Using Simulation Models to Train Clinicians in the Use of Point-of-care Ultrasound
Summary
The present protocol describes an ideal solution to train novices in the use of point-of-care ultrasound devices for the practical clinical skill of visually assessing distinct individual anatomical vascular conditions prior to and during an intended venous vascular cannulation using point-of-care ultrasound in a patient.
Abstract
The use of point-of-care ultrasound (POCUS) has shown to be a beneficial non-invasive vascular access assessment method by clinicians, which can provide critical elements of visual and measurable information that proves to be useful in the context of vascular access cannulation, in combination with the practical skill of the clinician performing the cannulation. However, the use of POCUS in this context is to practically train and enable individuals who are novices in using this technique to become proficient in performing this task subsequently on patients in a careful and successful way. The simulation of these vascular conditions may be useful to help healthcare professionals learn, understand, apply, and establish such practical skills for vascular cannulation safely to achieve the desired outcomes. This project intended, through the attendance of a half-day workshop, to establish skills to use POCUS in connection with simulation models and perform specific tasks to enable clinicians to use this method in their clinical practice for vascular access cannulation in patients. A mixed-methods longitudinal study design was used to evaluate the effect of a point-of-care ultrasound workshop for peripheral intravenous cannula insertion, including specific tasks for the participants to be performed on simulation models. A total of 81 individuals participated in 11 half-day workshops through 2021 and 2022. Offering a workshop that uses simulation models in combination with various POCUS devices is useful in establishing this newly learned skill in clinicians, such as measurements of depth, caliper, and direction of a vein with POCUS prior to cannulation providing essential anatomical facts to the operator, which increases the likelihood of first-time success in cannulation.
Introduction
Most patients being admitted to acute hospitals receive at least one peripheral intravenous catheter (PIVC), with the purpose of withdrawing blood, administration of fluids and/or medication, and for diagnostic purposes1. It is common that first-attempt insertions fail, and it has been reported that up to 50% of hospitalized patients have difficult intravenous access (DIVA)2. To alleviate this, the use of ultrasound-guided PIVC insertion (USGPIVC) has been demonstrated to improve insertion success rates, and training and practical education have been recommended for multiple healthcare professions3,4,5. Point-of-care ultrasound (POCUS) at the bedside is nowadays more frequently used to gain vascular access. POCUS has also been described as a useful tool for augmenting the teaching and learning of physical examination6. While several studies have described that training in USGPIVC is likely to enhance the skills of clinicians7,8,9,10, it has not yet been described in detail which specific elements of this training are the essential components to achieve the desired outcomes when applying POCUS for USGPIVC. To achieve this, a combined POCUS and USGPIVC training curriculum was developed covering the essential aspects of the training, which were considered as being the elemental aspects and learning objectives for USGPIVC workshops, including the theoretical background and practical hands-on aspects.
Training novices in the use of POCUS prior to and during vascular access cannulation requires an ideal simulation environment to enable effective learning success which replicates similar anatomical conditions as in a human anatomical environment11. Therefore, simulation models created from chicken breast and fluid-filled modeling balloons were found to be ideal and can be used to generate such a simulation model12. This approach teaches the learner the observational skill of assessing vascular conditions at an individual patient level first in a safe, simulated environment, which helps in the overall decision-making process of choosing the required cannula length, assessment of the vascular depth and width, and vessel direction for an individual patient. This allows for a critical assessment of any future patient's individual anatomical conditions, where a clinician may want to subsequently decide if the planned cannulation is likely to be successful or not. To obtain this information, POCUS-obtained images, when they are interpreted correctly, usually provide reliable and critical elements of information, which, in addition to the clinicians' experience and manual dexterity, are likely to lead to cannulation success.
In the second step, the learner is taught, in this simulative environment, the development of manual dexterity for using the ultrasound probe simultaneously with the manual skill of inserting a cannula, under vision, observing the POCUS screen and the insertion site, into the simulated blood vessel. This observational skill of constantly visualizing the simulated vessel and meticulously observing the needle tip during the insertion process is the most important aspect of the overall learning goal of this simulation activity until the needle tip is ultimately placed in the anatomical area of interest, in this case in the center of a simulated vessel. This process is crucial to avoid unintended and unnecessary vessel injury, tissue, bleeding, or extravasation, as this technique is intended to be subsequently used in a patient in clinical settings by the participant.
Some authors have previously recommended implementing and integrating ultrasound into the medical school curriculum, using low-cost simulation models and small teaching groups13. Others have recommended developing structured training programs followed by a hands-on session in a simulated environment14. It has also been described that the use of ultrasound helps with procedural success and may reduce risks for patients15. Others have observed that using POCUS and USGPIVC to train clinicians in the emergency department (ED) has increased the use of this approach in the short term. Still, there may also exist a lack of consistency in formalized education programs for vascular access7,16,17. In contrast, others have described that formalized vascular access training leads to improved adherence to best practices for PIVC insertion11.
The aim of this educational approach was to simulate a comparable visual and dexterity experience for learners so that they could replicate and apply this skill in a clinical setting and on patients in the future. An observational longitudinal mixed methods study approach was chosen, and electronic surveys were used to assess the confidence level of workshop participants using ultrasound (POCUS) in connection with peripheral venous cannulation. The surveys were first used in simulation models and subsequently used in the clinical specialty of workshop participants in admitted patients.
The workshop was divided into three parts. First, participants were introduced to some basic principles and theories of using ultrasound in the space of vascular access cannulation in an interactive learning environment. In a second approach, the workshop facilitator demonstrated the vascular access assessment approach using a simulator with a simulated artificial vessel created, demonstrating the observation of vessel depth, size, and direction through transverse and longitudinal view and observation using POCUS. This was followed by a demonstrated cannulation using POCUS and the simulator through the workshop facilitator, as participants were then invited to practice this task themselves on their individual simulators. At the workshop conclusion, participants were individually assessed on their skill of identifying and measuring vessel size, depth, and direction using transverse and longitudinal views in the simulator, followed by ultrasound-guided cannulation of the simulated vessel. After the workshop attendance, participants were invited to rate their confidence skills in using USPIVC in an electronic survey. At 8 weeks after the workshop attendance, participants were again invited to respond in an electronic survey if they had applied this adopted skill in their individual clinical setting.
Protocol
This study was approved by the Human Research Ethics Committee of Edith Cowan University, Reference Number REMS 2021-02489-STEINWANDEL. Informed consent was obtained from workshop participants, and a copy of a participant information sheet was provided. Only workshop participants who participated in one of the ultrasound workshops during the delivery period between the years 2021 and 2022 were invited to participate and included in this study. All subsequent workshop participants in 2023 and 2024 were excluded from participation in this study.
1. Creation and preparation of the simulation model12
- Cut a regular raw chicken breast with a sharp kitchen knife horizontally to allow for the insertion of three or more fluid-filled artificial blood vessels, which will simulate human blood vessels in this experiment.
- Preparation of the artificial blood vessels
- Fill modeling balloons (size 260Q) with cold rosehip tea or water prepared with red food color using a 50 mL catheter-tipped syringe. Fill the modeling balloon with the prepared fluid and remove any surplus air from the balloon.
- Push the fluid in the balloon and remove any air bubbles at the same time by repeatedly pushing the syringe in and out of the modeling balloon. When this repetitive process is completed, the balloon must be free of air bubbles and slightly pressurized.
- Tighten the modeling balloon with a knot to avoid any fluid leaks.
- Place the fluid-filled modeling balloon on the lower half of the chicken breast. Fold the other chicken breast half over (placed) on top. Wrap this chicken breast simulation model with a transparent film and place it on a tray (Figure 1).

Figure 1: Simulation model. The simulation model was created from raw chicken breast and fluid-filled modeling balloons (260Q). Please click here to view a larger version of this figure.
2. Simulated vascular access cannulation
- Place a charged POCUS device (portable or stationary) with a linear probe and a probe cover onto this simulation model of patient tissue prepared in step 1.
- Apply some ultrasound gel to the area of interest in the simulation model. Don a pair of non-sterile gloves.
3. Measurement of the depth and caliper of a vessel
- In a transverse view of the simulated blood vessel in the simulation model, visualize a simulated vessel and achieve a good vision of the vessel of interest by placing the ultrasound probe on top of the simulation model and centering the view of the vessel in the middle of the screen of the ultrasound device, where it will appear as a black circular structure. Ensure a reasonable size of the vessel can be identified, taking up at least 1/3rd of the screen.
- Place this vessel in the center of the screen of the POCUS device by moving the ultrasound probe across the simulation model so that the whole vascular structure is visible. Adjust image size and contrast settings on the ultrasound device, if required, to obtain optimal vision of the vessel and surrounding tissue, to distinguish between vessel space and surrounding tissue. Freeze the image by pressing the Freeze function button on the ultrasound device.
- On the frozen image, place digital markers, to indicate the depth of the center of the vessel. Place digital markers as well to measure the diameter (caliper) of the vessel (Figure 2).
NOTE: This information helps the observer make critical decisions on the size and length of cannula needed, which may be suitable for potentially reaching this particular blood vessel and enabling successful cannulation.
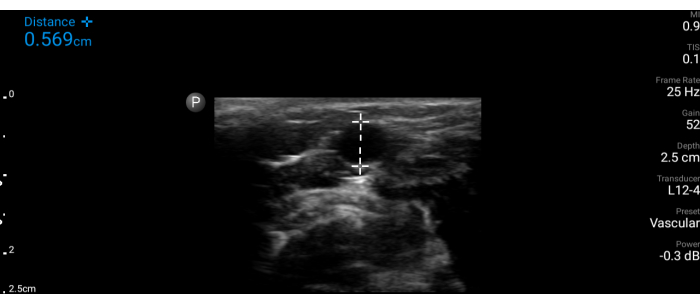
Figure 2: Vessel measurement. Measurement of the simulated vessel (transverse view) on the ultrasound screen. Please click here to view a larger version of this figure.
4. Observation of the direction of the vessel
- Rotate the ultrasound probe by 90° to obtain a longitudinal view of the blood vessel. This view allows the observer to make a decision on the direction of the vessel and the intended cannulation, providing crucial information prior to the subsequent process of the actual cannulation.
- Observe the direction of the vessel being aligned with the ultrasound probe. Once the vessel direction has been observed, use this to decide which direction of cannula placement might likely be useful and also successful for the insertion and placement of the cannula, even when the vessel might appear to be at a deeper level and may not be palpable or visible from the outside (Figure 3).

Figure 3: Longitudinal view of a simulated vessel. Please click here to view a larger version of this figure.
5. Cannulation of a deep vessel
NOTE: Combining all this information, a virtual picture of that vessel is created in the observer's mind; the process of vascular cannulation will follow.
- Place the linear probe in a transverse view of the vessel. Remove the protective needle cover of the PIVC cannula to commence the cannulation procedure.
- Place the transverse vision of the vessel centrally on the screen of the POCUS device. Slowly and carefully cannulate (perpendicular) in the middle of the linear probe the simulation model at an angle of around 40° and aim for the top (upper end) of the vessel.
- Advance the needle tip into the tissue of the simulation model and aim for the vessel. Try to identify visually the needle tip on the screen of the POCUS device while it is advanced through the tissue by simultaneously advancing the needle but also following the needle tip with the ultrasound probe (Figure 4).
- Final placement of the cannula with the use of POCUS
- Further advance the needle through the tissue towards the vessel and follow slowly with the ultrasound probe, the needle tip simultaneously in the same motion as the needle advancement.
NOTE: Through this the clinician can ensure that the needle tip is always visible in the desired anatomical space within the simulation model tissue and then moving towards the intravascular space. - Visualize the needle tip entering the intravascular space, then level the needle to a shallower angle and further advance the needle to finally rest it in the center of the vessel of the simulation model (final destination).
- Validate the position of the needle tip with the POCUS device by observing the needle tip on the screen by changing the angle of the ultrasound probe or moving the ultrasound in small (mm) increments back and forth until the needle tip visually disappears/reappears on the screen.
- Observe the opposite end of the PIVC for some evidence of red-colored fluid to confirm correct placement. Remove the stylet from the PIVC (Video 1).
- Further advance the needle through the tissue towards the vessel and follow slowly with the ultrasound probe, the needle tip simultaneously in the same motion as the needle advancement.

Figure 4: Transverse view of a simulated vessel. Please click here to view a larger version of this figure.
Video 1: Cannula advancement into the center of the vessel. Please click here to download this Video.
Representative Results
A total of 81 individuals participated in 11 half-day workshops between 2021 and 2022. Most participants were resident medical officers (n=43, 53%), followed by staff development/clinical nurses and clinical nurse consultants (n=19, 25.3%) with a mean of 8 years of clinical experience. Half of the participants (n=40, 49%) had only 2 years or less of clinical experience. There were also some other workshop participants, such as a nuclear medicine technologist, a dental sedationist, and a diagnostic radiologist. Almost a quarter of all participants were employed at satellite hemodialysis (HD) clinics. Ultrasound devices were available in almost all workplaces (n=75, 92.6%). Most participants (n=55,67.9%) were female and had a mean age of 35.7 years (Table 1).
Workshop participants followed the instructions of the workshop facilitator and were able, after some theoretical instructions and observing the facilitator demonstrating the task in a simulation model, to successfully perform the same cannulation process using a POCUS device in connection with the described simulation model step-by-step.
Workshop participants were invited to complete an electronic survey prior to and directly at the conclusion of the workshop, indicating their level of confidence in using POCUS for USGPIVC. These surveys consisted of 11 closed and three open-ended questions, and participants were able to rate their individual skills using ultrasound while cannulating on a 10-point rating scale, ranging from 0=not very skilled to 10= very proficient. Eight weeks after conclusion of the workshop, participants were again invited to complete a final survey to indicate any progress of their current skill in adopting and using the newly learned skill in their clinical practice. In this last survey, nine closed and two open-ended questions regarding their self-assessed USGPIVC skill were asked. Data analysis was done using SPSS statistics. The individually self-assessed cannulation skill using POCUS resulting from three different electronic surveys at three-time points were analyzed, and a Friedman Test and post hoc analysis were conducted using the Wilcoxon sign-ranked tests with a Bonferroni correction applied. A p-value < 0.05 was considered statistically significant, and content analysis was conducted on the responses to the open-ended questions (Figure 5, Table 2).
The self-evaluated cannulation skill exhibited a statistically meaningful variation across the three time periods (p < 0.001). Post hoc analysis unveiled a statistical distinction in self-assessed cannulation proficiency prior to the workshop when contrasted with the period immediately following the workshop (p < 0.001) and 8 weeks after the workshop (p < 0.001). However, no significant differences in self-evaluated cannulation skills using POCUS were observed between the immediate post-workshop period and the 8-week follow-up (p=.722). Some workshop participants reported on the conclusion of the workshop that at their workplace, management allows the use of ultrasound only before cannulations to help the clinician visualize vessels and to give the operator a better impression and understanding of specific individual vascular conditions before any cannulation attempt has been made.
Most workshop participants claimed that attending the workshop using simulation models had enhanced their clinical skill in using ultrasound for the purpose of cannulating a venous vessel significantly and that they gained more confidence in using this technique at the conclusion of the workshop, which further advanced after applying this technique in their clinical environment. At 8 weeks after the conclusion of the workshop, participants were again invited to complete another electronic version of the survey. Most participants were able to practice this newly learned skill subsequently in their clinical environment with patients, and as in most of their clinical institutions, POCUS devices were available (Table 3; Table 4).
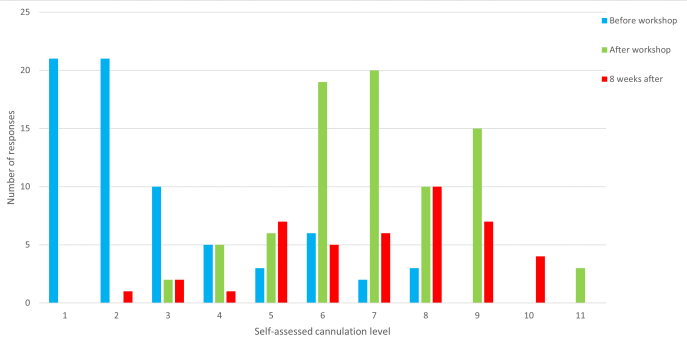
Figure 5: Self-assessment of cannulation skills. Level of self-assessed cannulation skill level provided by participants before, directly after (on conclusion) and 8 weeks after the workshop. Prior to the workshop, the Median was 1, IQR: 0 to 3; After the workshop, the Median was 6, IQR: 5 to 7; 8 weeks later, the Median: 6, IQR: 4 to 8. The n number is 81 before the workshop, 80 after the workshop, and 43 for 8 weeks after. This figure has been modified from8. Please click here to view a larger version of this figure.
Table 1: Baseline demographics. This table has been modified from8. Please click here to download this Table.
Table 2: Self-assessment of cannulation skills. The p-values of self-assessed cannulation skills by participants before, directly after, and 8 weeks after the workshop The n number is 81 before the workshop, 80 after the workshop, and 43 for 8 weeks after. This figure has been modified from8. Please click here to download this Table.
Table 3: Post-workshop evaluation. This table has been modified from8. Please click here to download this Table.
Table 4: Evaluation at 8 weeks after workshop completion. This figure has been modified from8. Please click here to download this Table.
Discussion
Vascular access cannulation of difficult venous conditions requires experience, manual dexterity, and continuous observation of the progress of the needle tip position while the cannula is advanced through human tissue into the intravascular space18. While the use of ultrasound has become more prevalent in the use in patients with difficult venous access2, it is also necessary that junior clinicians and novices become familiar with the use of ultrasound in connection with its use in the space of vascular access and receive adequate training17,19,20. It has not been reported anywhere in detail what specific essential elements are to be taught, understood, and practiced in the context of training for using POCUS and USGPIVC. To enable adequate training, the high-fidelity simulation models suggested by12 were used, and it was possible to demonstrate that the level of confidence in workshop participants using ultrasound in connection with vascular access cannulations increased after attending a workshop in a previous publication8. It can, therefore, be assumed that a similar approach, with the use of ultrasound in simulation models performing those training and education sessions, could be successful elsewhere when a similar approach is being used.
Several clinical guidelines have been created for the use of ultrasound in connection with vascular access proceedings21,22. At the same time, it has been reported that over the past few years, the number of available ultrasound devices in clinical areas has increased. Junior doctors require adequate practical education in using this technology, and face-to-face teaching using simulation models may be the most suitable approach to transferring expert knowledge to novices23. A series of multiple half-day workshops, with a duration of 4 hours each, performed at the local institution has already demonstrated that beginners can successfully apply this technique in their clinical area after sufficient training on the simulation models and under the guidance of an experienced facilitator8. This approach demonstrates that the step-by-step approach applied by the workshop facilitator guiding the participants through every single observational step during the POCUS-guided cannulation may be the key element to a successful cannulation outcome once this method is used in patients. As difficult vascular access conditions (DIVA) in highly complex patients have been previously described as generally increasing in multiple studies24,25,26,27,28, the use of POCUS and USGPIVC may offer a suitable solution to address the problem of failed cannulation during the first attempt in various clinical settings.
This step-by-step approach was first theoretically explained and discussed during the workshop, then practically demonstrated to participants before they practiced the same approach individually on the simulation models provided. Those education sessions were performed in small groups of a maximum of 15 participants, and each participant was provided with their own individual simulation model and a POCUS device to practice this procedure multiple times. Each simulation model had at least three simulated vessels inserted, which were neither palpable nor visible from the outside. This feature allowed for multiple cannulation attempts for each individual workshop participant in case a first cannulation attempt led to a leaking vessel in the simulation model, as only the repeated practice of using USGPIVC will lead to increased confidence in users of this technique. At the conclusion of the workshop, participants were assessed on their ability to identify a vessel in the simulation model, measure vessel depth, caliper, and direction, and were also assessed if they were able to cannulate the vessel in a transverse (out of plane) view. Almost all participants were able to demonstrate this ability at the conclusion of all the workshops and claimed that this exercise was useful for them.
While this workshop initially aimed at a broad and general audience of clinicians coming from various clinical areas and aiming at delivering a basic understanding of the principles of the use of ultrasound in the space of establishing vascular access, it may be necessary in the future to create a more specific approach of teaching for particular clinical areas. It has been previously recommended to include USGPIVC training in medical undergraduate curricula3, it may now also be essential to consider the different requirements of skills to be taught practically for clinicians working in various clinical settings and which need a different specialist skill set for the use of ultrasound in connection with the insertion of cannulas. It is obvious that a clinician caring for patients with chronic kidney disease will most likely use large bore cannulas in blood vessels with a larger diameter, compared to an anesthetist29 or a nurse30 who works in the pediatric setting. These different aspects of training requirements need to be considered when designing curricula, and they will also need to be considered when designing simulation models for training, as smaller vessels need to be more sophisticated, being simulated as realistic as possible and when compared to the clinical reality. Tailored and specific curricula aiming at establishing sound USGPIVC skills in clinicians in various settings need to be developed and designed to achieve the best possible outcomes in learners and ultimately result in improving clinical outcomes. This may ultimately lead to establishing the use of ultrasound in the space of best clinical practice for vascular access cannulation.
The reported approach from workshop participants that in some clinical areas where ultrasound is available but is only used prior to cannulations for visualization but not for the cannulations per se may be a useful suggestion for curriculum development. If this approach is being used in future workshops as an initial educational initiative for workplaces to increase the use and uptake of ultrasound when considering vascular access in clinical settings, it might pave the way for more general acceptance of this technique, USGPIVC, as best practice in the long term. This may also be included and influence the future versions of Infusion Therapy Standards of Practice27. Further, Simulation-Based Mastery Learning (SBML) and including USGPIVC in these concepts may help share and distribute the knowledge of this approach31,32. Despite some educational programs that have been developed and are applied today33, there exists no clear description of which particular elements are critical to be learned to be successful with USGPIVC. Is this related to critical information for the operator on the depth and size of a blood vessel? Is it information on the direction of a blood vessel? Is it about the manual dexterity of the operator in performing this task repeatedly and skillfully? Is it the combination of visual observation and manual dexterity that leads to the ultimate success of USGPIVC? It is obvious, that these elements require further research and clear identification and description in guiding future successful educational approaches in this space and to establish common principles for targeted curricula in this space, which should be common standard practice globally. It is also essential that clinicians adopt and understand that first-attempt cannulations are considered the best clinical practice through the use of simulation-based learning34. This has also been previously confirmed in a systematic review14.
This study also had some limitations. First, each of the simulation models had a distinct number of three fluid-filled modeling balloons per simulation model. It was expected that after the first cannulation attempt, each balloon (vessel) would leak and deflate and may not be able to be reused. Therefore, we placed three vessels into each simulator to allow for multiple attempts in various vessels, providing a repeated and similar experience for the individual and refining the operators' skills.
Secondly, the smallest achievable vessel size with the fluid-filled modeling balloons (size 260Q) was 0.6 cm, which may certainly not be reflective of what clinicians may encounter in real-life clinical scenarios and settings, especially in pediatric settings, but this was the minimum vessel size which was achievable under the given circumstances in the workshop settings and materials being available. The main teaching goal of the workshop was to transfer theoretical and practical knowledge and a basic understanding of vascular access cannulation using ultrasound, observing the needle tip at all times during the cannulation process to avoid unintended vessel injury, extravasation, or tissue damage.
The study findings may be limited to learners and clinicians in the geographical setting of Western Australia (WA), as other global areas may have different educational approaches or guidelines to achieve similar outcomes. Further, the study results may be partially skewed, as participants were keen learners and deliberately chose (voluntarily self-enrolling) to participate in the workshop, which may have contributed to a positive learning outcome. Future research studies are needed to evaluate if workshops with a longer duration (one or several full days) provide better learning outcomes. It may also be of particular interest to investigate which elements of teaching in USGPIVC workshops are critical to achieving the desired learning outcomes. Further, more research is required to reveal the attitude of senior clinicians towards adopting novel vascular access approaches such as through USGIPVC, as in this study, only junior clinicians participated (self-enrolled), and no senior clinicians were self-enrolling. Did this occur because senior clinicians already have skills in USGC? Or do senior clinicians not need this task in their daily practice? Is the landmark-cannulation approach more common in senior clinicians, and are they always successful with this approach due to their long-term clinical experience? In addition, it may be relevant to understand and learn more about clinicians' critical views and considerations on how they may determine and choose the most adequate and appropriate PIVC cannula for an individual patient after observing an individual's vascular conditions using POCUS, in conjunction with the required or planned therapeutic interventions such as required intravenous medication or smaller or larger amounts of intravenous fluids (larger sized cannulas for higher volumes of fluids).
A simulative teaching approach combining cannulation practice with point-of-care ultrasound (POCUS) instruction enhanced participants' skill levels over the duration of the workshop and retained improvements afterward, as demonstrated by this study. Some participants were able to apply this skill subsequently in their clinical setting. This educational activity could potentially promote the adoption of this novel approach by clinicians when patients present with difficult intravenous access (DIVA) conditions, as some workshop participants may be inclined to share their positive experiences with colleagues. The significant representation of junior doctors, comprising half of all participants, underscores the evident necessity for advanced training in scenarios where frontline healthcare workers encounter DIVA conditions while providing patient care. Furthermore, the creation and integration of evidence-based clinical guidelines specific to the use of POCUS for vascular access would be highly beneficial. By combining competency pathways and clinical guidelines, healthcare institutions can foster a culture of excellence, patient safety, and improved outcomes when employing POCUS for vascular access procedures.
Declarações
The authors have nothing to disclose.
Acknowledgements
The author would like to thank Dr. James Rippey, Sonologist at Sir Charles Gairdner Hospital, Nedlands, Western Australia, for guidance and instructions on how to create the used simulation model in the experiment. This project did not receive any institutional financial support.
Materials
| BD Insyte Autogard BC Pro shielded IV catheter with blood control technology (PIVC) | BD | 318054 | |
| Catheter tipped syringe 30 or 50 ml | BD Plastipak | 301229, 300865 | |
| Celeste Nitrile Powder Free Examination gloves sizes S/M/L (non-sterile) | Celeste | CLS121 | |
| Goliath Cling wrap | Goliath | ||
| modelling balloons 260 Q | Qualatex | 99321 | |
| Point-of care ultrasound device, eg. Philips Lumify or Vscan Air | Philips or GE Healthcare | https://www.usa.philips.com/healthcare/product/HC989605450382/lumify-c5-2-curved-array-transducer | |
| probe cover for Philips lumify | Philips | https://www.usa.philips.com/healthcare/product/HC989605450382/lumify-c5-2-curved-array-transducer | |
| raw chicken breast | |||
| Sunsonic Ultrasound Transmission Gel 250 ml | Sunsonic | LG250 | |
| Tasty Herbal Infusion Rosehip Tea | Tasty | ||
| Victorinox Fibrox Chef's Knife 20 cm | Victorinox | 40520 |
Referências
- Higgins, N., Iu, P., Carr, P., Ware, R., Van Zundert, A. Techniques to select site of insertion for a peripheral intravenous catheter with vessel locating devices using light, sounds or tactile actions (or palpations). J Clin Nurs. 30 (7-8), 1091-1098 (2021).
- Schults, J. A., et al. Peripheral intravenous catheter insertion and use of ultrasound in patients with difficult intravenous access: Australian patient and practitioner perspectives to inform future implementation strategies. PLoS One. 17 (6), e0269788 (2022).
- Armson, A. M., Moynihan, R., Stafford, N., Jacobs, C. Ultrasound-guided cannulation for medical students. Clin Teach. 18 (3), 295-300 (2021).
- Burton, S. O., et al. Use of point of care ultrasound (pocus) by intensive care paramedics to achieve peripheral intravenous access in patients predicted to be difficult: An out-of-hospital pilot study. Australas Emerg Care. 26 (2), 164-168 (2023).
- Dornhofer, K., et al. Evaluation of a point-of-care ultrasound curriculum taught by medical students for physicians, nurses, and midwives in rural Indonesia. J Clin Ultrasound. 48 (3), 145-151 (2020).
- JoVE Science Education Database. . Physical Examination IV. , (2024).
- Archer-Jones, A., et al. Evaluating an ultrasound-guided peripheral intravenous cannulation training program for emergency clinicians: An Australian perspective. Australas Emerg Care. 23 (3), 151-156 (2020).
- Steinwandel, U., Coventry, L. L., Kheirkhah, H. Evaluation of a point-of-care ultrasound (pocus) workshop for peripheral intravenous cannulation. BMC Med Educ. 23 (1), 451 (2023).
- Feinsmith, S., Huebinger, R., Pitts, M., Baran, E., Haas, S. Outcomes of a simplified ultrasound-guided intravenous training course for emergency nurses. J Emerg Nurs. 44 (2), 169-175.e2 (2018).
- Feinsmith, S. E., et al. Performance of peripheral catheters inserted with ultrasound guidance versus landmark technique after a simulation-based mastery learning intervention. J Vasc Access. 24 (4), 630-638 (2023).
- Bahl, A., et al. A standardized educational program to improve peripheral vascular access outcomes in the emergency department: A quasi-experimental pre-post trial. J Vasc Access. , 11297298231219776 (2024).
- Rippey, J. C., Blanco, P., Carr, P. J. An affordable and easily constructed model for training in ultrasound-guided vascular access. J Vasc Access. 16 (5), 422-427 (2015).
- Birrane, J., Misran, H., Creaney, M., Shorten, G., Nix, C. M. A scoping review of ultrasound teaching in undergraduate medical education. Med Sci Educ. 28 (1), 45-56 (2018).
- Van Loon, F. H. J., Scholten, H. J., Van Erp, I., Bouwman, A. R. A., Daele, A. T. M. D. V. Establishing the required components for training in ultrasoundguided peripheral intravenous cannulation: A systematic review of available evidence. Med Ultrason. 21 (4), 464-473 (2019).
- Spencer, T. R., Bardin-Spencer, A. J. Pre- and post-review of a standardized ultrasound-guided central venous catheterization curriculum evaluating procedural skills acquisition and clinician confidence. J Vasc Access. 21 (4), 440-448 (2020).
- Adhikari, S., Schmier, C., Marx, J. Focused simulation training: Emergency department nurses’ confidence and comfort level in performing ultrasound-guided vascular access. J Vasc Access. 16 (6), 515-520 (2015).
- Stone, R., Walker, R. M., Marsh, N., Ullman, A. J. Educational programs for implementing ultrasound guided peripheral intravenous catheter insertion in emergency departments: A systematic integrative literature review. Australas Emerg Care. 26 (4), 352-359 (2023).
- Thomas, S., Moore, C. L. The vanishing target sign: Confirmation of intraluminal needle position for ultrasound guided vascular access. Acad Emerg Med. 20 (10), e17-e18 (2013).
- Schott, C. K., et al. Retention of point-of-care ultrasound skills among practicing physicians: Findings of the VA national Pocus training program. Am J Med. 134 (3), 391-399.e8 (2021).
- Smith, C. Should nurses be trained to use ultrasound for intravenous access to patients with difficult veins. Emerg Nurse. 26 (2), 18-24 (2018).
- . AIUM practice parameter for the use of ultrasound to guide vascular access procedures. J Ultrasound Med. 38 (3), E4-E18 (2019).
- Keogh, S., Mathew, S., Alexandrou, E. . Peripheral intravenous catheters: A review of guidelines and research. , (2019).
- Lian, A., Rippey, J. C. R., Carr, P. J. Teaching medical students ultrasound-guided vascular access – which learning method is best. J Vasc Access. 18 (3), 255-258 (2017).
- Armenteros-Yeguas, V., et al. Prevalence of difficult venous access and associated risk factors in highly complex hospitalised patients. J Clin Nurs. 26 (23-24), 4267-4275 (2017).
- Van Loon, F. H. J., et al. The modified a-diva scale as a predictive tool for prospective identification of adult patients at risk of a difficult intravenous access: A multicenter validation study. J Clin Med. 8 (2), 144 (2019).
- Yalcinli, S., Akarca, F. K., Can, O., Sener, A., Akbinar, C. Factors affecting the first-attempt success rate of intravenous cannulation in older people. J Clin Nurs. 28 (11-12), 2206-2213 (2019).
- Nickel, B., et al. . Infusion therapy standards of practice. , (2024).
- Coritsidis, G. N., et al. Point-of-care ultrasound for assessing arteriovenous fistula maturity in outpatient hemodialysis. J Vasc Access. 21 (6), 923-930 (2020).
- Ballard, H. A., et al. Use of a simulation-based mastery learning curriculum to improve ultrasound-guided vascular access skills of pediatric anesthesiologists. Paedia Anaesthesia. 30 (11), 1204-1210 (2020).
- Russell, C., Mullaney, K., Campbell, T., Sabado, J., Haut, C. Outcomes of a pediatric ultrasound-guided short peripheral catheter training program and hands-on poultry simulation course. J Infusion Nurs. 44 (4), 204-215 (2021).
- Feinsmith, S. E., et al. Performance of peripheral catheters inserted with ultrasound guidance versus landmark technique after a simulation-based mastery learning intervention. J Vasc Access. 24 (4), 630-638 (2021).
- Amick, A. E., et al. Simulation-based mastery learning improves ultrasound-guided peripheral intravenous catheter insertion skills of practicing nurses. Simulation in Healthcare. 17 (1), 7-14 (2022).
- Bahl, A., Mielke, N., Diloreto, E., Gibson, S. M. Operation stick: A vascular access specialty program for the generalist emergency medicine clinician. J Vasc Access. , (2024).
- Oh, E. J., Lee, J. -. H., Kwon, E. J., Min, J. J. Simulation-based training using a vessel phantom effectively improved first attempt success and dynamic needle-tip positioning ability for ultrasound-guided radial artery cannulation in real patients: An assessor-blinded randomized controlled study. PLoS One. 15 (6), e0234567 (2020).

.
