Assembly and Operation of a Cooling Stage to Immobilize C. elegans on Their Culture Plates
Summary
This paper describes protocols for constructing and operating a cooling stage to immobilize C. elegans on their original cultivation plates en masse.
Abstract
High-resolution in vivo microscopy approaches can reveal subtle information and fine details inside the model animal Caenorhabditis elegans (C. elegans), but require strong animal immobilization to prevent motion blur in the images. Unfortunately, most current immobilization techniques require substantial manual effort, rendering high-resolution imaging low-throughput. Immobilization of C. elegans is greatly simplified by using a cooling approach that can easily immobilize entire populations directly on their cultivation plates. The cooling stage can establish and maintain a wide range of temperatures with a uniform distribution on the cultivation plate. In this article, the whole process of building the cooling stage is documented. The aim is that a typical researcher can build an operational cooling stage in their laboratory following this protocol without difficulty. Utilization of the cooling stage following three protocols is shown, and each protocol has advantages for different experiments. Also shown is an example cooling profile of the stage as it approaches its final temperature and some helpful tips in using cooling immobilization.
Introduction
High-resolution optical microscopy provides an indispensable tool for studying in vivo biological structures at the subcellular level. Many biological studies require submicron resolution imaging to resolve subtle anatomical details, including neuron morphology1,2, membrane structure3,4, and protein localization5,6. A high-resolution image requires an exposure time of several milliseconds to seconds, depending on the imaging modality and probe7,8. To achieve optimal results, it is essential to carefully plan and conduct microscopy-based experiments. Crucial to this effort is an efficient animal preparation method that facilitates high-resolution imaging.
The nematode C. elegans is a widely utilized model organism for studying many biological processes9. This small animal is typically cultivated on nematode growth medium (NGM) agar plates, and they reproduce rapidly by self-fertilization, making them well-suited for large-scale studies. Their transparency and a wide array of labeling techniques allow the straightforward visualization of their internal anatomy10,11. The fine structures in C. elegans are ideal for studying biological processes at the subcellular level, such as neuron regeneration12, neuron degeneration13, and cell division14. Such studies necessitate imaging at submicron resolution and animal immobilization strong enough to prevent image blur. Strong immobilization is especially crucial for techniques involving multiple images in space or time, such as 3D image stacks (i.e., z-stacks) and time-lapse imaging. Any animal movement between the exposures can obscure the result. For C. elegans, strong immobilization typically involves manual manipulation of individual animals and mounting them on slides with an anesthetic15,16. These time- and labor-intensive procedures make large-scale experiments very difficult. An immobilization strategy where animals are directly and reversibly immobilized on their original cultivation plates could enable high-throughput high-resolution imaging.
Cooling immobilization of C. elegans has been shown in a few studies but is not widely utilized. It is usually combined with a microfluidic device to further restrain animals17,18,19. However, microfluidic devices are complex, require significant operational training, and cannot be easily integrated with typical solid cultivation workflows of C. elegans experiments. Thus, microfluidics are not widely utilized for C. elegans immobilization. Presented here, in conjunction with the Chung Laboratory's recent publication20, is the introduction of a new cooling immobilization approach using a thermoelectric cooling stage (Figure 1) to address these shortcomings. With the cooling stage, a typical 60 mm polystyrene cultivation plate can be cooled down to any target temperature (Tset) between -8 °C to room temperature. This cooling stage approach can readily and reversibly immobilize an entire animal population with minimal user effort, eliminating 98% of the animal processing time20.
Below, the procedures for building a cooling stage from scratch are described. Except for the machining of parts and 3D printing, the whole procedure is expected to take 4 h without the requirement of special tools or expertise. Then, three different cooling strategies with varying cooling rates and user efforts to immobilize C. elegans on a typical upright microscope are further described. The preferred strategy may depend on the user application. The protocols for those three cooling immobilization strategies are described in detail.
Protocol
1. Manufacturing and preparing each component of the cooling stage
NOTE: The cooling stage comprises several components (see Table of Materials). Most of the components are off-the-shelf. The sapphire window requires a custom order, while the copper plate, holding bracket, and isolation plate can be manufactured on-site with a computer numerical control mill or 3D printer. After the initial manufacturing, the later assembly process takes around 2-3 h.
- Use a computer numerical control mill to machine the copper plate from a 170 mm x 120 mm x 3 mm, 99.9% pure copper metal sheet (Figure 2A). The 2D drawing for this manufacturing is provided in the Supplementary File 1. Use fine-grit sandpaper to remove any sharp edges and dirty residue.
- To manufacture the holding bracket and the isolation plate, use a 3D printer and 1.75 mm diameter polylactic acid (PLA) filament (Figure 2B,C). For better quality, the 3D printer should provide a layer height finer than 0.2 mm. 3D models are provided in Supplementary File 2 and Supplementary File 3.
2. Constructing the water-cooling assembly
- Prepare the platinum-cured silicone tube, pump tank, copper cooling block, and radiator (Figure 3A) for building the water-cooling assembly. Prepare a razor blade, scissors, and hex key ready for use. Be aware of electrical hazards due to the use of water throughout assembly.
- Cut the silicone tube into three sections with suggested lengths of 40 cm, 50 cm, and 80 cm. Adjust the length when needed.
- Plug the silicone tube sections from step 2.2 into the ports of the radiator, pump tank, and copper cooling block, as shown in Figure 3B. Ensure all the connections are watertight. The water-cooling assembly is now built.
- Prepare the water-cooling assembly, a 12 V power supply, three red and three black jumper wires, a breadboard, and 500 mL of purified water.
- Ensure the workbench is clear of liquid for electrical safety.
- Connect the pump tanks and the radiator's wires to the 12 V power supply through the breadboard (Figure 3C). The breadboard is used for convenience.
NOTE: For a more permanent and safe connection, researchers can replace the breadboard with soldering wires. - Open the pump tank cap using a flathead screwdriver. Use a funnel to add water until the pump tank is about 80% full (Figure 3D). Do not cap the pump tank after this filling.
- Power up the water-cooling assembly by plugging in the 12 V power supply or switching it on (if a switch is present). After powering up, the water will flow inside of the assembly and the fans on the radiator should blow.
- Due to the water flow from the pump tank, the liquid level in the tank will drop. Add more water to the pump tank until it stabilizes at nearly 2/3 full (Figure 3E).
- Shake the radiator to get rid of air bubbles and then cap the cooling tank.
- Turn off the power supply before going to the next step.
3. Testing Peltier cold and hot surfaces
NOTE: The Peltier, a key component of the cooling stage, is a solid-state active heat pump that transfers heat from one side to the other21. One surface of the Peltier becomes hot, and the other surface becomes cold when providing electric power. By default, Peltier manufacturers mark the cold surface before selling, but it is still helpful to manually test it before assembling.
- Prepare the tunable power supply and the Peltier, as shown in Figure 4A.
- Ensure the tunable power supply is off to prevent possible electrical hazards.
- Connect the red wire of the Peltier to the positive output and the black wire to the negative output of the tunable power supply with alligator clips, that are provided with the power supply (Figure 4B).
- Turn on the tunable power supply and set it to around 2 V by modulating both the voltage and current knobs on the top row of the power supply. Immediately use a bare finger to feel the two surfaces of the Peltier. One surface becomes cold within a few seconds.
- After identifying which surface is cold, immediately turn off the power supply and disconnect the Peltier.
- Use a marker to indicate the cold surface for future assembly.
4. Constructing the assembly to cool the Peltier using water-cooling assembly
- As shown in Figure 4A, prepare the switched-off water-cooling assembly, the Peltier (cold surface marked), and the thermal paste (for improved thermal conduction).
- Clean all the surfaces of the copper cooling block with 70% ethanol (or other cleaner solution) in the water-cooling assembly.
- Apply around 0.4 g of thermal paste to one surface of the copper water-cooling block and ensure this surface orientation will prevent the tubes from crossing or bending when facing down. Use a glove to protect the skin and try to distribute the thermal paste thinly and evenly (Figure 4C).
- Similarly, clean the hot surface of the Peltier, then apply the thermal paste to the surface (Figure 4D).
- Connect the Peltier hot surface to the copper cooling block surface with thermal paste. Apply pressure to ensure it is secure. Follow the orientation of the wires on the Peltier and the tubes of the copper cooling block, as shown in Figure 4E. Clean the excess thermal paste.
- Keep both the 12 V power supply and the tunable power supply off. Connect the Peltier to the tunable power supply, as in section 3.
- Recheck both the electrical and water-cooling assembly connections, and then turn on the 12 V power supply and tunable power supply sequentially.
- Gradually turn the tunable power supply to 12 V. With the suggested Peltier, the current should be around 7.3 A.
- Wait for 2 min; the temperature of the Peltier cold surface should become colder than -35 °C. Measure this temperature with an infrared thermometer (Figure 4F). Do not touch the cold surface to prevent injury to the hands.
- Check all the connections and components if the temperature cannot reach below -30 °C. Air bubbles inside the water-cooling assembly is a possible reason for suboptimal cooling performance.
- To ensure safety in later steps, turn off the tunable power supply, wait 1 min, then turn off the 12 V power supply.
5. Constructing copper plate and sapphire window assembly
- Prepare the copper plate, the 80 mm diameter sapphire window, thermal paste, a 4 inch wide tape, and a sharp blade for cutting (Figure 5A).
- Carefully clean the copper plate and the sapphire window with 70% ethanol and use fine-grit sandpaper to smooth rough surfaces.
- Apply the thermal paste on three inner surfaces, as shown in Figure 5B. Ensure the thermal paste covers all three surface areas but is not too thick, around 0.5 mm.
- Lay the copper plate on the benchtop protected with printer paper. The paper makes the later cleanup easier.
- Insert the sapphire window into the copper plate hole (Figure 5C). Ensure the sapphire does not rotate during insertion to prevent the thermal paste from moving to other areas. Remove excess thermal paste.
- Adhere the 4 inch wide tape to the top surface of the copper plate-sapphire window assembly (the surface that has the square depression area, as shown in Figure 5D). Avoid air bubbles between the tape and copper surfaces during pasting by guiding the adhesion slowly from one side to the other.
- Cut out the specified blue dashed areas of the tape using a sharp blade, following Figure 5E. Cutting exposes the two thread holes, the square depression, and 70 mm diameter area of the sapphire window.
- Tape the bottom surface of the copper plate-sapphire window assembly and then repeat the cutting procedure (sapphire area only) on this surface, as shown in Figure 5F.
NOTE: Now, the sapphire window is fixed to the copper plate, and the copper surfaces are protected from rust.
6. Cooling stage final assembling
- Ensure all essential sub-assembly and components are ready.
- Apply about 0.4 g of thermal paste to the square depression of the copper plate (Figure 6A).
- Apply about 0.4 g of thermal paste to the cold surface of the Peltier. Note that the Peltier is already attached to the copper cooling block (Figure 6B).
- Connect the Peltier cold surface to the copper plate depression with downward pressure. Clean all excess thermal paste (Figure 6C).
- Mount the 3D-printed bracket on the top of the copper cooling block, and then use a hex key to tighten two 8-32, 0.5 inch long screws to fix the bracket to the copper plate (Figure 6D). Use low torque tightening so that the printed bracket does not break or deform to ensure proper thermal conduction from the Peltier to the copper.
- Place the copper plate into the 3D-printed isolation base for thermal isolation from the benchtop or microscope base during operation (Figure 6D).
- The cooling stage is assembled and ready to use (Figure 6E).
- For microscopy, place the completed cooling stage on an upright microscope platform (Figure 7A).
- Assembly of the cooling stage is completed. Further details are available from the Chung Laboratory's companion publication, which fully characterize the detailed strategies and animal movement20.
NOTE: In the following sections, slow, fast, and abrupt cooling protocols are discussed. N2 hermaphrodites at L4 or young adult age were used to produce the following data. The slow cooling strategy is useful for immobilizing 20 °C cultivated N2 animals at 6 °C; 15 °C-cultivated N2 animals are most strongly immobilized at 1 °C20. A brief comparison between these three cooling protocols is shown in Table 1.
7. Slow cooling immobilization protocol
- Move the cultivation plate with a lid on to a 4 °C refrigerator.
- After moving the cultivation plate to the refrigerator, turn on the cooling stage's 12 V power supply and set the tunable power supply voltage to 5.5 V.
- After the lidded cultivation plate has stayed in the 4 °C refrigerator for 1 h, transfer the plate immediately to the cooling stage and remove the lid (Figure 7A). Such cultivation plates are usually around 6 °C. The pre-cooled stage is stable and cold enough to maintain the agar surface at 6 °C.
- If the agar surface temperature changes, as measured or by noting animal movement, slightly adjust the voltage until it stabilizes at 6 °C.
- Animals are properly immobilized at the time of transfer.
8. Fast cooling immobilization protocol
NOTE: The fast cooling strategy is the most basic immobilization method (see Movie 1); however, agar plates idly occupy the stage for an extended time while reaching the Tset. Also, when a strong immobilization is needed and the Tset is 6 °C, the idle time is extended to around 1 h20.
- Turn on the cooling stage's 12 V power supply and set the tunable power supply voltage to around 12 V. Wait for 10 min.
- Bring a cultivation plate from its incubator directly to the cooling stage and remove the lid.
- Once the agar surface temperature decreases to (Tset + ΔT) °C, adjust the tunable power supply to Vset and wait until the agar reaches Tset. The Vset is the appropriate voltage to stabilize the agar at Tset. The ΔT is a variable that prevents overcooling. See Table 2 for the combination of Tset, ΔT, and Vset.
NOTE: The data presented in Table 2 pertains specifically to the Chung Laboratory, and therefore it should be noted that experimental parameters may vary based on the unique environmental and usage conditions of each individual experiment. - Animals are immobilized when the agar reaches Tset. Immobilization improves with time until ~50 min after the initiation of cooling.
9. Abrupt cooling immobilization protocol
NOTE: The abrupt cooling strategy consumes the most user time but immobilizes animals the most rapidly from their cultivation temperature.
- Turn on the cooling stage's 12 V power supply and turn the tunable power supply voltage to around 12 V. Keep for 10 min.
- Bring an unoccupied agar plate to the cooling stage. Use step 8.3 in the fast cooling immobilization protocol to stabilize the agar surface temperature at Tset.
- Move the animals from their original cultivation plate to the cooled plate sitting on the cooling stage.
- Based on the small animal size, the animals are expected to cool to Tset in seconds and be immobilized. Immobilization improves with time until ~50 min after the initiation of cooling.
10. Revival of animals after cooling immobilization
- Move the cooled culture plate back to the original incubator or to room temperature.
- Wait for 20 min to 1 h until all the worms on the plate revive to their normal crawling and feeding behavior.
Representative Results
Cooling temperature measurement
For the initial cooling immobilization experiments, it is important to track the agar surface temperature to ensure the animals can be properly immobilized. Future experiments that are replicated from the initial one can utilize the same parameters, usually without frequent temperature tracking. For temperature measurement, the thermocouple tip of the thermometer is sterilized using 70% ethanol solution, waiting until the ethanol fully evaporates before using. Then, the thermocouple tip is inserted 1 mm into the NGM agar to ensure an accurate temperature reading. The thermometer tip is held using a clamp holder or other holders (Figure 7B).
Temperature measurement with an infrared camera
The cooling stage is designed to ensure the temperature distribution in the plate's central 40 mm diameter area is uniform. A forward-looking infrared (FLIR) camera is used to image the temperature distribution on the agar surface. The maximum temperature difference is around 1 °C when the Tset is 1, 3, or 6 °C (Figure 8A).
Assessing the cooling rate with the fast cooling strategy
The fast cooling strategy is used to characterize the cooling rate of a stage at 12 V. A 20 °C plate is placed on the cooling stage and a thermocouple thermometer is used to track the surface temperature. The stage cools down the 20 °C plates to 6 °C in 6 min, to 1 °C in 10 min, and eventually stabilizes below -7 °C in around 40 min (Figure 8B).
Using the cooling stage on an upright microscope platform
An upright microscope typically comprises an objective for imaging, a stage for sample holding, and illumination. This cooling stage is designed for use on a typical upright microscope stage with easy insertion and removal (Figure 8C). When cooling immobilization is needed for imaging or screening, the cooling stage is simply placed on the microscope stage to finish the installment and vice versa.
Immobilization of worms on the cooling plate is shown in Movie 1.
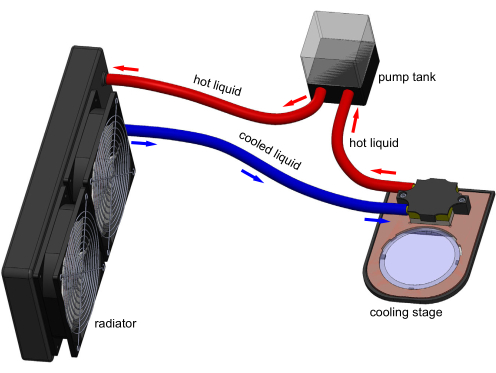
Figure 1: 3D model of the cooling stage apparatus. Electronic connections are not shown for clarity. A tank pumps water through the cooling block to remove heat transferred by the Peltier embedded in the stage. A typical 60 mm polystyrene cultivation plate can sit on the transparent sapphire window and be cooled by stage. Model generated in Solidworks. Please click here to view a larger version of this figure.
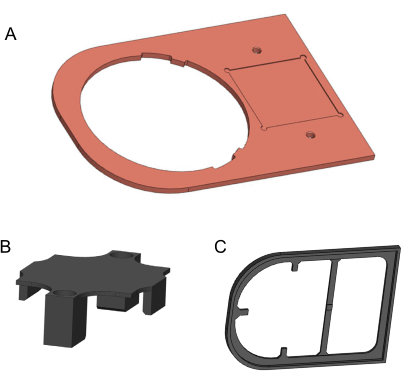
Figure 2: 3D models of components to be manufactured. (A) Copper plate. (B) 3D-printed holding bracket. (C) 3D-printed isolation plate. Models generated in Solidworks. Please click here to view a larger version of this figure.
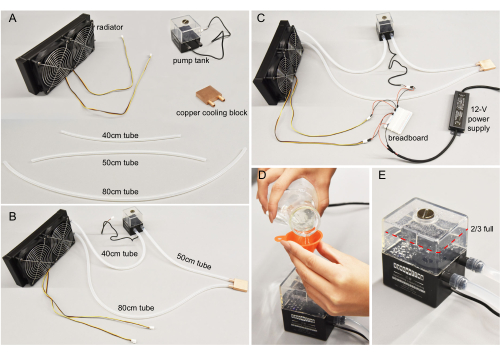
Figure 3: Water-cooling assembly. (A) Individual components. Tubes cut to specified lengths. (B) Water-cooling components connected. (C) Wires connecting the pump tank and the radiator to the 12 V power supply. In general, red wires connect to positive end, and black wires to the negative end. (D) Purified water poured into the pump. (E) The tank filled to more than two-thirds for optimal pump efficiency. Please click here to view a larger version of this figure.
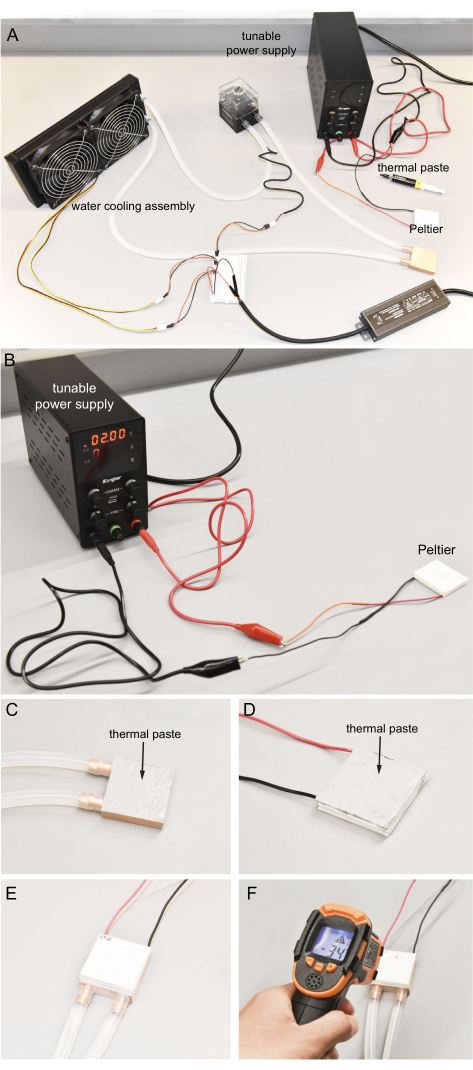
Figure 4: Connecting the Peltier and water-cooling assembly. (A) Components to operate the Peltier. (B) Utilizing the tunable power supply to determine the hot and cold sides of the Peltier. For safety, no more than 2 V is used. (C) Even application of thermal paste to the surface of copper block. (D) Even application of thermal paste to the Peltier hot surface. (E) Hot side of the Peltier pressed onto the copper block with thermal paste. (F) Infrared thermometer used to measure the Peltier cold surface temperature. Ideally, the cold temperature can reach near -35 °C. Please click here to view a larger version of this figure.
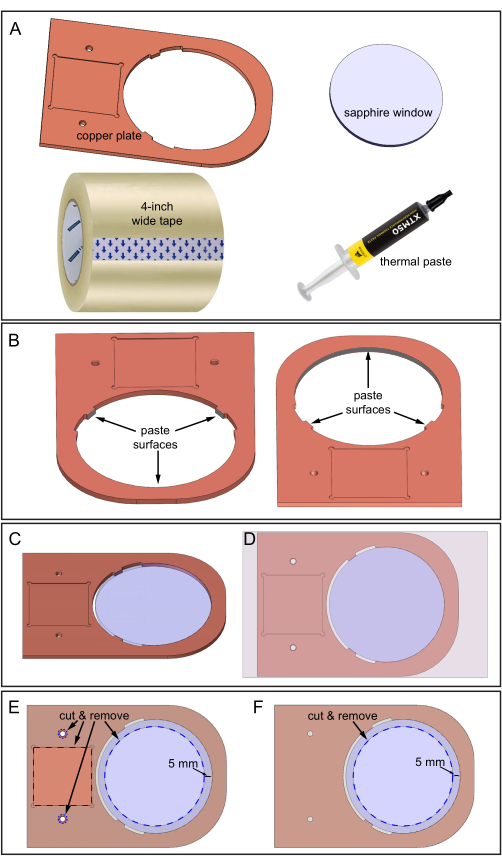
Figure 5: Assembling the copper plate and sapphire window. (A) Components required. (B) Thermal paste applied to three inner surfaces of the copper plate where the sapphire window will contact. Two downward-looking views of the copper plate showing the location of the three surfaces. (C) Sapphire window in the copper plate hole. (D) Tape applied to the top surface of the assembly. (E) Top-side: Blue dashed lines indicate the locations to cut and remove tape: square depression, two holes, and a 70 mm diameter sapphire area. (F) Bottom-side: Tape is cut and removed as shown. Please click here to view a larger version of this figure.
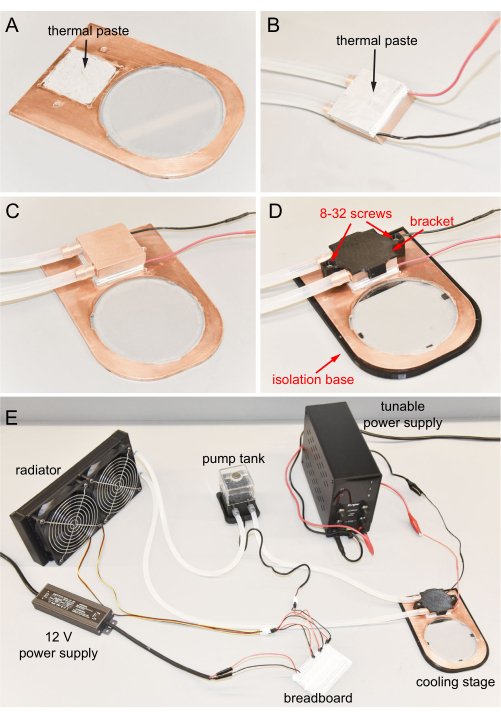
Figure 6: Cooling stage final assembly. (A) Thermal paste applied to the depression of the copper plate. (B) Thermal paste applied to the cold side of the Peltier. (C) Cold surface of the Peltier connected to the depression. (D) Copper cooling block fixed to the copper plate using screws. Cooling stage in the isolation base. (E) Completed cooling stage. Please click here to view a larger version of this figure.
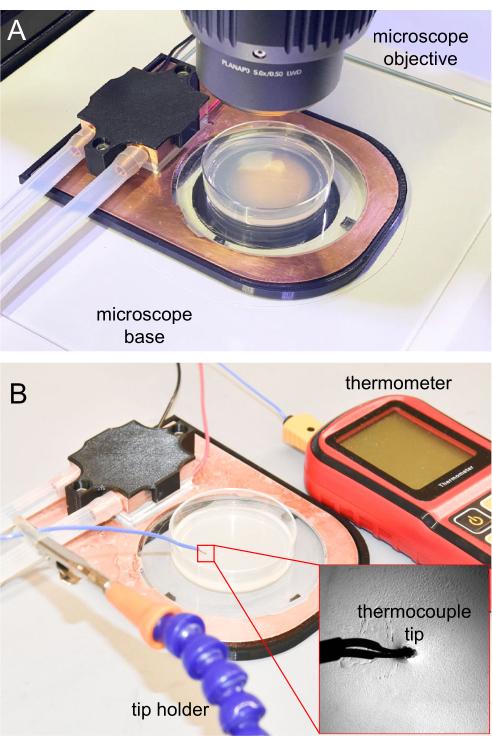
Figure 7: Cooling stage on the microscope and thermocouple measurement. (A) Cooling stage placed on the microscope base for imaging. The sapphire window is transparent, allowing transillumination. (B) Thermocouple thermometer used to measure the NGM agar surface temperature. The tip inserted around 1 mm into the NGM agar. Please click here to view a larger version of this figure.
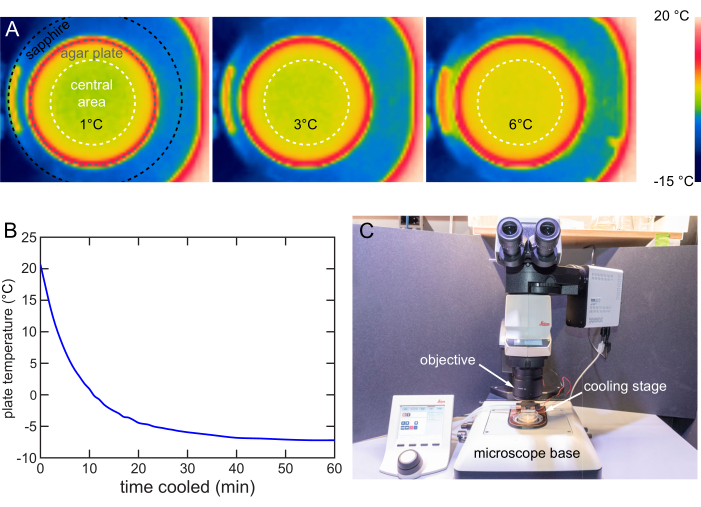
Figure 8: Cooling stage characterization and usage. (A) Thermal images showing the agar surface cooled to 1, 3, and 6 °C. Even temperature distribution within the central 40 mm area (white dashed circle). (B) Temperature of the NGM agar surface over time on the cooling stage at 12 V. The NGM agar surface can be cooled below -7 °C. Temperature measured by the method in Figure 7B. (C) Cooling stage in use on a typical upright microscope. The cooling stage can be easily installed or removed. Please click here to view a larger version of this figure.
| slow cooling | fast cooling | abrupt cooling | |
| stage occupation | minimum | long | medium |
| time until animals are immobilized | long | medium | very short |
| immobilization strength | strong | medium | medium |
| user effort | minimum | slightly more than minimum | maximum |
Table 1: Cooling strategies comparison.
| Tset (°C) | ΔT (°C) | Vset (V) |
| 1 | 2 | 8 |
| 2 | 3 | 7.4 |
| 3 | 4.5 | 7 |
| 4 | 5.5 | 6.5 |
| 5 | 6 | 5.9 |
| 6 | 6 | 5.5 |
Table 2: Parameters to achieve the desired temperature in the fast cooling strategy.
Supplementary File 1: Copper plate in metric. A2D drawing for machining the copper plate. Please click here to download this File.
Supplementary File 2: Holding bracket. A 3D drawing of a holding bracket that can be opened or modified by Solidworks and exported to 3D printing software. Please click here to download this File.
Supplementary File 3: Isolation plate. A 3D drawing of an isolation plate that can be opened or modified by Solidworks and exported to 3D printing software. Please click here to download this File.
Movie 1: Cooling video. Immobilization worms on the NGM agar plate at 2 °C. The plate was cooled down from room temperature to 2 °C, and stayed at 2 °C for several minutes. Then, the cooling stage was turned off and plates began to warm up to room temperature naturally. The video is speeded up by 10x to fit a 1 h video in 6 min. Please click here to download this Movie.
Supplementary Table 1: Price estimation Please click here to download this File.
Discussion
The cooling stage manufacturing, assembling, and usage is shown in this manuscript. Most of the components are off-the-shelf items that can be purchased online. Some components, like the copper plate and the sapphire window, need a custom order and may take up to 1 month to fabricate. Other components that can be 3D-printed are easily fabricated in most research institutions (Supplementary Table 1). The assembling process needs only a few tools and can be quickly done by a non-expert in a few hours. Thus, most biological laboratories should be able to easily implement this device.
The cooling stage and the cooling immobilization approach possess several significant improvements over existing immobilization methods, carefully detailed in the original publication20. In brief, the cooling stage enables the strong immobilization of large populations of C. elegans of all ages, including embryos and dauers, on their typical culture plates under standard microscopy workflows. It eliminates the need for complex hardware setups, like microfluidics, while providing a stronger immobilization effect. Additionally, it minimizes the possible toxic chemical exposure to animals and researchers since no chemicals are used, while providing a similar immobilization effect. These technical capabilities enable the broad application of this device and approach to many experiments that require high-resolution in vivo microscopy on large numbers of animals.
There are some critical steps during the building of the device, including all thermal paste application and the wide tape to fix the sapphire window to the cooper plate. The thermal paste ensures strong thermal conductivity by replacing gaps with a low-thermal resistance material. To achieve the desired cooling performance, the paste needs to be properly introduced between all abutting/contacting surfaces, including the Peltier cold surface to the copper plate, the Peltier hot surface to the copper cooling block, and the copper plate to the sapphire window. The wide tape applied to the stage isolates the copper plate to prevent heating from air and condensation, which leads to rust. It also strengthens the connection between the sapphire window and the copper plate. Thus, both applying thermal paste and the wide tape require extra care.
In an actual cooling immobilization experiment, the parameters provided in this manuscript, such as voltages and times, depend on the specific properties of the cultivation plates and stage, such as the amount of agar in the plates, the stage's efficiency, and the ambient temperature and humidity. In future modifications, a feedback controller could be installed, like a proportional-integral-derivative (PID), to actively adjust the voltage input to the cooling stage to achieve desired temperature and stabilize it.
There are several limitations of this cooling stage immobilization, carefully detailed in the original publication20. In brief, animals raised at different temperatures are immobilized to different degrees, which may need extra fine-tuning. Also, this current cooling stage is not designed for an inverted microscope. Further, imaging or screening on a cultivation plate directly may introduce contamination to the plate.
We are designing new versions of the cooling stage suitable for different imaging platforms, including compound upright microscopes and inverted microscopes. These new designs will allow direct animal cooling immobilization on culture plates during imaging on these platforms. The imaging on these cooling stages will use long working distance air immersion objectives, similar to the upright configuration. Nowadays, air immersion objectives can have a numerical aperture of up to 0.9, which provide around a 300 nm resolution for green fluorescence protein imaging. Thus, the combination of a new cooling stage with a microscope could permit submicron resolution fluorescence imaging routinely.
We also provide some helpful tips for using the cooling stage according to our experience. For instance, individuals should check whether there are any air bubbles inside the water-cooling assembly. Air bubbles degrade the cooling to the Peltier hot surface and thus degrade the cooling effectiveness of the cooling stage. If air bubbles are present, the 12 V power supply should be turned on to make the water flow and all the components of the water flow should be shaken. Air bubbles can be flushed out from trapped areas and vented by the pump tank. Researchers should ensure that the water flow tubing is not bent or crossed when assembling the water-cooling assembly. Tube bending or crossing may prevent the adequate flow of water and reduce cooling efficacy. Tube connections should be properly fit and tight. If necessary, a soft tube with a different diameter can be used instead to ensure tightness. Paste should not be applied, even if the connection is not tight enough, as paste may introduce clogs during future usage. The room humidity affects the cooling performance and introduces condensation and ice on the cooling stage. Before placing a cultivation plate on the cooling stage, it is recommended to use a paper tissue to remove condensation or use a heatsink to quickly remove ice that has formed on the sapphire window. The pump tank and radiator fans can cause small vibrations in the microscope if they work on the same table. Microscope vibration blurs the image acquired and thus should be avoided. A cushion can be used to mechanically insulate the tank and radiator, or they can be placed on a separate nearby table. The cooling stage can become a heating stage by reversing the electrical connection to the Peltier.
Declarações
The authors have nothing to disclose.
Acknowledgements
We acknowledge Noah Joseph (Northeastern Bioengineering Department) for the copper plate machining.
Materials
| 12-V power supply | ANYTITI | ledpower00 | output DC 12V +/-0.5V, 5A power 60W |
| 8-32 screw | arbitrary | for bracket fixation | |
| bracket | N/A | N/A | 3D printed using 1.75mm PLA filament. See supplementary for 3D model. |
| breadboard | DEYUE | 7545924028 | 400 pin solderless board kit for DIY electric connection |
| copper cooling block | Kalolary | Kalolary-Heatsink001 | 40*40mm internal fin thickness 0.5mm |
| copper plate | arbitrary | N/A | Machined from a 170x120x3 mm 99.9% pure copper sheet. See supplementary for 2D drawing for manufacturing. |
| digital thermocouple thermometer | Proster | 4333090752 | dual channel thermometer with two K-type thermocouple probes measuring range -50-300°C accuracy ±1.5% resolution 0.1°C /°F < 1000° |
| isolation base | N/A | N/A | 3D printed using 1.75mm PLA filament. See supplementary for 3D model. |
| jumper wires | arbitrary | for electronic connection | |
| multistage peltier | DigiKey | TEC1-12706 | thermoelectric cooling device size 40*40*7.05 mm Umax 16.1 V Imax 8.5 A ΔTmax @ Th 85°C @ 27°C Qmax @ Th 51.6W @ 27°C resistance 1.65 Ω |
| Nalgene 50 Platinum-Cured Silicone Tubing | ThermoScientific | 14-176-332E | ultrasoft tube durometer hardness Shore A, 50 inner diameter 1/4 in outer diameter 9.5 mm |
| packaging tape | arbitrary | 4 inch wide to cover the copper plate | |
| pump tank | Yosoo | SC-300T | input power DC 12V flow rate 300L/h max |
| radiator | DIYhzWater | 10463 | 12 pipe aluminum heat exchanger cooling water drain row with two 120mm fans |
| sapphire window | Altos Photonics, Inc. | N/A | Contact Altos for custom order size Ø 80mm, 3mm thick surface quality 60-40s/d uncoated |
| thermal paste | Corsair | XTM50 | reduce thermal impedance between surfaces thermal conductivity 5.0W/mK |
| tunable power supply | Kungber | DY-SPS3010B | voltage range 0 – 30V current range 0 – 10A linear Power Supply with 4-Digits coarse and fine adjustments with alligator leads |
Referências
- Wearne, S. L., et al. New techniques for imaging, digitization and analysis of three-dimensional neural morphology on multiple scales. Neurociência. 136 (3), 661-680 (2005).
- Zhou, Z., Sorensen, S., Zeng, H., Hawrylycz, M., Peng, H. Adaptive image enhancement for tracing 3D morphologies of neurons and brain vasculatures. Neuroinformatics. 13 (2), 153-166 (2015).
- Parthasarathy, R., Groves, J. T. Optical techniques for imaging membrane topography. Cell Biochemistry and Biophysics. 41 (3), 391-414 (2004).
- Chan, C. Y., Faragalla, Y., Wu, L. -. G. Illuminating membrane structural dynamics of fusion and endocytosis with advanced light imaging techniques. Biochemical Society Transactions. 50 (4), 1157-1167 (2022).
- Chen, Y., Periasamy, A. Characterization of two-photon excitation fluorescence lifetime imaging microscopy for protein localization. Microscopy Research and Technique. 63 (1), 72-80 (2004).
- Chen, Y., Mills, J. D., Periasamy, A. Protein localization in living cells and tissues using FRET and FLIM. Differentiation. 71 (9-10), 528-541 (2003).
- Frigault, M. M., Lacoste, J., Swift, J. L., Brown, C. M. Live-cell microscopy-tips and tools. Journal of Cell Science. 122 (6), 753-767 (2009).
- Schneckenburger, H., et al. Light exposure and cell viability in fluorescence microscopy. Journal of Microscopy. 245 (3), 311-318 (2012).
- Brenner, S. The genetics of Caenorhabditis elegans. Genética. 77 (1), 71-94 (1974).
- Hobert, O., Loria, P. Uses of GFP in Caenorhabditis elegans. Green Fluorescent Protein: Properties, Applications, and Protocols. 47, 203-226 (2005).
- Emmons, S. W., Yemini, E., Zimmer, M. Methods for analyzing neuronal structure and activity in Caenorhabditis elegans. Genética. 218 (4), (2021).
- Chung, S. H., et al. Novel DLK-independent neuronal regeneration in Caenorhabditis elegans shares links with activity-dependent ectopic outgrowth. Proceedings of the National Academy of Sciences. 113 (20), E2852-E2860 (2016).
- Caldwell, K. A., Willicott, C. W., Caldwell, G. A. Modeling neurodegeneration in Caenorhabditis elegans. Disease Models & Mechanisms. 13 (10), (2020).
- Pintard, L., Bowerman, B. Mitotic cell division in Caenorhabditis elegans. Genética. 211 (1), 35-73 (2019).
- Bargmann, C. I., Avery, L. Laser killing of cells in Caenorhabditis elegans. Methods in Cell Biology. 48, 225-250 (1995).
- Fang-Yen, C., Gabel, C. V., Samuel, A. D. T., Bargmann, C. I., Avery, L. Laser microsurgery in Caenorhabditis elegans. Methods in Cell Biology. 107, 177-206 (2012).
- Chung, K. H., Crane, M. M., Lu, H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nature Methods. 5 (7), 637-643 (2008).
- Rohde, C. B., Yanik, M. F. Subcellular in vivo time-lapse imaging and optical manipulation of Caenorhabditis elegans in standard multiwell plates. Nature Communications. 2, 271 (2011).
- Guo, S. X., et al. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nature Methods. 5 (6), 531-533 (2008).
- Wang, Y. L., Grooms, N. W. F., Jaklitsch, E. L., Schulting, L. G., Chung, S. H. High-throughput submicron-resolution microscopy of Caenorhabditis elegans populations under strong immobilization by cooling cultivation plates. iScience. 26 (2), 105999 (2023).
- Zhao, D., Tan, G. A review of thermoelectric cooling: Materials, modeling and applications. Applied Thermal Engineering. 66 (1-2), 15-24 (2014).

