Generating Human Pancreatic Tissue Slices to Study Endocrine and Exocrine Pancreas Physiology
Summary
This protocol describes how to generate human pancreas slices from deceased organ donors to study cell function under near-physiological conditions. This innovative approach enables the investigation of normal and structurally damaged islets and the intricate interplay between endocrine and exocrine compartments.
Abstract
It is crucial to study the human pancreas to understand the pathophysiological mechanisms associated with type 1 (T1D) and 2 diabetes (T2D) as well as the pancreas endocrine and exocrine physiology and interplay. Much has been learned from the study of isolated pancreatic islets, but this prevents examining their function and interactions in the context of the whole tissue. Pancreas slices provide a unique opportunity to explore the physiology of normal, inflamed, and structurally damaged islets within their native environment, in turn allowing the study of interactions between endocrine and exocrine compartments to better investigate the complex dynamics of pancreatic tissue. Thus, the adoption of the living pancreas slice platform represents a significant advancement in the field. This protocol describes how to generate living tissue slices from deceased organ donors by tissue embedding in agarose and vibratome slicing as well as their utilization to assess functional readouts such as dynamic secretion and live cell imaging.
Introduction
Studies on islet physiology are fundamental for understanding the pathogenesis of diabetes and developing new therapeutic approaches. So far, research has relied on isolated islets, which exposes the islets to mechanical and enzymatic stresses, likely causing changes in cell physiology; moreover, it is not possible to evaluate islet function in the context of their natural tissue environment, which is likely impacted by exocrine and vascular cells among others1. When studying pancreas from donors with T1D, there is the challenge that their islets are difficult to isolate and might become fragmented during the isolation, which might produce a selection effect on islets that may not represent the population in vivo2. Moreover, islets will be separated from their complex environment and cellular connections, especially from the infiltrating immune cells that are found in inflamed islets and are more abundant in the islet periphery. Thus, while isolated islets are a cornerstone tool in diabetes research, there are limitations. In response, we introduce a groundbreaking protocol for generating living pancreas slices, offering a solution to these challenges.
The recent development and adoption of pancreatic tissue slicing techniques is considered a breakthrough for our ability to explore the intricate biology and functions of the pancreas. This innovation has opened new avenues for dynamic studies of islet physiology and interactions among endocrine, exocrine, neural, vascular, and immune cells within their natural anatomical context. Unlike conventional approaches, this in vitro setting preserves much of the organ's cytoarchitecture, allowing for a closer approximation to its native biology. Initially developed in mice by Speier and Rupnik in 20033, this method has demonstrated its utility to assess calcium imaging, electrophysiology, and hormone secretion for both intra- and inter-cellular signaling4,5,6,7,8,9. The pancreas slice platform was then applied to the study of human pancreas tissue obtained via surgical biopsy4,10,11,12. Our group demonstrated the feasibility of obtaining and utilizing pancreas slices from cadaveric organ donors through the activity of the Network for Pancreatic Organ Donors with Diabetes (nPOD)13. nPOD provides pancreas tissues to approved investigators conducting research about human type 1 diabetes, and since adopting the pancreas slice platform nPOD routinely generates and distributes living pancreas slices14,15,16,17. Since the implementation of the pancreas slice platform in 2020, nPOD has successfully distributed tissue slices from 43 donors (including 12 donors with T1D) to numerous investigators. Using these slices, researchers performed groundbreaking research on critical aspects of islet function and explored the interplay between islets and the vasculature, nervous system, and immune cells in the context of T1D13,18,19,20,21,22,23,24. Numerous studies have highlighted the limitations of traditional approaches and underscored the significance of techniques that can capture the dynamic interplay within the pancreas25,26,27. The adaptability of slicing techniques from mouse to human pancreas, coupled with their integration into programs like the Network for Pancreatic Organ Donors with Diabetes (nPOD), exemplifies the growing acknowledgment of the method's potential to unlock valuable insights into diseases such as Type 1 diabetes.
Protocol
Human pancreatic sections from tissue donors of both sexes were obtained via the Network for Pancreatic Organ Donors with Diabetes (nPOD) tissue bank, University of Florida. Organ tissue from deceased individuals without identifiers have been determined to be non-human subjects research in accordance with organ donation laws and regulations and classified as Non-Human Subjects by the University of Florida Institutional Review Board (IRB; IRB no. 392-2008), waiving the need for consent. nPOD tissues specifically used for this project were approved as nonhuman by the University of Florida IRB (IRB20140093).
NOTE: For readers entirely new to the slice technique and its applications, such as perifusion and calcium imaging, it might be advisable to gain practical experience and develop basic skills using mouse or rat slices3,28 before working with human samples.
1. Preparations
NOTE: These preparations should be performed prior to tissue arrival.
- Prepare HEPES buffer by mixing 125 mM NaCl, 5.9 mM KCl, 2.56 mM CaCl2, 1 mM MgCl2, 25 mM HEPES, 0.1% BSA, 2 mM L-alanine, L-arginine, and L-glutamine. Adjust pH to 7.4 and filter sterilize.
- Add glucose and or other stimuli to the buffer to prepare solutions for perifusion experiment. For baseline buffer add 5.5 mM glucose. This buffer will be used for slicing procedures, slice collection, and perifusion preparations.
- Prepare at least two dishes for slice collection by adding aprotinin (10 µg/mL) to the baseline buffer.
- Prepare 3.8 % low melting point agarose solution in HEPES buffer without BSA and amino acids (125 mM NaCl, 5.9 mM KCl, 2.56 mM CaCl2, 1 mM MgCl2, 25 mM HEPES) and keep it at 37 °C.
- Assemble the vibratome, by placing a fresh blade in the holder. If applicable set the blade angle to 15° (if using Leica VT1200S). If applicable, calibrate the vibratome.
- Switch on the perifusion machine, select number of chambers and program protocol.
- Place all necessary solutions in the perifusion machine, prime the system and keep solutions heated. The machine does calculate the necessary volumes of each solution used for the protocol.
2. Tissue processing
NOTE: Slices should be generated immediately after receipt. Any delay might cause difficulties in the procedure and lead to tissue degradation.
- Place pancreas tissue in a dish with baseline buffer under a stereo microscope (Figure 1A). Gently remove connective, fibrotic, and adipose tissues using forceps and scissors.
- Cut tissue into multiple small pieces of approximately 0.5 cm3 (Figure 1B) using scissors or a scalpel. Blot dry pancreas pieces on tissue paper.
- Transfer 4 pieces into a 35 mm petri dish and fill dish with agarose solution until all pieces are fully submerged. Allow agarose to fully solidify.
- Carefully cut pieces out of the agarose. Run the scalpel along the edge of the dish to remove the agarose and carefully separate tissue blocks. Ensure pieces are surrounded by a thin layer of agarose.
3. Slicing
- Glue tissue pieces on the metal plate of the vibratome by placing them upside down.
- Mount the plate into the tray and fill the tray with baseline buffer (HEPES containing 5.5 mM baseline glucose).
- Set vibratome to automated slicing at 120 µm and adjust start and end position.
- Move the blade shortly above the tissue and start slicing at slow speed (0.1 mm/s) and amplitude at 0.8 mm. Speed can be increased if tissue permits (Figure 1C).
- Collect slices by using curved forceps or a small brush. Accumulate slices in baseline buffer containing aprotinin (Figure 1D).
- Allow slices to rest for at least 1 h in baseline buffer with aprotinin. Place slices on a slow orbital shaker to allow flushing out of enzymes released by the cutting procedure.
4. Live/dead assay
NOTE: This is an optional assay that will show viability of tissue slices after procedure. However, slices cannot be reused once stained.
- Transfer a single slice in a well filled with baseline buffer.
- Add Fluorescein diacetate (FDA, 50 µg/mL), incubate for 1 min at room temperature and protect from light.
- Add Propidium iodide (PI,50 µg/mL), incubate for 1 min at room temperature and protect from light.
- Transfer slice to another well filled with PBS to wash for 1 min.
- Transfer slices into a Petri dish or mount on a glass slide with cover slip for imaging (Figure 2A,B).
5. Perifusion
NOTE: This protocol describes how to perform dynamic perifusion for tissue slices, however it is suitable for isolated islets as well. Islet chambers will need preparation with filter paper and bead solution before loading the islets as described in the user manual of the machine. However, islet and slice chamber can be used together in the same experiment.
- Choose 3 slices and trim the agarose down to a minimum under a stereomicroscope using brush and scalpel.
- Add a drop of baseline buffer (HEPES containing 5.5 mM baseline glucose) on the grid of the slice chamber and gently place one slice on the grid. Repeat for each of the 3 slices (Figure 3A). If slices are very small stack more than 3 chambers to increase the islet count. Do not place more than one slice per chamber.
NOTE: Number of islets per slice varies greatly between donors and depends on slice size (10-100 islets/slice). A total of 3 slices have been proven sufficient to measure both insulin and glucagon secretion. - For isolated islets, use a minimum of 30 islets per column for insulin secretion. Recommended to use 100 islets for glucagon detection. Assemble individual slice chamber parts from top to bottom.
- Connect slice chamber to the inflow and outflow tubing's in the machine and start the protocol (Figure 3C).
- Use chamber heating and tray cooling pump during the protocol. Change collection plates during the protocol and store at 4 °C if quantification is performed the same day or store at -80 °C until then.
- When protocol is done, remove chambers, disassemble, and collect slices for lysis (3% HCl in absolute Ethanol) or fixation (in 4% Paraformaldehyde).
- Clean the machine by flushing it with water, 10% bleach, water and air.
- Quantify hormone secretion by using commercially available detection kits.
NOTE: Figure 4 shows absolute levels of insulin and glucagon secretion of 3 slices to estimate concentration ranges.
6. Calcium imaging
- Prepare Calcium dye (e.g., Fluo4-AM, Calbryte) solution according to manufacturer instruction.
- Dilute dye in baseline HEPES buffer solution and add aprotinin (10 µg/mL).
- Transfer a single slice and incubate for 30-60 min on an orbital shaker at room temperature and protect from light.
- During incubation time, prepare slice imaging setup by filling the perifusion syringes and priming of tubings (Figure 5A,B).
- Connect all devices, switch on heater, and start pump flow. Recommended to use both in-flow heating and platform heater and flow rate of 0.5 mL/min.
- Gently place a single slice in the imaging chamber and secure using a harp.
- Use a low magnification objective to identify imaging area. Using reflection helps to identify islet area (Figure 5C).
- Switch to a higher magnification objective (e.g., 20x or 40x) and adjust position according to experimental setup.
NOTE: To ensure optimal cell viability and islet integrity, it is recommended that the optical sections should be 1-2 cell layers below the cutting surface. - Set z-stack and or tile scan position. Set imaging interval and recording duration. Here, a resolution of at least 256 x 256 was used to allow discrimination of individual cells, an imaging interval of 5-10 s and a z-stack range (if applicable) of 30-60 µm with an interval of 10 µm between planes (one cell layer). See Figure 6 for detailed imaging settings.
NOTE: Set imaging parameter to get best resolution and maximum area but avoid bleaching the sample. - Start imaging and switch solution inflow according to experiment. Once done, collect slices and fix them for immunohistochemistry.
Representative Results
When the protocol is executed successfully, 1 g of pancreas tissue yields approximately 100-200 slices. Subsequently, these slices should undergo stereomicroscope inspection to identify those rich in islets before proceeding with functional assessments. Viability, determined by labeling with Fluorescein Diacetate (FDA) and Propidium Iodide (PI), is expected to reach 80%-90% (Figure 2A). Viability may be notably lower at the cutting surface due to cell damage during the slicing process. In viable slices, dynamic hormone secretion (Figure 2B) is observed, resulting in robust insulin release upon exposure to high glucose and membrane depolarization with Potassium chloride (KCl).
Conversely, when there are delays during the slicing procedure or the tissue quality is suboptimal, the outcomes differ significantly. The number of obtained slices may decrease, and the viability assessment may indicate a higher proportion of non-viable cells labeled with PI (Figure 2C). In these cases, the functional assessment indicates a diminished or even absent response to high glucose and membrane depolarization (Figure 2D). The data from suboptimal experiments illustrate the importance of precise execution in a timely manner and tissue quality, as they can lead to reduced tissue viability and impaired functionality. These negative outcomes serve as a valuable reminder of the critical factors to consider in the success of this method.
Common findings in calcium imaging are visualized as a heat map, as depicted in Figure 5D, or as separate traces for individual cells, as presented in Figure 5E. It is important to emphasize that the dye labels all cell types indiscriminately, hence specific stimuli are essential for distinguishing cells based on their responses. To identify viable cells, we employ KCl and sort the cells based on responses that exceed the average baseline response by more than two standard errors. Moreover, slices can be fixed and stained, enabling identification of cell types.
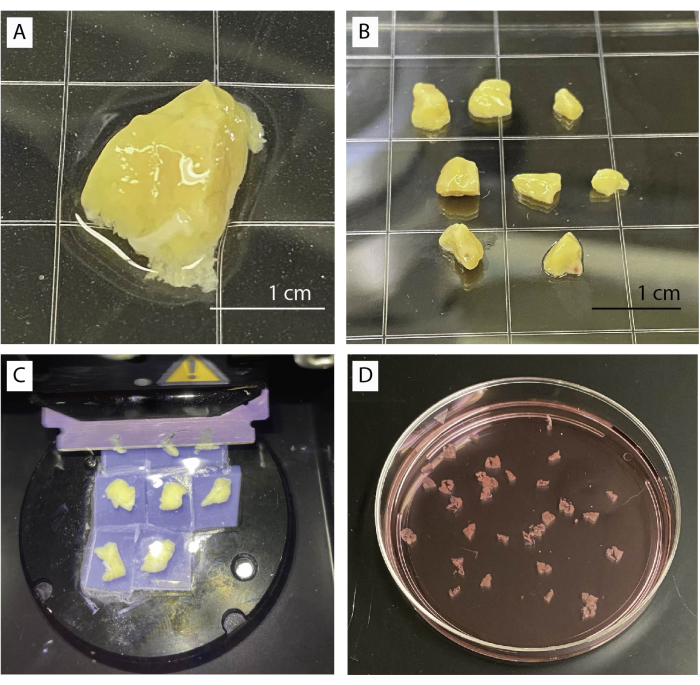
Figure 1: Tissue processing and slicing. (A) 1 g of unprocessed pancreas tissue. (B) Cleaned pancreas pieces ready for agarose embedding. (C) Slicing procedure using a vibratome. (D) Freshly cut human pancreas slices. Please click here to view a larger version of this figure.
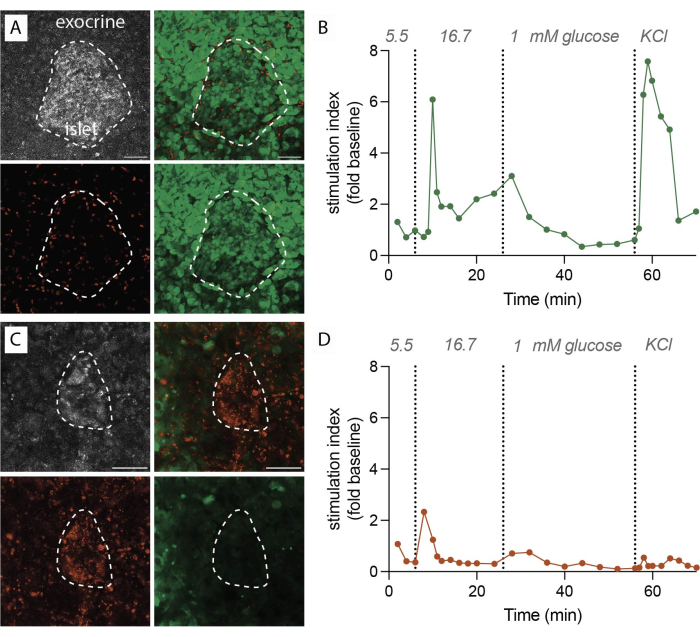
Figure 2: Tissue viability and insulin secretion data. (A) Representative images for viable tissue slices. Maximum intensity projection of reflected laser light (grey, top left), PI for dead cells (red, bottom left), FDA for living cells (green, bottom right), and merged images for live and dead cells (top right). Dotted line indicates an islet. Scale bar 50 µm. (B) Dynamic insulin secretion of human pancreatic tissue slices from a single nondiabetic donor. The insulin kinetics show a first phase peak response after 6 min of high glucose stimulation, followed by a plateau second phase. Data is normalized to the average baseline secretion at 5.5 mM glucose (stimulation index, fold change). Secretion has been performed on slices from the same donor as shown in (A). (C) Representative images for non-viable tissue slices. Maximum intensity projection of reflected laser light (grey, top left), PI for dead cells (red, bottom left), FDA for living cells (green, bottom right), and merged images for live and dead cells (top right). Dotted line indicates an islet. Scale bar 50 µm. (D) Dynamic insulin secretion of human pancreatic tissue slices from a single nondiabetic donor. The insulin kinetics demonstrate a clear loss of both glucose stimulated insulin secretion and membrane depolarization with KCl. Data is normalized to the average baseline secretion at 5.5 mM glucose (stimulation index, fold change). Secretion has been performed on slices from the same donor as shown in (C). Please click here to view a larger version of this figure.
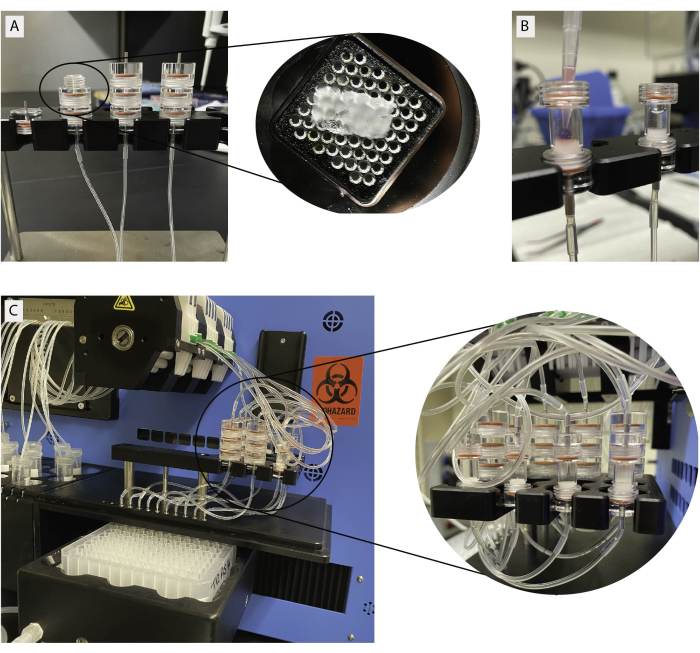
Figure 3: Tissue loading for dynamic perifusion. (A) Stacked slice chambers. Individual slices are loaded on a metal grid as shown in the insert. (B) Loading of isolated islets in islet chambers. Slice and islet chambers connected to perifusion machine. Please click here to view a larger version of this figure.
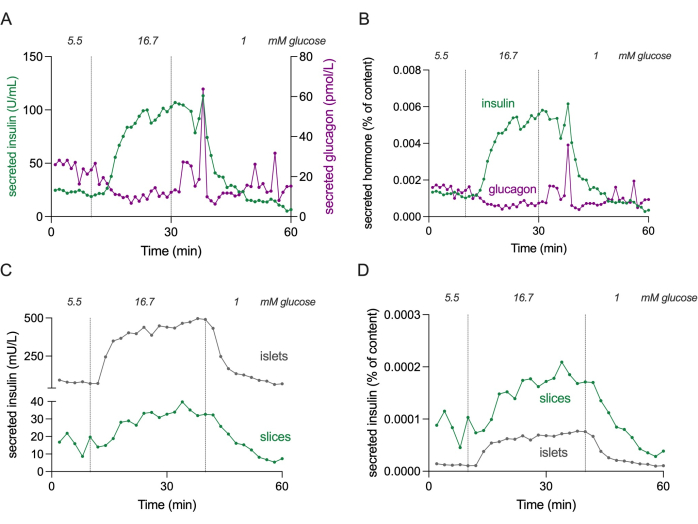
Figure 4: Hormone secretion in slices and isolated islets. Data shown in panels (A) and (B) originate from a different donor compared to panels (C) and (D). (A) Absolute hormone secretion from 3 pancreatic tissue slices from a single nondiabetic donor. Insulin secretion is shown in green and glucagon secretion in magenta. (B) Hormone secretion shown in (A) normalized to total hormone content (% of content). Content is measured from all 3 slices used in the experiment. (C) Dynamic insulin secretion of isolated islets (100 islets) and pancreatic tissue slices (3 slices) from the same nondiabetic donor. Data is shown in absolute numbers (mU/L). (D) Insulin secretion shown in (C) normalized to total insulin content (% of content). Please click here to view a larger version of this figure.
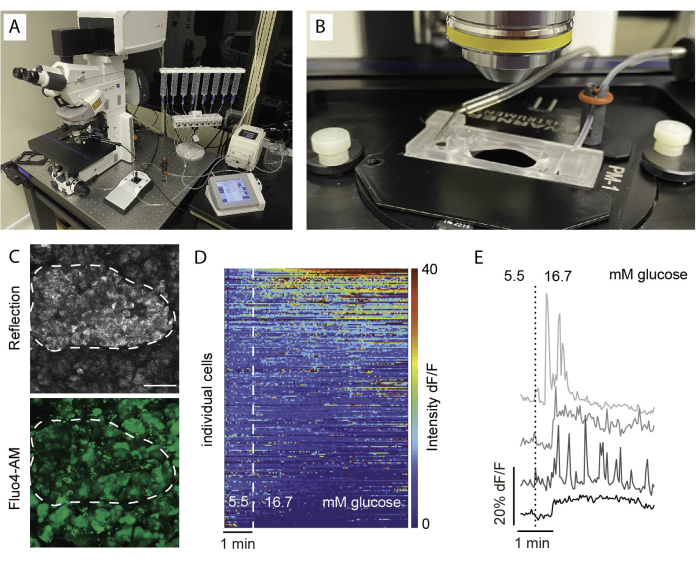
Figure 5: Imaging setup and expected results. (A) Setup for functional imaging with an upright confocal microscope and a perifusion setup. (B) Imaging chamber connected to inflow and outflow. (C) Z stack of confocal images of an islet within a tissue slice from a healthy human donor showing reflected light (top) and Fluo4 signal (bottom). Reflection is used to identify islets within the slice (dotted line). (D) Heatmap showing in vitro Ca2+ dynamics of islet cells expressed as the fluorescent intensity of Fluo4 normalized to the basal signal intensity at 5.5 mM glucose and stimulation with high glucose (16.7 mM). Each row represents a single cell followed over time in the x-axis and their response in magnitude change (%) of the fluorescent intensity over baseline (dF/F) shown in the color scale from blue (low intensity) to red (high intensity). (E) Representative traces of 4 individual cells showing a response to high glucose. Please click here to view a larger version of this figure.
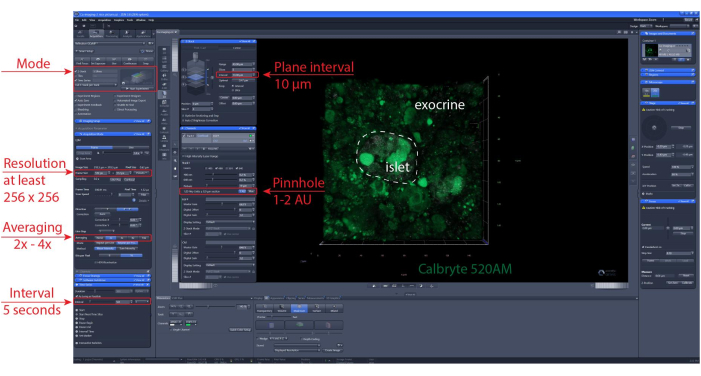
Figure 6: Calcium imaging settings. Screenshot of the software of representative settings chosen to perform time-lapse recordings of 40 µm (XYZT imaging). Please click here to view a larger version of this figure.
Discussion
Here we present a protocol to generate viable pancreas tissue slices and their use for functional readouts like dynamic hormone secretion and functional imaging. Similar to human islet isolation, the success of the slice procedure is influenced by various factors, including donor characteristics, tissue shipment time, and tissue quality25,29. Therefore, it is crucial to carefully select tissue samples for the experiment and keep ischemia times to a minimum. In this context, other potential sources of human tissue besides cadaveric donors should be carefully considered. Including surgical donors offers the option to combine functional slice data with relevant in vivo information from the same patient, strengthening the translational relevance11. However, this tissue source brings in other factors as biopsies are usually from older patients undergoing pancreatectomy mostly due to a localized tumor. Notably, pancreas biopsies are not performed in the context of diabetes.
When performing the actual slicing procedure, timely tissue processing is critical. Slices can be stored for several hours, as outlined in this protocol, or cultured for extended periods, as described by Qadir et al.19. At present, this protocol stands as the sole method for sustaining slice viability over prolonged durations however, future efforts should assess functional changes across diverse culture times and draw comparisons with isolated islets, from the same donor.
The most critical step in the process is careful tissue preparation before embedding in agarose. Large ducts and fibrotic tissue can complicate the slicing process and potentially lead to tissue blocks breaking out of the agarose. If this occurs and the pieces remain reasonably sized, they can be reprocessed and embedded for slicing. Maintaining good tissue quality and careful processing greatly enhances the slicing procedure’s efficiency and yields the maximum quantity and quality of slices. The time elapsed from tissue preparation to slice generation should not exceed 2-3 h, as longer intervals significantly impact tissue viability.
During slice perifusion, a simple yet critical step is the precise trimming of the slice to ensure a perfect fit into the chamber. This allows for the proper bathing of the tissue and uninterrupted flow. Once the protocol is initiated, it is essential to avoid further manipulation of the chambers to prevent unwanted spikes in hormone release. Sufficient buffer should be prepared, and tubing should reach the solution’s bottom to prevent air suction and chambers running dry.
For calcium imaging it is important to choose the area of interest carefully, depending on experimental needs and design. It is important to minimize photobleaching by choosing imaging parameters that reduce light exposure or shorten the overall protocol time. Like dynamic hormone secretion, maintaining proper solution flow and a heated setting is crucial, as slices require near-physiological conditions for optimal function (e.g., 37 °C).
Pancreatic tissue slices effectively maintain the structural integrity of the pancreas, preserving cell-cell connections between its diverse cell types. Consequently, they provide an alternative to working with isolated islets, facilitating the concurrent exploration of both endocrine and exocrine functions and their interplay. To assess the interplay of individual cell types, it is crucial to discriminate between them. Staining afterward is one option, but it has limitations in terms of available fluorescent probes and the challenge of locating exact cell layers. Therefore, it is advisable to incorporate cell-specific stimuli into the protocol to enable cell discrimination based on responses. For discriminating between cell types in mouse or human slices, effective stimuli include adrenaline for alpha cells21,30, ghrelin for delta cells31, cerulein for acinar cells5,32, bile acids for ductal cells33, and norepinephrine for vascular cells22. Similar stimuli can be employed for measuring secretory responses. While imaging studies focus on individual cells, secretion studies analyze the collective response. Hence, it is crucial to include an ample number of slices to detect the desired outcomes. The optimal quantity may vary between cell types, with three slices proving sufficient for endocrine cells; however, it is advisable to use more slices to ensure detectability rather than risk missing valuable information.
Like any other method, tissue slices have limitations that should be considered when interpreting results. Stimulus application targeting specific cell types may lead to effects on other cell types in the slice, potentially triggering feedback loops. However, those are also important to study and thus responses measured can be more representative of a physiological response. For selective cell targeting, traditional in vitro protocols may be used. Importantly acinar cells contain pancreatic enzymes that can break down proteins and digest the tissue slice, resulting in cellular degradation within a matter of hours. To maintain viability, the consistent use of trypsin inhibitors is essential when slices are in a static state, even though their application may interfere with the successful transfer of viruses employed for labeling purposes.
Compared to islet isolation, donor variability and tissue quality can impact both the quantity and viability of the obtained slices. Insufficient viability post-slicing may result in a short lifespan and hinder the ability to culture the slices. Furthermore, islet counts can vary significantly among donors, making it challenging to estimate islet content before conducting experiments. Consequently, careful selection of donor acceptance criteria and the implementation of appropriate normalization methods, such as the percentage of hormone content for secretion or fold change to baseline, are crucial. For consistent results, it is advised to assess tissue slice viability before the experiment. Moreover, incorporating relevant control stimuli (e.g., KCl) into the experiment is recommended. In cases of individual cell analysis, such as imaging, pre-sorting cells based on their response to these control stimulations can be implemented. Despite the challenges mentioned, slices offer a valuable augmentation to current research methods.
The protocol described can be used as starting point for several applications, and pancreas slices can be manipulated, and responses examined after a variety of stimuli. We also direct readers to numerous research studies utilizing mouse or human pancreas slices, providing valuable insights for those planning their experiments. In the future, it is possible that potential therapies may be investigated using pancreatic slices or that disease mechanisms may be modeled.
Declarações
The authors have nothing to disclose.
Acknowledgements
This research was performed with the support of the Network for Pancreatic Organ donors with Diabetes (nPOD; RRID:SCR_014641), a collaborative type 1 diabetes research project supported by JDRF (nPOD: 5-SRA-2018-557-Q-R) and The Leona M. & Harry B. Helmsley Charitable Trust (Grant#2018PG-T1D053, G-2108-04793). The content and views expressed are the responsibility of the authors and do not necessarily reflect the official view of nPOD. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org/for-partners/npod-partners/. The authors are grateful to the donors and their families for their invaluable contribution. This work was supported by the American Diabetes Association 4-22-PDFPM (J.K.P.) and the Leona M. and Harry B. Helmsley Charitable Trust (grant 2015-PG-T1D-052).
Materials
| Alanine | Sigma | A7627 | amino acid |
| AlexaFluor 488 goat anti-guineapig | Invitrogen | A11073 | secondary antibody |
| AlexaFluor 546 goat anti-rat | Thermo Fisher | A11077 | secondary antibody |
| AlexaFluor 647 goat anti-mouse | Thermo Fisher | A21235 | secondary antibody |
| Aprotinin | Sigma | A1153 | inhibitor |
| Arginine | Sigma | A5006 | amino acid |
| Calbryte 520 AM | AAT Bioquest | 20651 | Calcium dye |
| Fluo4-AM | Invitrogen | F14201 | Calcium dye |
| Fluorescein diacetat | Sigma | F7378 | viability marker |
| Glucagon | Sigma | G2654 | primary antibody |
| Glucagon ELISA (human kit) | Mercodia | 10-1271-01 | ELISA for hormone detection |
| Glutamine | Sigma | G3126 | amino acid |
| Insulin | Dako | A-0546 | primary antibody |
| Insulin ELISA (human) | Mercodia | 10-1113-01 | ELISA for hormone detection |
| Low melting point agarose | Sigma | A9414 | |
| LSM 900 | Zeiss | confocal microscope | |
| Pancreas Slice Chamber | Biorep Technologies | PERI-PSC-001 | slice chamber for perifucion |
| Pancreas Slice Chamber Extender Kit | Biorep Technologies | PERI-PSC-EXT | slice chamber for perifucion |
| Pancreas Slice Chamber Perforated Plate | Biorep Technologies | PERI-PSC-PP | slice chamber for perifucion |
| Perifusion system with automated tray handling | Biorep Technologies | PERI4-02-230-FA | |
| Propidium iodide | Life technologies | P1304MP | dead marker |
| Semi-automatic vibratome VT1200S | Leica | 14048142066 | |
| Somatostatin | Millipore | MAB354 | primary antibody |
Referências
- Abdelli, S., et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 53 (11), 2815-2823 (2004).
- Lyon, J., et al. Research-focused isolation of human islets from donors with and without diabetes at the alberta diabetes institute isletcore. Endocrinology. 157 (2), 560-569 (2016).
- Speier, S., Rupnik, M. A novel approach to in situ characterization of pancreatic beta-cells. Pflugers Arch. 446 (5), 553-558 (2003).
- Cohrs, C. M., et al. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology. 158 (5), 1373-1385 (2017).
- Marolt, U., et al. Calcium imaging in intact mouse acinar cells in acute pancreas tissue slices. PLoS One. 17 (6), 0268644 (2022).
- Stozer, A., et al. Confocal laser scanning microscopy of calcium dynamics in acute mouse pancreatic tissue slices. J Vis Exp. (170), e62293 (2021).
- Stozer, A., Dolensek, J., Rupnik, M. S. Glucose-stimulated calcium dynamics in islets of langerhans in acute mouse pancreas tissue slices. PLoS One. 8 (1), e54638 (2013).
- Weitz, J. R., et al. Mouse pancreatic islet macrophages use locally released atp to monitor beta cell activity. Diabetologia. 61 (1), 182-192 (2018).
- Speier, S., Yang, S. B., Sroka, K., Rose, T., Rupnik, M. Katp-channels in beta-cells in tissue slices are directly modulated by millimolar atp. Mol Cell Endocrinol. 230 (1-2), 51-58 (2005).
- Chen, C., Cohrs, C. M., Stertmann, J., Bozsak, R., Speier, S. Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab. 6 (9), 943-957 (2017).
- Cohrs, C. M., et al. Dysfunction of persisting beta cells is a key feature of early type 2 diabetes pathogenesis. Cell Rep. 31 (1), 107469 (2020).
- Marciniak, A., et al. Using pancreas tissue slices for in situ studies of islet of langerhans and acinar cell biology. Nat Protoc. 9 (12), 2809-2822 (2014).
- Panzer, J. K., et al. Pancreas tissue slices from organ donors enable in situ analysis of type 1 diabetes pathogenesis. JCI Insight. 5 (8), e134525 (2020).
- Campbell-Thompson, M., et al. Network for pancreatic organ donors with diabetes (npod): Developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 28 (7), 608-617 (2012).
- Kaddis, J. S., Pugliese, A., Atkinson, M. A. A run on the biobank: What have we learned about type 1 diabetes from the npod tissue repository. Curr Opin Endocrinol Diabetes Obes. 22 (4), 290-295 (2015).
- Pugliese, A., et al. New insight on human type 1 diabetes biology: Npod and npod-transplantation. Curr Diab Rep. 14 (10), 530 (2014).
- Pugliese, A., et al. The juvenile diabetes research foundation network for pancreatic organ donors with diabetes (npod) program: Goals, operational model and emerging findings. Pediatr Diabetes. 15 (1), 1-9 (2014).
- Liang, T., et al. Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology. J Biol Chem. 292 (14), 5957-5969 (2017).
- Qadir, M. M. F., et al. Long-term culture of human pancreatic slices as a model to study real-time islet regeneration. Nat Commun. 11 (1), 3265 (2020).
- Huber, M. K., et al. Observing islet function and islet-immune cell interactions in live pancreatic tissue slices. J Vis Exp. (170), e62207 (2021).
- Panzer, J. K., Tamayo, A., Caicedo, A. Restoring glutamate receptor signaling in pancreatic alpha cells rescues glucagon responses in type 1 diabetes. Cell Rep. 41 (11), 111792 (2022).
- Mateus Goncalves, L., Almaca, J. Functional characterization of the human islet microvasculature using living pancreas slices. Front Endocrinol (Lausanne). 11, 602519 (2020).
- Doke, M., et al. Dynamic scrna-seq of live human pancreatic slices reveals functional endocrine cell neogenesis through an intermediate ducto-acinar stage. Cell Metab. 35 (11), 1944-1960 (2023).
- Mateus Goncalves, L., et al. Pericyte dysfunction and impaired vasomotion are hallmarks of islets during the pathogenesis of type 1 diabetes. Cell Rep. 42 (8), 112913 (2023).
- Hanley, S. C., Paraskevas, S., Rosenberg, L. Donor and isolation variables predicting human islet isolation success. Transplantation. 85 (7), 950-955 (2008).
- Hart, N. J., Powers, A. C. Use of human islets to understand islet biology and diabetes: Progress, challenges and suggestions. Diabetologia. 62 (2), 212-222 (2019).
- Henquin, J. C. The challenge of correctly reporting hormones content and secretion in isolated human islets. Mol Metab. 30, 230-239 (2019).
- Panzer, J. K., Caicedo, A. Protocol to generate and utilize pancreatic tissue slices to study endocrine and exocrine physiology in situ from mouse and human tissue. STAR Protoc. 4 (3), 102399 (2023).
- Toso, C., et al. Factors affecting human islet of langerhans isolation yields. Transplant Proc. 34 (3), 826-827 (2002).
- Hamilton, A., et al. Adrenaline stimulates glucagon secretion by tpc2-dependent ca(2+) mobilization from acidic stores in pancreatic alpha-cells. Diabetes. 67 (6), 1128-1139 (2018).
- Adriaenssens, A. E., et al. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 59 (10), 2156-2165 (2016).
- Marciniak, A., Selck, C., Friedrich, B., Speier, S. Mouse pancreas tissue slice culture facilitates long-term studies of exocrine and endocrine cell physiology in situ. PLoS One. 8 (11), e78706 (2013).
- Gal, E., et al. A novel in situ approach to studying pancreatic ducts in mice. Front Physiol. 10, 938 (2019).

