), 25570-25577 (1995).
- Luque, A. et al. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region(355-425) of the common β1 chain. Journal of Biological Chemistry. 271 (19), 11067-11075 (1996).
- Mould, A. P., Akiyama, S. K., Humphries, M. J. The inhibitory Anti-β1 integrin monoclonal antibody 13 recognizes an epitope that is attenuated by ligand occupancy: evidence for allosteric inhibition of integrin function. Journal of Biological Chemistry. 271 (34), 20365-20374 (1996).
- Spiess, M. et al. Active and inactive β1 integrins segregate into distinct nanoclusters in focal adhesions. Journal of Cell Biology. 217 (6), 1929-1940 (2018).
- Yang, S. et al. Relating conformation to function in integrin α5β1. Proceedings of the National Academy of Sciences. 113 (27), E3872-E3881 (2016).
- Shattil, S. J., Hoxie, J. A., Cunningham, M., Brass, L. F. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. Journal of Biological Chemistry. 260 (20), 11107-11114 (1985).
- Shattil, S. J., Motulsky, H. J , Insel, P. A., Flaherty, L., Brass, L. F. Expression of fibrinogen receptors during activation and subsequent desensitization of human platelets by epinephrine. Blood. 68 (6), 1224-1231 (1986).
- Carreño, R. et al. 2E8 binds to the high affinity i-domain in a metal ion-dependent manner: a second generation monoclonal antibody selectively targeting activated LFA-1. Journal of Biological Chemistry. 285 (43), 32860-32868 (2010).
- Keizer, G. D., Visser, W., Vliem, M., Figdor, C. G. A monoclonal antibody (NKI-L16) directed against a unique epitope on the alpha-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. The Journal of Immunology. 140 (5), 1393-1400 (1988).
- Lefort, C. T. et al. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood. 119 (18), 4275-4282 (2012).
- van Kooyk, Y. et al. Activation of LFA-1 through a Ca2(+)-dependent epitope stimulates lymphocyte adhesion. Journal of Cell Biology. 112 (2), 345-354 (1991).
- Mould, A. P. et al. Conformational changes in the integrin a domain provide a mechanism for signal transduction via hybrid domain movement. Journal of Biological Chemistry. 278 (19), 17028-17035 (2003).
- Chigaev, A. et al. Real-time analysis of conformation-sensitive antibody binding provides new insights into integrin conformational regulation. Journal of Biological Chemistry. 284 (21), 14337-14346 (2009).
- Njus, B. H. et al. Conformational mAb as a tool for integrin ligand discovery. Assay and Drug Development Technologies. 7 (5), 507-515 (2009).
- Chigaev, A., Wu, Y., Williams, D. B., Smagley, Y., Sklar, L. A. Discovery of very late antigen-4 (VLA-4, α4β1 integrin) allosteric antagonists. Journal of Biological Chemistry. 286 (7), 5455-5463 (2011).
- Ghigo, A., De Santi, C., Hart, M., Mitash, N., Swiatecka-Urban, A. Cell signaling and regulation of CFTR expression in cystic fibrosis cells in the era of high efficiency modulator therapy. Journal of Cystic Fibrosis. 22, S12-S16 (2023).
- Van Goor, F., Yu, H., Burton, B., Hoffman, B.J. Effect of ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. Journal of Cystic Fibrosis. 13 (1), 29-36 (2014).

