Surgical Bone Implantation Technique for Rat Tibia Models of Diabetes and Osteoporosis
Summary
The placement of implants in a rat model is an essential experimental procedure for clinical research. This study presents a comprehensive surgical protocol for implanting titanium implants into the tibia of rat models with diabetes and osteoporosis.
Abstract
The rat has long served as a valuable animal model in implant dentistry and orthopedics, particularly in studying the interactions between biomaterials and bone tissue. The rat’s tibia is frequently chosen due to its easy surgical access through thin tissue layers (skin and muscle) and the flattened shape of its medial face, facilitating the surgical insertion of intraosseous devices. Additionally, this model enables the induction of specific diseases, mimicking various clinical conditions to assess biological responses to different implant conditions like geometry, surface texture, or biological cues. However, despite its robust cortical structure, certain intraosseous devices may require adaptations in design and size for successful implantation. Therefore, establishing standardized surgical methods for manipulating both soft and hard tissues in the implantation region is essential for ensuring proper implant or screw device placement, particularly in fields like implant dentistry and orthopedics. This study included eighty Sprague Dawley rats divided into two groups based on their respective diseases: Group 1 with osteoporosis and Group 2 with Type 2 Diabetes. Implantations were performed at 4 weeks and 12 weeks, with the same surgeon following a consistent surgical technique. A positive biological response was observed, indicating complete osseointegration of all implants placed. These results validate the success of the surgical protocol, which can be replicated for other studies and serve as a benchmark for the biomaterials community. Notably, osseointegration values remained stable at both 4 weeks and 12 weeks for both disease models, demonstrating a durable integration of the implant over time and emphasizing the establishment of an intimate bone connection as early as 4 weeks.
Introduction
The common choice of rats as experimental subjects is due to the fact that they are easy to breed and relatively inexpensive compared to larger animal models. The emergence of new procedures, such as the reliable reproduction of a disorder, e.g., osteoporosis or diabetes, makes this model especially useful for analyzing the potential use of treatments and/or the influence of the disease in the biological response to drugs and surgical devices or procedures1,2.
The rat's bone mass gain occurs mostly during the first 6 months of life, although some researchers believe that the long bone grows constantly for at least a year with a progressive increase in length1. With aging, there is a transition from modeling to remodeling, which does not occur in all cases equally throughout the bones2. Female Sprague Dawley rats grow more slowly than male rats and achieve a lower peak in weight than male rats1. Continuous bone elongation and varied bone remodeling dynamics in rats are factors that have to be taken into account when addressing human health issues; however, it has not yet been possible to find any experimental research that shows either lifelong rat bone development or the species' inability to remodel bone1. If the experimentation starts around 10 months of age, a margin of at least 1 mm from the growth plate of the tibia should be left intact due to this longitudinal bone growth, an issue to be considered in dental implant studies2. Hormones are also a key parameter in bone research since at 8 months of age, male rats were found to have 22% greater bone width and 33% greater breaking strength than females in the tibia3.
The reliable reproduction of a disorder is thus very important in orthopedics and implant dentistry since osseointegration of an orthopedic screw or a dental implant is a complex process that depends on numerous factors influencing the systemic response to the device implantation into the bone. Systemic disorders like osteoporosis and diabetes are known to affect the success rate in orthopedics and implant dentistry, so the reliable reproduction of those disorders in rat models can be applied to explore ways to overcome these limitations.
The rat tibia, due to the easy surgical access, moderate bone volume, and the flat shape on the medial plate, makes it suitable for surgical bone implantation experiments4,5, and it has been used in numerous research studies exploring the effects of implant surface on osseointegration4,5,6. A growing number of studies assess the effects on osseointegration of coatings and substances added to the implant surface in both healthy animals7 and in compromised animals affected by diabetes or osteoporosis8,9,10,11,12,13,14.
The number of implant devices placed in one rat's tibia is limited and can differ depending on the type of study. Depending on the number of implants or study conditions, the dimensions of the devices must be adapted to minimize surgical trauma. In studies with one implant, a nearly human-size implant can be placed (2.0 mm in diameter and 4 to 5 mm in length), and bi-cortical anchorage can be achieved6,7,15,16. The dimensions of the implants in multi-implant protocols should adopt an appropriate implant size (1.5 mm in diameter and 2.5 mm in length)4,17.
The present study aims to describe a standardized surgical protocol for titanium implant placement on the tibia of two rat models: the osteoporosis and the diabetes rat model. Moreover, this study permits testing the surgical protocol to assess different types of implant surface biofunctionalization and its effect on osseointegration.
A sample of 80 rats was divided into two groups. In group 1, 40 ovariectomized Sprague Dawley females and 5 sham animals were selected, with a mean weight of 484 g and a mean age of 12 weeks. Based on vendor recommendations (see Table of Materials), three months after neutering, the experiment started. This waiting period ensured the disappearance of sex hormones. Osteoporosis was confirmed at the time of surgery based on micro-computed tomography (micro-CT) bone analysis, which reflected an average of 20% bone loss compared to the sham group. Group 2 consisted of 40 BBDR (Bio Breeding Diabetes Resistant) genetically modified Sprague Dawley rats with type II diabetes. The mean weight was 730 g, and the average age was 12 weeks. Prior to surgery, the diabetic status was confirmed with three consecutive days of glucose measurements with results higher than 200 mg/dL. Glucose was measured with a glucometer in 6 h of fasting, and a blood drop was collected by tail puncture.
Grade 3 titanium implants measuring 2 mm in length and 1.8 mm in diameter were used. All implants were ultrasonically cleaned in cyclohexane (3 times for 2 min), acetone (once for 1 min), deionized water (3 times for 2 min), ethanol (3 times for 2 min), and acetone (3 times for 2 min) using an ultrasound bath (230 VAC, 50/60 Hz, 360 W). Then, the samples were dried with nitrogen gas, and a nitrogen beam at 0.5 bar was applied directly onto the samples. Prior to implantation, the implants were first soaked in deionized water and then sterilized by immersion in 70% ethanol (v/v) for 10 min. After this, the implants were transferred to sterile microcentrifuge tubes, and kept under sterile conditions until the surgery.
Protocol
All experimental procedures were conducted in accordance with the European Community Guidelines for the protection of animals used for scientific purposes (Directive 2010/63/EU) as implemented in Spanish law (Royal Decree 53/2013) and Generalitat de Catalunya regulations (Decree 214/97). Ethics approval for all animal procedures and handling was obtained from the Ethics Committee for Animal Experimentation of the Vall D'Hebron Institut de Recerca (registration number 72/18 CEEA). For the osteoporotic model, female Sprague Dawley rats with an average weight of 484 g and an average age of 12 weeks were utilized. As for the diabetic model, genetically modified female BBDR (Bio Breeding Diabetes Resistant) rats with an average weight of 730 g and an average age of 12 weeks were employed. All animals were sourced from a commercial supplier. The specific details of the animals, reagents, and equipment utilized in the study are listed in the Table of Materials.
1. Anaesthesia/pharmacology and preparation of animals
- Administer presurgical analgesia using buprenorphine at 0.05 mg/kg and meloxicam at 2 mg/kg through subcutaneous injections 10-15 min before starting the surgical procedure.
- Perform intra-surgical anesthesia with inhaled isoflurane: initiate at 5% in fresh air during induction and maintain at 3%. Induce anesthesia in a rat chamber and maintain the isoflurane supply with a conical nose adaptation during surgery.
2. Preparation for the surgery
- Measure the anesthetized animal's body temperature with a rectal probe and use an electronically controlled heating pad for thermal support throughout the surgical procedure. Eye ointment must be applied prior to surgery beginning to avoid corneal dryness.
NOTE: If necessary, the ointment must be reapplied after checking eye dryness. - Clean the skin of the knee thoroughly, ensuring sterility. Trim hair with an electric shaver and apply hair removal cream to eliminate any remaining fur.
- Obtain an aseptic surgical field by cleaning the knee skin in a pattern of iodine and 70% (v/v) ethanol using sterile swaps, starting from inside and moving outward the incision line without retracing. Perform a minimum of three consecutive cleaning rotations (iodine-ethanol-iodine).
- Isolate the operating Field by positioning a sterile surgical drape fenestrated over the animal, exposing the leg through the central opening (Figure 1).
3. Surgery
- Surgical exposure
- Make a full-thickness skin incision of approximately 1 cm in length vertically along the proximal border of the anteromedial face of the tibia, in the metaphysis region to expose the bone (Figure 2).
- Stabilize the leg and pull the skin taut against the underlying bone while making the incision, ensuring a clean incision stays in the correct location. Manage by cleaning the expected light bleeding with a compress soaked in saline solution (Figure 2).
NOTE: The rat skin is thin and sagging or loose. Stabilization of the skin is crucial and required. - Fully detach the area using small periosteal elevators (Figure 3).
- Expose the bone until identification of the insertion of the tibialis cranialis muscle, the gracilis, and the gastrocnemius lateral head muscle in the posterior border of the medial aspect of the tibia, as a fibrous white tissue firmly adhered to the bone (Figure 3).
NOTE: It is important to identify this group of muscular insertions to enable the implant to be placed in a bone region with similar characteristics and stimuli across the entire sample, regardless of the rat's size.
- Drilling process
- Begin the drilling process at the correct region between the proximal tibial crest and the posterior limit of the medial face of the tibia bone, contiguous to the insertion of the tibialis cranialis muscle, the gracilis, and the gastrocnemius lateral head muscle, avoiding any muscle injury.1
NOTE: The correct location should be 5 mm ± 2 mm from the tibial plateau. - Drill with a maximum of 150 rpm (rotations/min) under saline solution irrigation at a temperature close to 20 °C with a surgical electric motor with a 20:1 reduction contra-angle.
NOTE: Only two drills were required. - Start with a lance pilot drill (Figure 4) at a depth of 2.4 mm under saline solution irrigation.
NOTE: Each drill had a maximum of 10 uses. - As a second drill, use (Figure 4) a twist design drill at a depth of 2.4 mm with a diameter of 1.6 mm under saline solution.
NOTE: Each drill had a maximum of 10 uses.
- Begin the drilling process at the correct region between the proximal tibial crest and the posterior limit of the medial face of the tibia bone, contiguous to the insertion of the tibialis cranialis muscle, the gracilis, and the gastrocnemius lateral head muscle, avoiding any muscle injury.1
- Implant placement
- Insert the implant with an intermediary piece (Figure 5) attached to the 20:1 reduction contra-angle.
- Before placing the implant, clean the implant from any residual chemical sterilant by rotating it in the contra-angle with simultaneous saline irrigation for 10 s (Figure 5).
- Place the titanium implant (2 mm lengthwise and 1.8 mm in diameter) using the intermediate piece at 20 rpm while monitoring real-time torque value, registering the maximum insertion torque.
NOTE: An initial difficulty in inserting the implant is expected due to the difference between the final drill and the implant, as well as the cylindrical profile of the implant; however, this initial difficulty is quickly normalized as soon as the implant penetrates the initial cortical bone. - Finish implant insertion before it completely passes the cortical bone where it is inserted, that is, the flat medial face of the tibia.
NOTE: At the final moment of implant insertion, it is important to leave the implant slightly outside the cortical bone or level with the cortical bone where it is inserted, that is, the flat medial face of the tibia, to ensure primary stability (Figure 6).
- Wound closure
- Suture the muscle tissue borders with simple internal sutures using a 4/0 monofilament synthetic resorbable suture (Glyconate) (Figure 7).
- Perform skin closure with an intradermal suture using a 4/0 monofilament synthetic resorbable suture (Glyconate) (Figure 7).
4. Micro-CT scan
- After completing the surgery and still under general anesthesia, conduct a micro-CT scan to confirm proper implant placement.
- Remove the rat from the surgery bed and place it on the scanning bed. Locate the operated leg using the micro-CT live scan mode and center the Field of View on the implant.
NOTE: Recommended acquisition parameters are Field of view of 5 mm, spatial resolution of 0.0001 mm3, 50 kV, 200 µA, and acquisition time of 3 min. - Once the scan is acquired, confirm the correct distance between the proximal aspect of the implant and the surface of the tibial plateau, according to step 5.2.1.
NOTE: This value will be helpful for the standardization of the technique (Figure 8).
5. Postoperative care
- Following imaging acquisition, return the rat to its cage and monitor until full recovery.
NOTE: This takes approximately 5-10 min, depending on the animal model. Diabetic rats are expected to remain anesthetized longer and have a longer recovery time due to the metabolic changes associated with diabetes. - Administer postoperative analgesia 8 h after surgery using the same dose and technique as previously described. Repeat this dosing pattern of analgesia at 48 h.
- Suture removal time
- Examine the surgical wound daily for infection, suture integrity, or other issues, and remove sutures 15 days post-surgery.
6. Euthanasia
- Euthanize the animals using a CO2 chamber, according to the National Institutes of Health (NIH) guidelines, with a CO2 fill rate of 30%-70% of the chamber volume/min after the implantation time (4 weeks or 12 weeks).
7. Postsurgical analysis
- For both models (osteoporosis and diabetes), remove the tibia by disarticulation after euthanasia for further analysis.
NOTE: Regarding the micro-CT analysis, data allowed for calculating bone-implant contact (BIC). The CT acquisition was conducted using the previously described parameters, and the BIC analysis was performed by dividing the bone area (mm2) by the implant area (mm2) calculated at the cortical region, adapting a protocol already described in the literature18. Representative images are shown for each model, osteoporosis (Figure 9) and diabetes (Figure 10).
Representative Results
Surgical phase
It is important to mention that both animal models used in this study present certain constraints due to the induced diseases. These constraints regarding the manipulation of hard and soft tissues are reflected during the surgical procedure.
In the diabetic model, the rat is larger, making it difficult to stabilize the legs during surgical procedures. This increases the surgical time and, consequently, the anesthesia time, which requires a longer recovery time and, therefore, requires greater vigilance in the postoperative period.
As for the osteoporotic model, the requirements were very different, being more concentrated at the time of bone manipulation. In this case, it is possible to observe greater bleeding during the drilling procedure. In addition, the implant placement process becomes more difficult. Some complications may occur, such as small cracks in the bone, altering the progression of the implant during its entry and making it more unstable due to the lack of bone resistance on the lateral walls of the implant. This limitation requires more manual pressure and stabilization during placement, making it more difficult to control until the correct positioning of the implant is achieved.
Regarding the maximum implant insertion torque, relatively different values were observed for the two diseases (Table 1). In general, the maximum average insertion torque observed for the osteoporotic model was higher than for the diabetic model. With these results, it is interesting to understand the effect that diabetes has on bone density and bone resistance to implant insertion, as this condition appears to have a more severe effect on the mechanical properties of bone compared to osteoporosis.
Postsurgical analysis
The adequate placement of the titanium implants after 4 weeks and 12 weeks of implantation was analyzed by micro-CT imaging. Representative images are provided for both osteoporotic (Figure 9) and diabetic (Figure 10) rats. Overall, the implants were correctly implanted through the cortical and trabecular tibiae, showing intimate and continuous contact with the cortical part, and without any signs of inflammation or adverse reactions, thus proving the success of the surgical protocol for both models of pathology.
Collectively, the analysis of the images also allowed for calculating the ratio of bone area (mm2) versus implant area (mm2), referred to as V(BIC), at the cortical region, and comparing the performance of the surgical procedure over time and within models. As shown in Figure 11, V(BIC) values remained constant for the osteoporotic model at 4 weeks and 12 weeks, indicating a stable integration of the implant over time and highlighting that an intimate connection with bone is ensured already at 4 weeks. Of note, very similar values were obtained for the diabetic model, thus showing the adequacy of the study protocol for both models of pathology.

Figure 1: Surgical field. The surgical field showing the correct position of the rat and exposure of the area to be intervened, adaptation of the conical adaptor to the airways for maintenance of the anesthesia during the procedure, as well as the instruments necessary for the intervention. Please click here to view a larger version of this figure.

Figure 2: Surgical incision. Correct stabilization of the rat's paw and, at the same time, fixing the thin mobile skin to obtain a correct incision. Please click here to view a larger version of this figure.
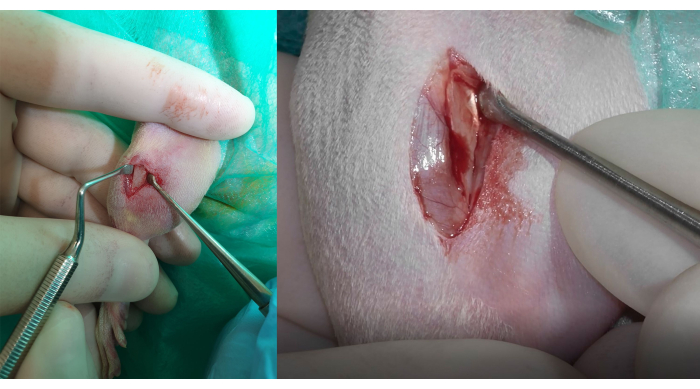
Figure 3: Bone exposure and muscle insertion Identification. Left side: total detachment of the tissues with the consequent bone exposure. To preserve and avoid damage to the insertion of the tibialis cranialis muscle, the gracilis, and the gastrocnemius lateral head muscles, it is important to correctly identify the fibrous white tissue firmly adhered to the bone, visible in the figure on the right side. Please click here to view a larger version of this figure.

Figure 4: Drilling sequence. The drilling process sequence begins with the lance pilot drill on the left, followed by the twist drill on the right. To ensure a stable and safe drilling procedure, it is important to maintain both leg and soft tissue stabilization during drilling. Please click here to view a larger version of this figure.

Figure 5: Preparation for implant insertion. The implant is inserted on an intermediate piece to be submitted to the cleaning process with a saline solution while the implant rotates at 20 rpm. This intermediate piece allows the insertion of the implant into the bone with an electronic device. This device ensures control of the implant insertion speed and records the real-time insertion torque. Please click here to view a larger version of this figure.

Figure 6: Titanium implant placement. Placing the implant within the bone requires its placement on the flat face of the medial bone, away from the proximal border of the anterior-medial face of the bone. Please click here to view a larger version of this figure.
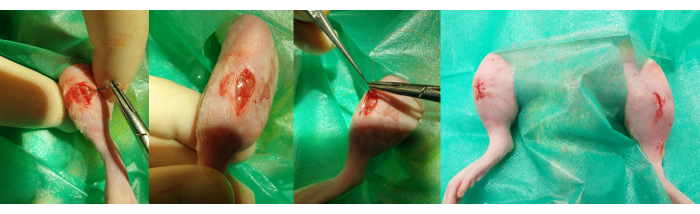
Figure 7: Wound closure. The steps for closing the wound are observed in 2 different planes. Left: the first, deeper plane is made with simple stitches to approximate the edges of the muscle tissue. Right: the second, more superficial plane is carried out with an intradermal suture to approximate the skin while preventing the knot from being in an externalized position. Please click here to view a larger version of this figure.
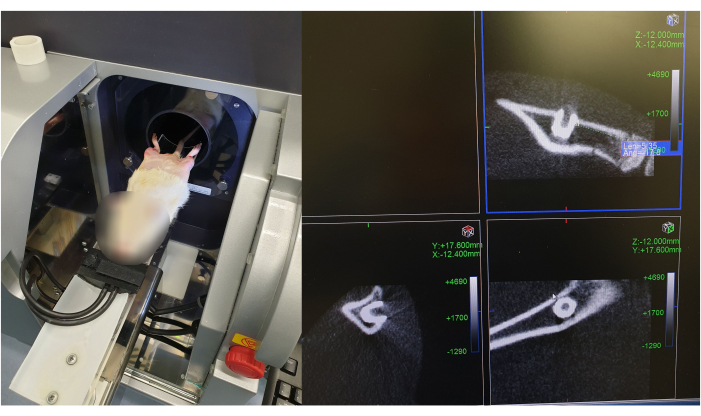
Figure 8: Micro-CT scan of implant placement after surgical procedure. The figure illustrates the positioning of the rat in the Micro-CT scanner, and the images of the implant position are divided into three sections, corresponding to the x-, y-, and z-views, respectively. The implant is pointed with a red arrow in the different views. Please click here to view a larger version of this figure.

Figure 9: Micro-CT scan of an implant placed in the tibia of an osteoporotic rat. Representative postsurgical micro-CT images of titanium implants placed in the tibiae of osteoporotic rats after (A) 4 weeks and (B) 12 weeks of implantation. Each image is divided into three sections, corresponding to the x-, y- and z-views, respectively. Please click here to view a larger version of this figure.
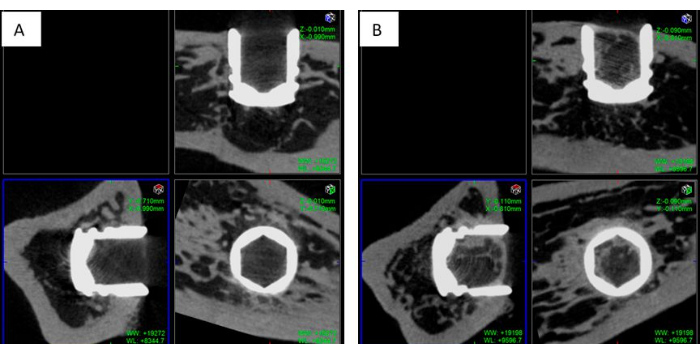
Figure 10: Micro-CT Scan of an implant placed in the tibia of a diabetic rat. Representative postsurgical micro-CT images of titanium implants placed in the tibiae of osteoporotic rats after (A) 4 weeks and (B) 12 weeks of implantation. Each image is divided into three sections, corresponding to the x-, y- and z-views, respectively. Please click here to view a larger version of this figure.

Figure 11: Bone-implant contact (BIC) analysis. V(BIC) values calculated as bone area (mm2) *100/ implant area (mm2) from micro-CT images for the osteoporotic (Osteo) and diabetic (Diab) models after 4 weeks (4 w) and 12 weeks (12 w) post-implantation. Please click here to view a larger version of this figure.
| Maximum implant insertion torque [N·cm] | ||
| Model | Right leg | Left leg |
| Osteoporosis | 6.11 ± 0.96 | 6.49 ± 1.34 |
| Diabetes | 4.79 ± 1.70 | 4.90 ± 1.76 |
Table 1: Maximum insertion torque. Differences in maximum torque values obtained during implant insertion between diabetic rats and osteoporotic rats. Diabetic rats present lower maximum torque values than osteoporotic rats.
Discussion
Although the rat is a widely used model for studying osseointegration, it is important to define and describe a reproducible surgical technique for adequately placing implants. Such a technique could serve as a guide for the scientific community. Moreover, the fact that certain diseases, such as osteoporosis and diabetes, alter bone metabolism implies stronger demands for correctly designing surgical procedures. The rat compares favorably with other animal models since it presents the main features of both osteoporosis (spontaneous and low-impact fractures) and diabetes and diet-induced obesity without requiring costly specialized facilities and long, expensive experiments, and postoperative control and maintenance19. The rat's small size, high fecundity, and short life facilitate large-scale, time-efficient experiments while meeting the three R requirements of animal experiments (replacement, reduction, and refinement)20.
Despite the considerable amount of scientific literature in the field, there are only a few scientific articles describing detailed surgical techniques. Yet, the vast majority omits crucial surgical information, thus preventing the possibility of replicating the techniques described.
In this surgical protocol, several critical steps need to be considered throughout the surgical procedure, such as the location of the incision, the precise location for drilling and implant placement, the drilling technique, the exact dimensions of the implant, and the final drill used for implant insertion.
In the literature, the exact location of the incision is often imprecise, lacking a scientific basis for its placement. The incision should be made in a different region from that of the implant placement. It is important to avoid placing the incision and consequent suture directly above the implanted area to maintain the integrity of the periosteum detached above the implant. An incision and initial detachment of the full-thickness flap can disrupt the blood supply and damage the periosteum, potentially leading to a disturbance in periosteal cell activity during new bone formation, as observed in other studies21. This approach aims to optimize peri-implant bone formation and minimize trauma, poor healing, fibrosis, or the risk of bacterial colonization in the suture area. In this method, the incision is detailed and made in the proximal tibial crest, ensuring it is not directly above the implant placement site.
When determining the region for implant placement, it's crucial to ensure uniform bone density across the sample. Typically, measurements are referenced to the tibial plateau. Given the longitudinal size variation among rats, an anatomical reference is necessary to consistently place implants in the same region. In this method, the area adjacent to the insertion of the tibialis cranialis, gracilis, and gastrocnemius lateral head muscles serves as a reliable anatomical reference. This region, characterized by muscular insertions, is expected to exhibit homogeneous bone density throughout the sample.
When discussing the drilling method, it's crucial to provide accurate details about the type/dimensions of the drill(s), the drilling speed (usually measured in rpm), and the temperature of the saline solution. These factors are essential in preventing an extensive necrotic area and subsequent formation of fibrous tissue22,23, as highlighted in several studies focused on drilling-induced necrotic reactions. However, some articles exceed these biological limits24 in their drilling methods, while others omit this crucial information entirely4,25,26,27,28. This study emphasizes the meticulous precautions taken during drilling procedures to reduce surgical trauma and tissue necrosis. We opted for a safety drilling procedure with a speed capped at 150 rpm, a level considered safe in terms of bone temperature even without saline solution. This approach is effective in terms of drilling speed and allows for good drill control during the procedure29,30. Additionally, we maintained irrigation and cooling using a saline solution at approximately 20 °C to further mitigate any potential thermal effects.
Another relevant piece of information that is frequently omitted is the size of the implant used. This omission makes it impossible to interpret the relationship between the size of the final drill and the size of the implant. This discrepancy is crucial in assessing the stability of the implant, which in a deficit may lead to instability, while in excess, it may cause excess pressure and an ischemic-necrotic reaction.
It's crucial to note that for primary stability to be achieved, there should be a slight difference in diameter between the final drill and the implant, with the implant being slightly larger in diameter. This critical surgical parameter for attaining primary stability is not always consistent in the literature, as some methodologies feature a final drill with a larger diameter than the implant placed4. Given that this region is characterized by muscular insertions, a homogeneous and robust bone density is expected throughout the sample. This characteristic helps avoid the primary limitation reported for rat tibial interventions, namely the longitudinal growth of the tibia over time31. Additionally, this method allows for temperature control, addressing one of the causes of complications and subsequent morbidity23. However, similar to other studies, this study also faces a limitation concerning the need to adapt the device due to variations in tibia size4,17.
Another concern related to the number of animals used is the importance of selecting an appropriate sample size. This is crucial for maximizing the impact of research outcomes while minimizing animal usage. Such a strategy not only improves the efficiency and reliability of studies but also emphasizes the ethical considerations in scientific research. Continued efforts should be made to optimize this efficiency.
In summary, the present method provides a detailed description of all surgical procedures and allows for the observation of various surgical challenges in both diseases. Furthermore, this protocol stands out as a valid method because all samples with direct contact with the bone achieved stable V(BIC) values. However, it differs from the majority of experimental studies in the literature by presenting a detailed and justified technique, thereby allowing for exact reproduction. Consequently, it can be used to study the effect of different implant surfaces in various diseases, including those affecting bone density.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank the Spanish State Research Agency for financial support through projects PID2020-114019RBI00 and PID2021-125150OB-I00.
Materials
| 22 G needles+A2:C30 | Terumo | NN-2238R | |
| 4/0 monofilament synthetic resorbable suture | Braun ( MonoSyn) | ||
| 5 mL, 10 mL syringes | Braun | 4617100V-02 4606051V | |
| Adson forceps | Antão Medical | Ref: A586 | |
| BBDR ( Biobreeding Diabetes Resistant ) Sprague Dawley Rats | Janvier Labs | ||
| Betadine | Mylan | ||
| Buprecare | Animalcare (UK) | ||
| Castroviejo Caliper 0-40 mm 15 cm angled | UL AMIN Industries | ||
| Castroviejo Needle Holder | Antão Medical | Ref: AM1702 | |
| Dental surgery scissors curved and straight | Antão Medical | AMA603 / AMA600 | |
| Electric shaver | Oster Pro 3000i | 34264482227 | |
| Extra Fine Graefe Forceps | F.S.T | Ref: 11150-10 | |
| Gauze pads | COVIDIEN | 441001 | |
| Glucometer | Menarini (Italy) | ||
| Helicoidal Drill / OSTEO-PIN DRILL Ø1.6 mm | soadco | Ref. OS-8001 | |
| Implants / SCREW OSTEO-PIN Ø1.8 x 2.0 mm | soadco | Ref. OS-3 | |
| Isoflo | Le Vet Pharma (Netherlands) | ||
| Lance pilot drill / Lanceolate Drill (DS) | soadco | Ref. 10 02 01 T | |
| Latex gloves – Surgical gloves sterile | Hartmann | Ref: 9426495 | |
| Lucas Surgical Curette | Antão Medical | Ref: AMA940-3 | |
| Metacam | Boehringer Ingelheim(Germany) | ||
| Micro forceps straight | nopa | Ref: AB 542/12 | |
| Micro-CT scan( Quantum Fx microCT ) | Perkin Elmer (US) | ||
| Osteoporotic Sprague Dawley females Rats | Janvier Labs | ||
| Periosteal elevator - Molt 2-4 | Antão Medical | Ref: A1564 | |
| Physiologic solution for Irrigation | Hygitech | Ref:10238 | |
| Scalpel Blade Carbon Steel 15C | Razor Med | Ref: 02846 | |
| Sterile Gauze Swabs | Alledental | Ref: 270712 | |
| Sterile Irrigation system | Hygitech | Ref:HY1-110001D | |
| Sterile towels (1 piece per animal) | Dinarex | 4410 | |
| Surgical contra-angle handpiece | W&H | Ref: WS-75 LED G | |
| Surgical contra-angle handpiece | W&H | SN 08877 | |
| Surgical contra-angle handpiece | W&H | SN 01309 | |
| Surgical Electric Motor | WH Implantmed Type: SI-1023 | Ref: 30288000 | |
| Surgical scalpel handle | AsaDental | Ref: 0350-3 | |
| Towel clamps | Xelpov surgical | AF-773-11 | |
| Ultrasonic device | J.P. Selecta, Abrera, Spain |
References
- Turner, R. T., et al. Animal models for osteoporosis. Rev Endocr Metab Disord. 2 (1), 117-127 (2001).
- Jee, W. S., Yao, W. Overview: Animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact. 1 (3), 193-207 (2001).
- Kim, B. T., et al. The structural and hormonal basis of sex differences in peak appendicular bone strength in rats. J Bone Miner Res. 18 (1), 150-155 (2003).
- Alenezi, A., Galli, S., Atefyekta, S., Andersson, M., Wennerberg, A. Osseointegration effects of local release of strontium ranelate from implant surfaces in rats. J Mater Sci Mater Med. 30 (10), 116 (2019).
- Blanc-Sylvestre, N., Bouchard, P., Chaussain, C., Bardet, C. Pre-clinical models in implant dentistry: Past, present, future. Biomedicines. 9 (11), 1538 (2021).
- Schliephake, H., et al. Functionalization of titanium implants using a modular system for binding and release of VEGF enhances bone-implant contact in a rodent model. J Clin Periodontol. 42 (3), 302-310 (2015).
- Mafra, C. E. S., et al. Effect of different doses of synthetic parathyroid hormone (1-34) on bone around implants: A preclinical rat model. Braz Dent J. 30 (1), 43-46 (2019).
- Rybaczek, T., Tangl, S., Dobsak, T., Gruber, R., Kuchler, U. The effect of parathyroid hormone on osseointegration in insulin-treated diabetic rats. Implant Dent. 24 (4), 392-396 (2015).
- Zou, G. K., et al. Effects of local delivery of bfgf from plga microspheres on osseointegration around implants in diabetic rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 114 (3), 284-289 (2012).
- Zhang, J., et al. Effect of nerve growth factor on osseointegration of titanium implants in type 2 diabetic rats. Int J Oral Maxillofac Implants. 31 (5), 1189-1194 (2016).
- Kuchler, U., et al. Intermittent parathyroid hormone fails to stimulate osseointegration in diabetic rats. Clin Oral Implants Res. 22 (5), 518-523 (2011).
- Hashiguchi, C., Kawamoto, S., Kasai, T., Nishi, Y., Nagaoka, E. Influence of an antidiabetic drug on biomechanical and histological parameters around implants in type 2 diabetic rats. Implant Dent. 23 (3), 264-269 (2014).
- Han, Y., et al. Sustained topical delivery of insulin from fibrin gel loaded with poly(lactic-co-glycolic acid) microspheres improves the biomechanical retention of titanium implants in type 1 diabetic rats. J Oral Maxillofac Surg. 70 (10), 2299-2308 (2012).
- De Molon, R. S., et al. Impact of diabetes mellitus and metabolic control on bone healing around osseointegrated implants: Removal torque and histomorphometric analysis in rats. Clin Oral Implants Res. 24 (7), 831-837 (2013).
- Simon, M. M., et al. A comparative phenotypic and genomic analysis of c57bl/6j and c57bl/6n mouse strains. Genome Biol. 14 (7), 82 (2013).
- Cirano, F. R., et al. Effect of curcumin on bone tissue in the diabetic rat: Repair of peri-implant and critical-sized defects. Int J Oral Maxillofac Surg. 47 (11), 1495-1503 (2018).
- De Oliveira, M. A., et al. The effects of zoledronic acid and dexamethasone on osseointegration of endosseous implants: Histological and histomorphometrical evaluation in rats. Clin Oral Implants Res. 26 (4), 17-21 (2015).
- Vandeweghe, S., Coelho, P. G., Vanhove, C., Wennerberg, A., Jimbo, R. Utilizing micro-computed tomography to evaluate bone structure surrounding dental implants: A comparison with histomorphometry. J Biomed Mater Res B Appl Biomater. 101 (7), 1259-1266 (2013).
- Kleinert, M., et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. 14 (3), 140-162 (2018).
- Grimm, H., et al. Advancing the 3Rs: Innovation, implementation, ethics and society. Front Vet Sci. 10, 1185706 (2023).
- Melcher, A. H. Role of the periosteum in repair of wounds of the parietal bone of the rat. Arch Oral Biol. 14 (9), 1101-1109 (1969).
- Barrak, I., et al. Heat generation during guided and freehand implant site preparation at drilling speeds of 1500 and 2000 rpm at different irrigation temperatures: An in vitro study. Oral Health Prev Dent. 17 (4), 309-316 (2019).
- Kniha, K., et al. Effect of thermal osteonecrosis around implants in the rat tibia: Numerical and histomorphometric results in context of implant removal. Sci Rep. 12 (1), 22227 (2022).
- Da Silva, J. P., et al. Apoptosis in bone defect of diabetic rats treated with low intensity laser: Radiological and immunohistochemical approach. International Journal of Morphology. 35, 178-183 (2017).
- Zeller-Plumhoff, B., et al. Analysis of the bone ultrastructure around biodegradable Mg-χGd implants using small angle X-ray scattering and X-ray diffraction. Acta Biomater. 101, 637-645 (2020).
- Bruns, S., et al. On the material dependency of peri-implant morphology and stability in healing bone. Bioact Mater. 28, 155-166 (2023).
- De Morais, J. A., et al. Effect of diabetes mellitus and insulin therapy on bone density around osseointegrated dental implants: A digital subtraction radiography study in rats. Clin Oral Implants Res. 20 (8), 796-801 (2009).
- Aydemir Celep, N., Kara, H., Erbas, E., Dogan, E. Radioprotective role of amifostine on osteointegration of titanium implants in the tibia of rats. J Vet Sci. 24 (3), 35 (2023).
- Delgado-Ruiz, R. A., et al. Slow drilling speeds for single-drill implant bed preparation. Experimental in vitro study. Clin Oral Investig. 22 (1), 349-359 (2018).
- Abdel Motagly, M., El Khadem, A., Abdel Rassoul, M. Assessment of low-speed drilling without irrigation, versus convencionaldrilling with irrigation regarding heat generation and peri-implant marginal bone loss (randomised clinical trial). Alex Dent J. 46 (2), 33-38 (2021).
- Lelovas, P. P., Xanthos, T. T., Thoma, S. E., Lyritis, G. P., Dontas, I. A. The laboratory rat as an animal model for osteoporosis research. Comp Med. 58 (5), 424-430 (2008).

.
