A Mouse Model for Corneal Neovascularization by Alkali Burn
Summary
This protocol focuses on alkali burn-induced corneal neovascularization in mice. The method generates a reproducible and controllable corneal disease model to study pathological angiogenesis and the associated molecular mechanisms and to test new pharmacological agents to prevent corneal neovascularization.
Abstract
Corneal neovascularization (CoNV), a pathological form of angiogenesis, involves the growth of blood and lymph vessels into the avascular cornea from the limbus and adversely affects transparency and vision. Alkali burn is one of the most common forms of ocular trauma that leads to CoNV. In this protocol, CoNV is experimentally induced using sodium hydroxide solution in a controlled manner to ensure reproducibility. The alkali burn model is useful for understanding the pathology of CoNV and can be extended to study angiogenesis in general because of the avascularity, transparency, and accessibility of the cornea. In this work, CoNV was analyzed by direct examination under a dissecting microscope and by immunostaining flat-mount corneas using anti-CD31 mAb. Lymphangiogenesis was detected on flat-mount corneas by immunostaining using anti-LYVE-1 mAb. Corneal edema was visualized and quantified using optical coherence tomography (OCT). In summary, this model will help to advance existing neovascularization assays and discover new treatment strategies for pathologic ocular and extraocular angiogenesis.
Introduction
The cornea is an avascular tissue that maintains its transparency by establishing an angiogenic privilege1,2. Damage to the cornea can result in inflammation and the development of blood and lymph vessels, as well as fibrosis3. Corneal neovascularization (CoNV) leads to visual impairment and is the second leading cause of blindness worldwide4. CoNV affects about 1.4 million people in the United States per year5. CoNV can be induced by various factors, including chemical burns, infections, inflammation, and hypoxia3,6. Chemical burns are one of the most common ocular emergencies, and they account for about 13.2% of ocular trauma and require immediate assessment and treatment7. Chemical burns could be alkali or acid burns, but alkali burns cause more severe injury, as alkali penetrates deeper into the tissue8.
Mouse models of alkali burn are widely used to study CoNV and wound healing. Compared to the corneal pocket angiogenesis model9,10, alkali burn models are relatively straightforward to create and can also be used to study corneal inflammation, fibrosis, and epithelial proliferation. These models are also more closely related to clinical chemical burns than corneal suture models of angiogenesis11. With alkali burn, the otherwise avascular cornea develops blood vessels due to inflammation and an imbalance in anti-angiogenic and pro-angiogenic factors1,2. The drawbacks of corneal alkali burn models are the difficulties in controlling the area and severity of the alkali burn, the variation in corneal neovascularization, and unintentional burning of the adjacent tissues due to excess alkali solution. The purpose of this study is to describe a controlled corneal alkali burn model in mice using filter paper pre-soaked in sodium hydroxide solution. This model could be used to study angiogenic factors, anti-angiogenic therapeutic reagents, and other factors and reagents that could modulate inflammation and fibrosis.
Protocol
All the animal work, including the experimental procedures and euthanasia, was approved by the Institutional Animal Care and Use Committee (IACUC) at Baylor College of Medicine with Protocol Number AN-8790.
1. Preparation of 1 N NaOH
- Add 4 mL of sterile deionized water to a 15 mL centrifuge tube. Weigh out 400 mg of sodium hydroxide (NaOH), and add to the tube carefully.
- Dissolve the NaOH by slowly stirring the solution using a glass rod. Make up the volume to 10 mL by adding sterile deionized water to the tube, and mix again by gently inverting the tube up and down. Close the cap tightly, and store the solution at room temperature.
- Prepare the fresh solution every month because the concentration of the NaOH solution may be reduced by the solution absorbing carbon dioxide in the air.
- Always gently mix the NaOH solution before use.
CAUTION: Prepare the solution inside a chemical hood, and wear appropriate personal protective equipment (PPE).
2. Preparation of the 4% paraformaldehyde (PFA) solution
- Add 30 mL of 1x phosphate-buffered saline (PBS) to a glass beaker. Weigh out 4 g of paraformaldehyde (PFA), and add it to the beaker.
- Keep the beaker on a hot plate at 60 °C with stirring. Add the 1 N NaOH solution dropwise to raise the pH until the solution clears.
- Check and adjust the pH to 7.4 using 1 N hydrochloric acid (HCl). Adjust the final volume to 50 mL with 1x PBS.
- Cool and filter the solution. Store the solution at 4 °C.
CAUTION: Prepare the solution in a fume hood while wearing appropriate PPE.
3. Preparing the ketamine/xylazine cocktail
- Prepare the ketamine/xylazine cocktail by adding 0.8 mL of ketamine (stock concentration: 100 mg/mL) and 0.16 mL of xylazine (stock concentration: 100 mg/mL) to 9.4 mL of saline.
- Store the cocktail in sterile injection bottles at room temperature (RT).
4. Alkali burn on the mouse cornea
- Inject meloxicam (4-6 mg/kg of body weight) subcutaneously 30 min prior to the procedure for pain relief. Anesthetize the mice (C57BL/6J, 6-8 weeks of age, male) using an i.p. injection of the ketamine/xylazine cocktail (Ketamine 80 mg/kg and Xylazine 16 mg/kg of body weight).
- Check the reflex response (pedal withdrawal) by pinching the toes of the mouse, and confirm the absence of the reflex. Apply one drop of topical anesthetic, 0.5% proparacaine, on the corneal surface of one eye, and a drop of artificial tears on the other eye.
- Using a 2 mm biopsy punch, punch out Whatman filter paper discs.
- Add 2 µL of 1N NaOH to a clean Petri dish. Put the 2 mm filter paper disc on the 1 N NaOH drop, and allow it to soak for 15 s.
- Pick up the filter paper with forceps, and apply the filter paper to the proparacaine-treated eye at the center of the cornea for 30 s.
NOTE: The filter paper must be touching only the center of the cornea, and care must be taken to avoid the movement of the filter paper once placed, as moving the filter paper can cause burns to the adjacent tissues. - Wash the eye by flushing with 20 mL of sterile saline solution in a sterile syringe.
NOTE: Care must be taken to ensure that the cornea, along with the conjunctival sac, is washed thoroughly to ensure no further damage to the cornea or surrounding tissue. Washing the conjunctival sac will further prevent symblepharon. - Wipe the excess saline gently from the eyes and the surrounding area using disposable soft wipes. Afterward, keep the mice in a recovery cage on a warm heating pad until ambulatory.
NOTE: Mice are monitored daily after the alkali burn for 3 days. If symptoms of pain or stress are observed, meloxicam (4-6 mg/kg of body weight) is administered subcutaneously.
5. Examination and assessment of neovascularization and opacity
- In anesthetized mice, examine the eyes under a dissection microscope on day 10 after the burn, and obtain images using a camera attached to the dissection scope to score the opacity and neovascularization.
NOTE: A regular dissection scope with a camera attached is sufficient. - While observing the cornea through the dissection microscope, score the opacity after the burn based on the following scale12:
0 = No opacity; clear cornea
1 = Mild opacity; slight haziness in the iris and pupil areas; iris and pupil easily visible
2 = Moderate opacity; iris and pupil barely visible
3 = Severe opacity; iris or pupil not visible
4 = Opaque cornea; iris and pupil not visible - While observing the cornea through the dissection microscope, score the CoNV based on the following scale12:
0 = No neovascularization; no new vessels from the limbus
1 = Mild neovascularization; new vessels originating from the limbus
2 = Moderate neovascularization; blood vessels originated from the limbus and growing toward the center of the cornea
3 = Severe neovascularization; blood vessels originating from the limbus and reaching and/or crossing the center of the cornea - Use a Student's t-test to statistically compare the opacity and neovascularization scores between the alkali burn and healthy eye groups.
- Euthanize the mice on day 10 by isoflurane exposure at 5% until 1 min after breathing stops, followed by cervical dislocation, and collect the corneas for flat-mount imaging.
6. Optical coherence tomography (OCT) imaging
- Take OCT images of the anterior segment of the eyes in anesthetized mice on day 10 after the burn. Perform OCT image acquisition as a volume scan using IR + OCT mode with a 30° field of view and 100% IR intensity.
- Quantify the thickness of the corneas using ImageJ software.
- To measure the thickness, use the Line Selection tool in ImageJ software to create a straight line between the anterior and posterior surfaces at the central cornea.
- Click on Analyze > Measure in the software tools to transfer the values to the data window.
- Copy the values to a spreadsheet file, and statistically compare the corneal thickness between the alkali burn and healthy eye groups using a Student's t-test.
NOTE: The thickness of the cornea is the distance from a point on the anterior corneal surface to the closest point on the posterior corneal surface at the corneal center.
7. Immunostaining for CoNV on flat-mount corneas
- Euthanize the mice on day 10 post alkali burn, and enucleate the eyes by blunt dissection.
- Pull the eyelids apart using the thumb and index fingers, and place forceps under the eye globe. Close the forceps, and gently pull the eyeball off from the orbit.
- Place the eyeballs in 1x PBS. For each eyeball, remove the cornea from the eye globe by first making an incision using a 30 G needle below the limbus area.
- Cut around the limbus area using corneal micro scissors, with the incision as the starting point, and slowly separate the cornea and limbus from the globe.
- Clean the corneas gently using a fine paintbrush to remove the iris. Fix the corneas in 4% paraformaldehyde for 1 h.
- Wash the corneas three times for 20 min each in 1x PBS at room temperature (RT).
- Incubate in a blocking buffer (1x PBS supplemented with 0.1% Triton-X 100 and 5% bovine serum albumin [BSA]) for 1 h at RT.
- Transfer the corneas to an antibody solution containing primary antibodies. Prepare the antibody solution in 1x PBS supplemented with 1% BSA, 0.1% Triton-X 100, Dylight550-conjugated anti-CD31 mAb (1:100), and Alexa Fluor488-conjugated anti-LYVE-1 mAb (1:100).
- Incubate for 3 days at 4 °C. Wash the corneas in 1x PBS three times for 20 min each.
- Stain the nuclei using Hoechst stain solution (1:1,000) for 5 min in the dark.
- Flatten the corneas with radial cuts, and mount them on a pre-cleaned glass slide using mounting medium and coverslips. Seal the coverslips with clear nail polish, and dry the slides overnight in the dark before analysis by confocal microscopy.
- Image the flat-mounted corneas using confocal microscopy by stitching individual Z-stack images; use a 10x objective, 488 nm and 561 nm lasers, and a resolution of 512 pixels x 512 pixels per slice on non-resonant galvano scanners.
- Quantify the density of CD31+ blood and LYVE-1+ lymph vessels using ImageJ software.
- To determine the vascular density, convert the confocal images to an 8-bit image.
- Choose Vascular Density from the Plugins.
- Choose the region of interest on the image, and click on OK. The measurements will open in a new data window.
- Copy the values to a spreadsheet file, and statistically compare the vascular density between the alkali burn and healthy eye groups using a Student's t-test.
NOTE: CD31, also called platelet endothelial cell adhesion molecule-1 (PECAM-1), is a cell adhesion molecule involved in angiogenesis and is highly expressed in the endothelial cells of early and mature blood vessels13. LYVE-1 (lymphatic vessel endothelial hyaluronan receptor-1) is a cell surface marker on lymphatic endothelial cells and can be used as a lymphangiogenesis marker14.
Representative Results
This study describes a method to induce corneal angiogenesis in the mouse eye by alkali burn. The images obtained with the dissection microscope (Figure 1A,B) demonstrated significantly elevated neovascularization and opacity scores in the corneas in the alkali burn group (P < 0.05; Figure 1C,D). The corneas that were collected on day 10 were further immunostained with anti-CD31 mAb for blood vessels and anti-LYVE-1 mAb for lymph vessels, respectively (Figure 2A–I). The alkali burn group showed significantly higher densities of blood and lymph vessels after 10 days (P < 0.001 and P < 0.05, respectively; Figure 2J,K). The thickness of the cornea, as imaged and quantified using OCT (Figure 3A,B), was observed to be significantly higher in the group with alkali burn (P < 0.01; Figure 3C).
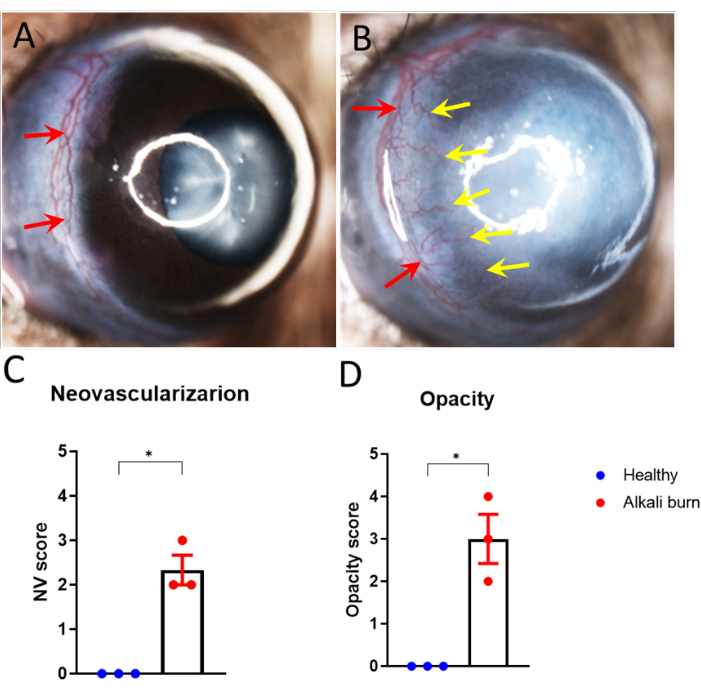
Figure 1: Alkali burn-induced corneal neovascularization and opacity. (A,B) Corneal neovascularization sprouted from the limbus vessels toward the corneal center in the (B) alkali-burned mouse eye (A) but not healthy eye 10 days after the injury. (C,D) Quantification of the (C) corneal neovascularization and (D) opacity in panels A and B (± SEM; t-test; *P < 0.05; n = 3 eyes, 1 eye/mouse). The red arrows represent the limbus, and the yellow arrow indicates the sprouting new vessels. Please click here to view a larger version of this figure.
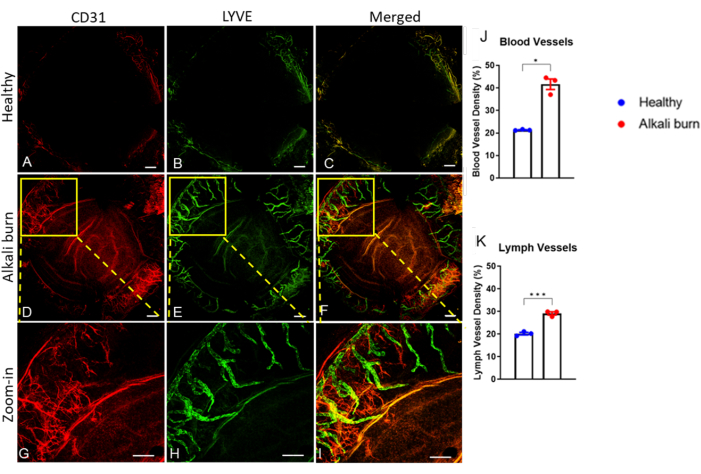
Figure 2: Corneal neovascularization and lymphangiogenesis caused by alkali burn. Immunohistochemistry revealed (A,D,G) blood and (B,E,H) lymph vessels using the anti-CD31 and anti-LYVE-1 mAbs, respectively. (A–C) The healthy mouse cornea. (D–I) The alkali-burned cornea 10 days post injury. (C,F,I) Superimposed images of CD31 and LYVE-1 signals. (G–I) Zoomed-in images for panels D–F. Scale bars = (A–F) 200 µm and (G–I) 500 µm. (J,K) Quantification of the blood and lymph vessel density in panels A–F, as indicated (± SEM; t-test; *P < 0.05; ***P < 0.001; n = 3 eyes, 1 eye/mouse). Please click here to view a larger version of this figure.
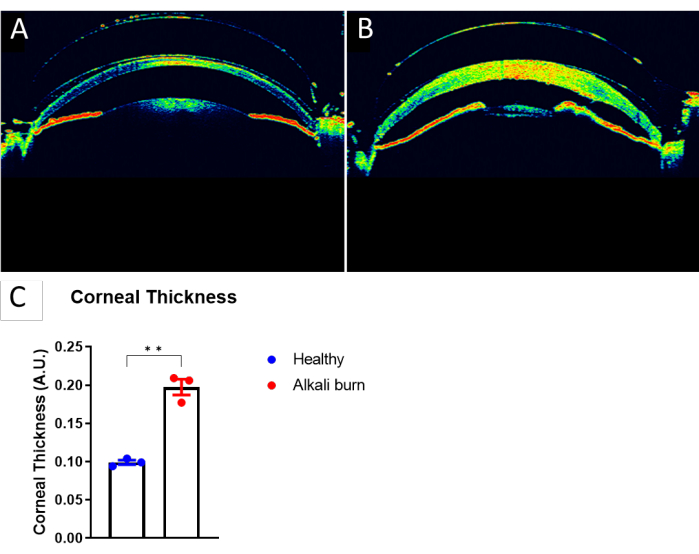
Figure 3: Increase in corneal thickness caused by alkali burn. (A) An OCT image of a healthy mouse eye. (B) An OCT image of the mouse cornea 10 days post alkali burn. (C) Quantification of the corneal thickness in panels A and B, as measured at the center of the cornea (± SEM; t-test; **P < 0.01; n = 3 eyes, 1 eye/mouse). Please click here to view a larger version of this figure.
Discussion
The cornea is an excellent tissue for studying angiogenesis and inflammation because it is accessible and avascular, meaning that neovascularization can be conveniently detected and documented. Corneal burn in rabbits, rats, and mice has been used to study corneal angiogenesis, inflammation and opacity, ulceration, perforation of the cornea, and fibrosis15,16,17. Moreover, the mouse model of corneal burn is valuable for testing various therapeutic strategies for angiogenesis and inflammation because mice have an immune system closely related to that of humans18. The availability of techniques to genetically manipulate the mouse genome also makes the species an excellent choice for this type of study19. The challenge in this research has been to develop a method of corneal burn that provides consistent, reproducible pathophysiology.
The alkali burn model is particularly useful for the pharmacological screening of drugs that modulate angiogenesis, inflammation, and fibrosis. The minimal requirements for reagents and resources, the simplicity of performing the alkali burn, and the benefits of the short duration of the protocol and the direct observation of the results make alkali burn on the mouse cornea a primary choice for pharmacological drug screening. However, a few precautions should be considered when performing this procedure to ensure consistency and reproducibility. Firstly, the filter paper must be placed at the center of the cornea to avoid burning other areas of the eye, especially the limbus, eyelids, and conjunctiva; secondly, the volume and concentration of NaOH should be appropriate to obtain consistent results from the alkali burn on the cornea. The filter must not be dripping wet but should have been soaked in the NaOH solution. The filter size and filter type and the normality and volume of the solution used in this method are optimized to avoid an overflow of NaOH. Using a different-sized filter paper or a higher or lower volume of NaOH would cause inconsistencies in the neovascularization. Thirdly, it is important to prevent the NaOH solution from absorbing CO2 in the room air by immediately tightening the tube cap of the solution after use and reducing the air/solution ratio. Care must be taken to use fresh alkali solutions to prevent inconsistencies in the neovascularization and to avoid corneal ulceration. Finally, extensive washing of all the NaOH solution from the eye and conjunctiva with saline is necessary to prevent further damage to the cornea and surrounding tissues of the eye. The thorough washing of the cornea and the adjacent tissues will also prevent symblepharon.
The protocol described here is an efficient and reliable method for studying the pathophysiology of corneal angiogenesis. This protocol can be further used to study corneal inflammation, fibrosis, and wound healing.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the SRB Charitable Corporation, National Institutes of Health (NIH) P30EY002520, and an unrestricted institutional grant from Research to Prevent Blindness (RPB) to the Department of Ophthalmology, Baylor College of Medicine. W.L. is supported by The Knights Templar Eye Foundation Endowment in Ophthalmology.
Materials
| 0.9% Sodium Chloride Injection | Hospira | KL-7302 | |
| 30 G Needle | McKesson | 16-N3005 | |
| A1R Confocal | Nikon Instruments | ||
| Anti-CD31 | Novus Biologicals | NB100-1642R | |
| Anti-LYVE-1 | Life technologies | 53-0443-82 | |
| ASM Module | Heidelberg Engineering | Anterior segment objective | |
| Biopsy Punch | McKesson | 16-1309 | |
| BSA | Thermoscientific | 9048-46-8 | |
| Coverslip | VWR International | 22X22-1-601640G | |
| Dissection Microscope | AmScope | SM-4TZ-30WY-10M3 | |
| Fluoromount-G | Electron Microscopy Sciences | 17984-25 | |
| Forceps | Fine Science Tools | 15000-02 | |
| Forceps | Fine Science Tools | 11049-10 | |
| Forceps | Fisherbrand | 12-000-157 | |
| Forceps | Roboz | RS-4905 | |
| Gonak Hypromellose | Akorn | 17478006412 | |
| GraphPad Prism 9 | GraphPad Sotware, Inc | ||
| Heating pad | K&H Pet Products | 100213018 | |
| Hoescht | Life Technologies | 62249 | |
| HRA + OCT Spectralis | Heidelberg Engineering | ||
| Insulin Syringe | Mckesson | 102-SN310C31516P | |
| Kimwipe | Kimberly Clark Professional | 34155 | |
| Micro Cover Glass | VWR | 48366-067 | |
| Microscissors | Roboz | RS-5110 | |
| Microscopic Slide | Fisherbrand | 12-550-15 | |
| NaOH | Sigma Aldrich | 55881-500G | |
| Neomycin and Polymyxin B Sulfates and Dexamethasone | Bausch & Lomb | 24208-0795-35 | |
| Normal Serum | Jackson Immuno | 008-000-121 | |
| Paraformaldehyde | Sigma Aldrich | 158127-500G | |
| PBS | Gibco | 20012-027 | |
| Proparacaine HCl | Bausch & Lomb | 24208073006 | |
| Saline | Henry Schein | 1531042 | |
| SMZ125 | Nikon Instruments | ||
| Syringe 10 mL | McKesson | 16-S10C | |
| Triton X-100 | Sigma Aldrich | TX1568-1 | |
| Whatmann Filter Paper | Cytiva | WHA1003323 |
Referências
- Ellenberg, D., et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Progress in Retinal and Eye Research. 29 (3), 208-248 (2010).
- Azar, D. T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Transactions of the American Ophthalmological Society. 104, 264-302 (2006).
- Rolfsen, M. L., et al. Corneal neovascularization: A review of the molecular biology and current therapies. Expert Review of Ophthalmology. 8 (2), 167-189 (2013).
- Skobe, M., Dana, R. Blocking the path of lymphatic vessels. Nature Medicine. 15 (9), 993-994 (2009).
- Lee, P., Wang, C. C., Adamis, A. P. Ocular neovascularization: An epidemiologic review. Survey of Ophthalmology. 43 (3), 245-269 (1998).
- Su, W., et al. Efficacious, safe, and stable inhibition of corneal neovascularization by AAV-vectored anti-VEGF therapeutics. Molecular Therapy – Methods & Clinical Development. 22, 107-121 (2021).
- Lasagni Vitar, R. M., et al. Epidemiology of corneal neovascularization and its impact on visual acuity and sensitivity: A 14-year retrospective study. Frontiers in Medicine. 8, 733538 (2021).
- Said, D. G., Dua, H. S. Chemical burns acid or alkali, what’s the difference. Eye. 34, 1299-1300 (2020).
- Muthukkaruppan, V. R., Auerbach, R. Angiogenesis in the mouse cornea. Science. 2 (4413), 1416-1418 (1979).
- Kenyon, B. M., et al. A model of angiogenesis in the mouse cornea. Investigative Ophthalmology & Visual Science. 37 (8), 1625-1632 (1996).
- Cursiefen, C., Maruyama, K., Jackson, D. G., Streilein, J. W., Kruse, F. E. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 25 (4), 443-447 (2006).
- Yoeruek, E., et al. penetration and efficacy of topically applied bevacizumab: Evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmologica. 86 (3), 322-328 (2008).
- DeLisser, H. M., et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. The American Journal of Pathology. 151 (3), 671-677 (1997).
- Johnson, L. A., Prevo, R., Clasper, S., Jackson, D. G. Inflammation-induced uptake and degradation of the lymphatic endothelial hyaluronan receptor LYVE-1. The Journal of Biological Chemistry. 282 (46), 33671-33680 (2007).
- Choi, H., et al. Comprehensive modeling of corneal alkali injury in the rat eye. Current Eye Research. 42 (10), 1348-1357 (2017).
- Chung, J. H., Fagerholm, P., Lindström, B. The behaviour of corneal epithelium following a standardized alkali wound. Acta Ophthalmologica. 65 (5), 529-537 (1987).
- Chang, J. H., Gabison, E. E., Kato, T., Azar, D. T. Corneal neovascularization. Current Opinion in Ophthalmology. 12 (4), 242-249 (2001).
- Alves da Costa, T., Lang, J., Torres, R. M., Pelanda, R. The development of human immune system mice and their use to study tolerance and autoimmunity. Journal of Translational Autoimmunity. 2, 100021 (2019).
- vander Weyden, L., White, J. K., Adams, D. J., Logan, D. W. The mouse genetics toolkit: Revealing function and mechanism. Genome Biology. 12 (6), 224 (2011).

