AlDeSense在基于醛脱氢酶1A1活性的卵巢癌细胞分层中的应用
Summary
测量活细胞中 ALDH1A1 活性的方法在癌症研究中至关重要,因为它是干性的生物标志物。在这项研究中,我们采用了亚型选择性荧光探针来确定一组五种卵巢癌细胞系中 ALDH1A1 活性的相对水平。
Abstract
癌症治疗后的复发通常归因于称为癌症干细胞(CSC)的肿瘤细胞亚群的持续存在,其特征在于其显着的肿瘤起始和自我更新能力。根据肿瘤的起源(例如卵巢),CSC表面生物标志物谱可能会有很大差异,这使得通过免疫组织化学染色 鉴定 此类细胞是一项具有挑战性的工作。相反,醛脱氢酶 1A1 (ALDH1A1) 因其在几乎所有祖细胞(包括 CSC)中的保守表达谱而成为鉴定 CSC 的极好标志物。ALDH1A1 亚型属于 19 种酶的超家族,负责将各种内源性和异生素醛氧化成相应的羧酸产物。Chan等人最近开发了AlDeSense,这是一种用于检测ALDH1A1活性的亚型选择性“开启”探针,以及一种非反应性匹配对照试剂(Ctrl-AlDeSense),用于解决脱靶染色。这种亚型选择工具已被证明是一种多功能化学工具,可检测 K562 骨髓性白血病细胞、乳腺球和黑色素瘤衍生的 CSC 异种移植物中的 ALDH1A1 活性。在本文中,通过额外的荧光法、共聚焦显微镜和流式细胞术实验展示了探针的实用性,其中在一组五种卵巢癌细胞系中测定了相对 ALDH1A1 活性。
Introduction
癌症干细胞(CSC)是具有干细胞样特性的肿瘤细胞亚群1。与非癌性对应物类似,CSC具有非凡的自我更新和增殖能力。与其他内置机制(例如ATP结合盒转运蛋白的上调)一起,CSC通常免于初始手术减瘤工作以及随后的辅助治疗2。由于CSCs在治疗耐药性3,复发4和转移5中的关键作用,已成为癌症研究的优先事项。尽管有多种细胞表面抗原(例如CD133)可用于鉴定CSC6,但利用细胞质中发现的醛脱氢酶(ALDH)的酶活性已成为一种有吸引力的替代方法7。ALDHs 是 19 种酶的超家族,负责催化反应性内源性和异生素醛氧化为相应的羧酸产物8。
一般来说,醛解毒对于保护细胞免受可能损害干细胞完整性的不良交联事件和氧化应激至关重要9。此外,1A1亚型控制视黄酸代谢,而视黄酸代谢又通过视黄醛信号传导影响干性10。AlDeSense 11,12 是一种基于活性的小分子传感 (ABS) 探针,用于选择性检测 ALDH1A1 活性,最近被开发出来。ABS设计通过化学变化而不是结合事件实现分析物检测,从而实现高选择性和减少脱靶响应13,14,15,16。亚型选择性荧光探针的设计原理依赖于供体光诱导电子转移(d-PeT)猝灭机制17,该机制源自醛官能团,用于抑制探针18的荧光特征。在 ALDH1A1 介导的转化为羧酸后,辐射松弛被解锁以产生高荧光产物。由于d-PeT猝灭从来都不是100%有效的,因此在通过开发Ctrl-AlDeSense(一种具有匹配的光物理特性(例如,量子产率)和细胞中相同细胞质染色模式的非响应试剂)建立该测定时,考虑了可能导致假阳性结果的残留荧光。当同时使用时,这种独特的配对可以通过荧光、分子成像和流式细胞术可靠地区分具有高 ALDH1A1 活性的细胞和低水平细胞。与传统的免疫组织化学方法相比,使用亚型选择性可活化染料有几个关键优势。例如,假设CSCs深埋在肿瘤中,因此相对于大抗体更容易被小分子接近19。此外,翻转的荧光产物不会共价修饰任何细胞成分,这意味着它可以通过洗涤循环轻松去除,使CSC处于未修饰状态。最后,开启反应仅识别活细胞和功能,与MTT测定非常相似,因为它依赖于NAD +辅因子。
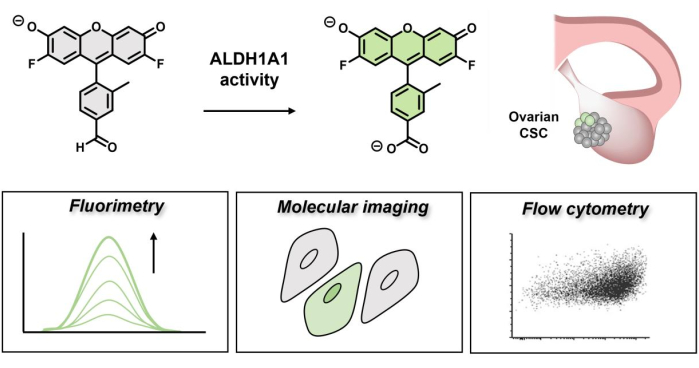
图 1:演示 AlDeSense 荧光开启的示意图。 这种亚型选择性染料由 ALDH1A1 激活,可用于通过荧光、分子成像和流式细胞术 鉴定 卵巢癌细胞中升高的 ALDH1A1 活性。 请点击此处查看此图的大图。
在过去的工作中,亚型选择性荧光探针测定成功地从K562人慢性白血病细胞、MDA-MB-231人乳腺癌细胞和B16F0小鼠黑色素瘤细胞中的ALDH低(ALDH-)细胞中分层了ALDH高(ALDH+)细胞。这一点很重要,因为对于许多癌症类型,高 ALDH1A1 蛋白表达意味着临床预后较差20。这假设 ALDH1A1 水平升高表明 CSC 可以逃避治疗、产生耐药性并传播到全身。然而,在卵巢癌的情况下,有研究报告了相反的发现(高 ALDH1A1 表达与改善患者生存率有关)21,22,23,24。虽然乍一看这似乎是矛盾的,但表达不一定与酶活性相关,酶活性可能受到肿瘤微环境变化(例如pH通量、氧梯度)、NAD+辅因子或醛底物的可用性、羧酸水平(产物抑制)以及可能改变酶活性的翻译后修饰的影响25.此外,卵巢癌分为五种主要的组织学类型(高级别浆液性、低级别浆液性、子宫内膜样、透明细胞和粘液),我们假设其 ALDH1A1 活性水平不同26。为了研究卵巢肿瘤中的 ALDH1A1 活性,采用亚型选择性荧光探针测定法鉴定属于上述不同组织学类型的五种卵巢癌细胞系中的 ALDH1A1+ 群体。本研究中测试的细胞系包括BG-1,Caov-3,IGROV-1,OVCAR-3和PEO4细胞,涵盖透明细胞和浆液组织型。本文强调了探针的多功能性和可推广性,以鉴定CSC,以便寻求在其他永生化癌细胞系以及患者样本中进行类似研究的研究人员使用CSC。AlDeSense的使用将揭示复杂组织微环境中CSC维持所涉及的生化途径,并可能作为确定预后和测量癌症侵袭性的临床工具。
Protocol
Representative Results
Discussion
泛选择性是许多 ALDH 探头的主要限制;然而,最近报道了几种亚型选择性实例32,33,34,35,36,37,38,39,40,41。本研究中使用的亚型选择性?…
Declarações
The authors have nothing to disclose.
Acknowledgements
这项工作得到了美国国立卫生研究院(R35GM133581至JC)和伊利诺伊州癌症中心研究生奖学金(授予SG)的支持。JC感谢Camille和Henry Dreyfus基金会的支持。作者感谢Thomas E. Bearrood博士为准备AlDeSense和AlDeSense AM股票所做的初步贡献。我们感谢奥利弗·皮查多·佩盖罗先生和约瑟夫·福尔扎诺先生协助制备各种合成前体。我们感谢Erik Nelson教授(UIUC分子与综合生理学系)的Caov-3,IGROV-1和PEO4细胞。我们感谢Paul Hergenrother教授(UIUC化学系)的BG-1细胞。我们感谢Carl R. Woese基因组生物学研究所的核心设施使用蔡司LSM 700共聚焦显微镜和相应的软件。我们感谢流式细胞术设施使用BD LSR II CMtO分析仪。我们感谢Sandra McMasters博士和细胞培养基设施在制备细胞培养基方面的帮助。
Materials
| 0.25% Trypsin, 0.1% EDTA in HBSS w/o Calcium, Magnesium and Sodium Bicarbonate | Corning | 25-050-CI | |
| 1x Phosphate Buffer Saline | Corning | 21-040-CMX12 | |
| AccuSpin Micro 17R | Fisher Scientific | 13-100-675 | |
| AlDeSense | Synthesized in-house | ||
| BG-1 | A gift provided by the Prof. Paul Hergenrother Lab, University of Illinois Urbana-Champaign | ||
| BioLite 25cm2 Flask | Thermo Fisher Scientific | 130189 | |
| Biosafety Cabinet 1300 series A2 | Thermo Fisher Scientific | ||
| Caov-3 | A gift provided by the Prof. Erik Nelson Lab, University of Illinois Urbana-Champaign | ||
| Cell homogenizer | Fisher Scientific | ||
| Centrifuge 5180R | Eppendorf | 22627040 | |
| Contrl-AlDeSense | Synthesized in-house | ||
| DMEM, 10% FBS, 1% P/S | Prepared by UIUC cell media facility | ||
| Falcon Round-Bottom Polystyrene Test Tubes with Cell Strainer Snap Cap, 5mL | Corning | 352003 | |
| FCS Express 6 | Provided by UIUC CMtO | ||
| FL microscope | EVOS | ||
| Fluorometer | Photon Technology International | ||
| Forma Series II Water-Jacketed CO2 Incubator | Fisher Scientific | 3110 | |
| IGROV-1 | A gift provided by the Prof. Erik Nelson Lab, University of Illinois Urbana-Champaign | ||
| ImageJ | NIH | ||
| Innova 42R Incubated Shaker | |||
| LSM 700 | Zeiss | ||
| LSR II | BD | ||
| Nunc Lab-Tek Chambered #1.0 Borosicilate Coverglass System | Thermo Fisher Scientific | 155383 | |
| OVCAR-3 | ATCC | HTB-161 | |
| PEO4 | A gift provided by the Prof. Erik Nelson Lab, University of Illinois Urbana-Champaign | ||
| Pierce Protease Inhibitor Tablets | Thermo Scientific | A32963 | |
| Poly-L-Lysine | Cultrex | 3438-100-01 | |
| Rocker | VWR | ||
| RPMI, 10% FBS, 1% P/S | Prepared by UIUC cell media facility | ||
| RPMI, 20% FBS, 1% P/S, 0.01 mg/mL Insulin | Prepared by UIUC cell media facility |
Referências
- Bonnet, D., Dick, J. E. Human acute myeloid leukaemia is organised as a heirarchy that originates from a primitive haematopoetic cell. Nature Medicine. 3 (7), 730-737 (1997).
- Begicevic, R. R., Falasca, M. ABC transporters in cancer stem cells: Beyond chemoresistance. International Journal of Molecular Sciences. 18 (11), 2362 (2017).
- Cojoc, M., Mäbert, K., Muders, M. H., Dubrovska, A. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Seminars in Cancer Biology. 31, 16-27 (2015).
- Islam, F., Gopalan, V., Smith, R. A., Lam, A. K. Y. Translational potential of cancer stem cells: A review of the detection of cancer stem cells and their roles in cancer recurrence and cancer treatment. Experimental Cell Research. 335 (1), 135-147 (2015).
- Li, F., Tiede, B., Massagué, J., Kang, Y. Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Research. 17 (1), 3-14 (2007).
- Kim, W. T., Ryu, C. J. Cancer stem cell surface markers on normal stem cells. BMB Reports. 50 (6), 285-298 (2017).
- Pors, K., Moreb, J. S. Aldehyde dehydrogenases in cancer: An opportunity for biomarker and drug development. Drug Discovery Today. 19 (12), 1953-1963 (2014).
- Jackson, B., et al. Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Human Genomics. 5 (4), 283-303 (2011).
- Vasiliou, V., Pappa, A., Petersen, D. R. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chemico-Biological Interactions. 129 (1-2), 1-19 (2000).
- Tomita, H., Tanaka, K., Tanaka, T., Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. 7 (10), 11018-11032 (2016).
- Anorma, C., et al. Surveillance of cancer stem cell plasticity using an isoform-selective fluorescent probe for aldehyde dehydrogenase 1A1. ACS Central Science. 4 (8), 1045-1055 (2018).
- Bearrood, T. E., Aguirre-Figueroa, G., Chan, J. Rational design of a red fluorescent sensor for ALDH1A1 displaying enhanced cellular uptake and reactivity. Bioconjugate Chemistry. 31 (2), 224-228 (2020).
- Chan, J., Dodani, S. C., Chang, C. J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nature Chemistry. 4 (12), 973-984 (2012).
- East, A. K., Lucero, M. Y., Chan, J. New directions of activity-based sensing for in vivo NIR imaging. Chemical Science. 12 (10), 3393-3405 (2021).
- Yadav, A. K., et al. Activity-based NIR bioluminescence probe enables discovery of diet-induced modulation of the tumor microenvironment via nitric oxide. ACS Central Science. 8 (4), 461-472 (2022).
- Yadav, A. K., et al. An activity-based sensing approach for the detection of cyclooxygenase-2 in Live Cells. Angewandte Chemie. 59 (8), 3307-3314 (2020).
- Ueno, T., et al. Rational principles for modulating fluorescence properties of fluorescein. Journal of the American Chemical Society. 126 (43), 14079-14085 (2004).
- Tanaka, F., Mase, N., Barbas 3rd, C. F. Design and use of fluorogenic aldehydes for monitoring the progress of aldehyde transformations. Journal of the American Chemical Society. 126 (12), 3692-3693 (2004).
- Thurber, G. M., Schmidt, M. M., Wittrup, K. D. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Advanced Drug Delivery Reviews. 60 (12), 1421-1434 (2008).
- Marcato, P., Dean, C. A., Giacomantonio, C. A., Lee, P. W. K. Aldehyde dehydrogenase its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 10 (9), 1378-1384 (2011).
- Meng, E., et al. ALDH1A1 maintains ovarian cancer stem cell-like properties by altered regulation of cell cycle checkpoint and DNA repair network signaling. PLoS One. 9 (9), e107142 (2014).
- Kaipio, K., et al. ALDH1A1-related stemness in high-grade serous ovarian cancer is a negative prognostic indicator but potentially targetable by EGFR/mTOR-PI3K/aurora kinase inhibitors. The Journal of Pathology. 250 (2), 159-169 (2020).
- Deng, S., et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 5 (4), e10277 (2010).
- Chang, B., et al. ALDH1 expression correlates with favorable prognosis in ovarian cancers. Modern Pathology. 22 (6), 817-823 (2009).
- Gardner, S. H., Reinhardt, C. J., Chan, J. Advances in activity-based sensing probes for isoform-selective imaging of enzymatic activity. Angewandte Chemie. 60 (10), 5000-5009 (2021).
- Reid, B. M., Permuth, J. B., Sellers, T. A. Epidemiology of ovarian cancer: a review. Cancer Biology and Medicine. 14 (1), 9-32 (2017).
- Tulake, W., et al. Upregulation of stem cell markers ALDH1A1 and OCT4 as potential biomarkers for the early detection of cervical carcinoma. Oncology Letters. 16 (5), 5525-5534 (2018).
- Nwani, N. G., et al. A novel ALDH1A1 inhibitor targets cells with stem cell characteristics in ovarian cancer. Cancers. 11 (4), 502 (2019).
- Roy, M., Connor, J., Al-Niaimi, A., Rose, S. L., Mahajan, A. Aldehyde dehydrogenase 1A1 (ALDH1A1) expression by immunohistochemistry is associated with chemo-refractoriness in patients with high-grade ovarian serous carcinoma. Human Pathology. 73, 1-6 (2018).
- Landen Jr, C. N., et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Molecular Cancer Therapeutics. 9 (12), 3186-3199 (2010).
- Condello, S., et al. β-catenin-regulated ALDH1A1 is a target in ovarian cancer spheroids. Oncogene. 34 (18), 2297-2308 (2015).
- Storms, R. W., et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proceedings of the National Academy of Sciences. 96 (16), 9118-9123 (1999).
- Duellman, S. J., et al. A bioluminescence assay for aldehyde dehydrogenase activity. Analytical Biochemistry. 434 (2), 226-232 (2013).
- Minn, I., et al. A red-shifted fluorescent substrate for aldehyde dehydrogenase. Nature Communications. 5, 3662 (2014).
- Dollé, L., Boulter, L., Leclercq, I. A., van Grunsven, L. A. Next generation of ALDH substrates and their potential to study maturational lineage biology in stem and progenitor cells. American Journal of Physiology. Gastrointestinal and Liver Physiology. 308 (7), 573-578 (2015).
- Yagishita, A., et al. Development of highly selective fluorescent probe enabling flow-cytometric isolation of ALDH3A1-positive viable cells. Bioconjugate Chemistry. 28 (2), 302-306 (2017).
- Maity, S., et al. Thiophene bridged aldehydes (TBAs) image ALDH activity in cells: Via modulation of intramolecular charge transfer. Chemical Science. 8 (10), 7143-7151 (2017).
- Koenders, S. T. A., et al. Development of a retinal-based probe for the profiling of retinaldehyde dehydrogenases in cancer cells. ACS Central Science. 5 (12), 1965-1974 (2019).
- Oe, M., et al. Deep-red/near-infrared turn-on fluorescence probes for aldehyde dehydrogenase 1A1 in cancer stem cells. ACS Sensors. 6 (9), 3320-3329 (2021).
- Yagishita, A., Ueno, T., Tsuchihara, K., Urano, Y. Amino BODIPY-based blue fluorescent probes for aldehyde dehydrogenase 1-expressing cells. Bioconjugate Chemistry. 32 (2), 234-238 (2021).
- Okamoto, A., et al. Identification of breast cancer stem cells using a newly developed long-acting fluorescence probe, C5S-A, targeting ALDH1A1. Anticancer Research. 42 (3), 1199-1205 (2022).

