Structure du ferrocène
English
COMPARTILHAR
Visão Geral
Source : Tamara M. Powers, département de chimie, Texas A & M University
En 1951, Kealy et Pauson ont signalé à la Nature , la synthèse d’un nouveau ferrocène composé organométallique. 1 dans leur rapport initial, Pauson a proposé une structure de ferrocène dans laquelle le fer est individuellement collé (liaisons sigma) à un atome de carbone de chaque ligand cyclopentadiène (Figure 1, la Structure I). 1 , 2 , 3 ce rapport initial conduit à intérêt largement répandu dans la structure du ferrocène, et beaucoup d’éminents scientifiques ont participé à l’élucidation de la structure de cette nouvelle molécule intéressante. Wilkinson et Woodward ont été prompts à suggérer une formalisation alternative où le fer est « en sandwich » entre deux ligands cyclopentadiène, avec liaison égale pour tous les atomes de carbone 10 (Figure 1, Structure II). 4 ici, nous allons synthétiser ferrocène et décider, basé sur des données expérimentales (IR et 1H RMN), qui, de ces structures sont observées. En outre, nous étudierons l’électrochimie du ferrocène en recueillant un voltamogramme cyclique. Au cours de cette expérience, nous introduire la règle des 18 électrons et discuter des électrons de valence, comptant pour les complexes de métaux de transition.
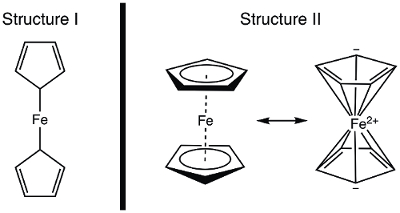
Figure 1. Deux structures proposées du ferrocène.
Princípios
Procedimento
Resultados
Ferrocene Characterization:
1H NMR (chloroform-d, 300 MHz, δ, ppm): 4.15 (s).
The 1H NMR spectrum of ferrocene clearly shows a single resonance, consistent with structure II.
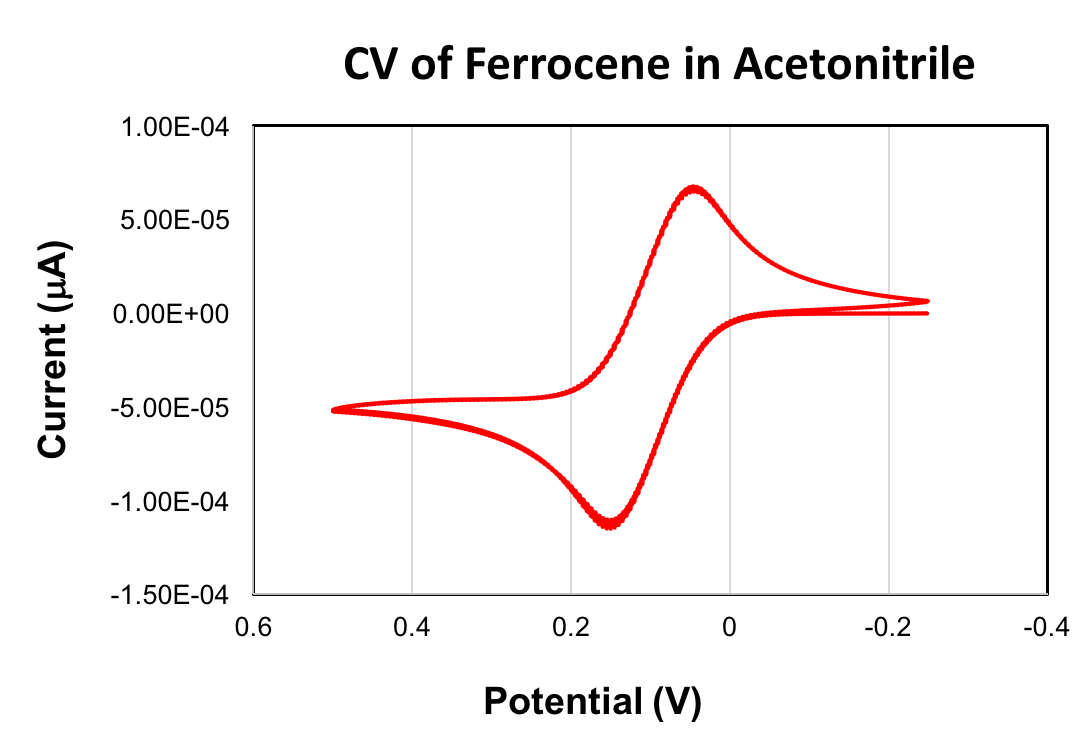
A CV of ferrocene is given below. The E1/2 value obtained for the oxidation of ferrocene was +90 mV (acetonitrile, scan rate 100 mV/s, 0.1 M (Bu4N)PF6, glassy carbon working electrode). The ferrocene/ferrocenium redox couple is commonly used as a reference in cyclic voltammetry. When used as a reference, the E1/2 value of ferrocene is set equal to 0 V.
Applications and Summary
In this video, we discussed ferrocene and the role it played in the development of organometallic chemistry. Ferrocene was synthesized and characterized by 1H NMR and IR spectroscopy. Both spectra are consistent with the 18 e− Structure II, where the iron is "sandwiched" between two cyclopentadiene ligands, with equal binding to all 10 carbon atoms (Figure 1, Structure II). Oxidation of ferrocene to ferrocenium cation was observed electrochemically.
In 1973, following the structural characterization of ferrocene, Wilkinson was one of two chemists awarded the Nobel Prize in Chemistry "for [his] pioneering work … on the chemistry of the organometallic, so called sandwich complexes".6 His work greatly influenced and expanded the emerging field of organometallic chemistry. While the first organometallic compound was prepared in 1849, it was only in the 1950's that significant advancements were made to understanding how metals can bond to carbon atoms. Today, the field of organometallic chemistry, or the chemistry of compounds that form metal-carbon bonds, is central to many applications. This includes: energy, dye-sensitized solar cells, catalysis, polymerization, drug discovery and synthesis, bioinorganic systems, and organic light-emitting diodes (OLEDs).7
Ferrocene itself also plays an active role in the field of organometallic chemistry. Ferrocene readily participates in electrophilic aromatic substitution; in fact, it is 100,000 times more reactive than benzene in these reactions. Ferrocene has found widespread application as a structural component of bidentate ligands in organometallic catalysis. For example, 1,1'-bis(diphenylphosphino)ferrocene (dppf) is a chelating ligand used in homogeneous catalysis. The ligand dppf chelates 1st, 2nd, and 3rd row transition metals including Ni, Pd, and Pt. [1,1'-Bis(diphenylphosphino) ferrocene]palladium(II) dichloride is an example of a palladium cross-coupling catalyst for C-C and C-heteroatom bond formation (Figure 5).8 In the video "MO Theory of Transition Metal Complexes", we will synthesize two metal complexes featuring dppf.
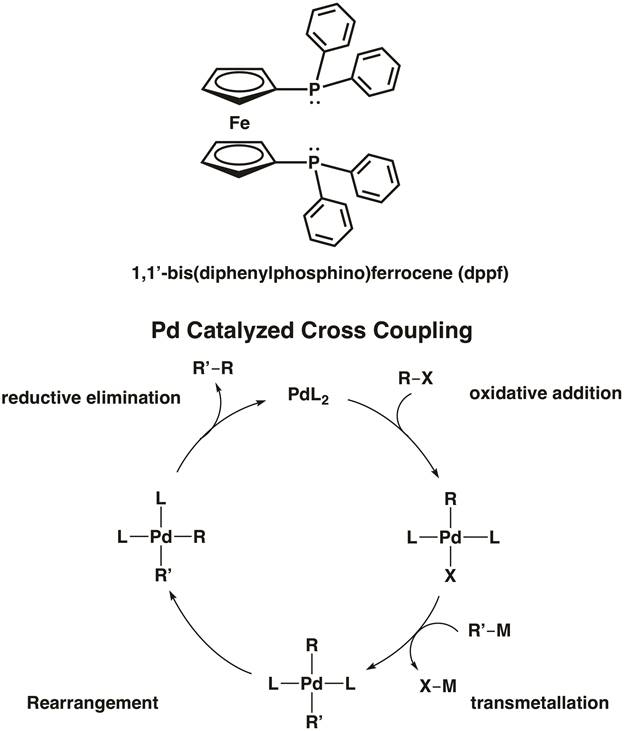
Figure 5. [1,1'-Bis(diphenylphosphino) ferrocene]palladium(II) dichloride is a cross-coupling catalyst for C-C and C-X bond formation.
Referências
1. Kealy, T. J., Pauson, P. L. A New Type of Organo-Iron Compound. Nature. 168 (4285), 1039-1040 (1951).
2. Pauson, P. L. Ferrocene—how it all began. J Organomet Chem. 637, 3-6 (2001).
3. Seeman, J. I., Cantrill, S. Wrong but seminal. Nat Chem. 8 (3), 193-200 (2016).
4. Wilkinson, G., Rosenblum, M., Whiting, M. C., Woodward, R. B. The Structure of Iron Bis-cyclopentadienyl. 74, 2125-2126 (1952).
5. Green, M. L. H., Parkin, G. Application of the Covalent Bond Classification Method for the Teaching of Inorganic Chemistry. J Chem Educ. 91 (6), 807-816 (2014).
6. Press Release. http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1973/press.html.
7. Crabtree, R. H. The Organometallic Chemistry of the Transition Metals, 6th ed. John Wiley & Sons. Hoboken. 2014.
8. Gildner, P. G., Colacot, T. J. Reactions of the 21st Century: Two Decades of Innovative Catalyst Design for Palladium-Catalyzed Cross-Couplings. Organometallics. 34, 5497-5508 (2015).
Transcrição
Structure determination of organometallic compounds is crucial for understanding the reactivity of a given molecule. Various models and techniques enable scientists to elucidate compounds in question, as for example ferrocene.
Ferrocene, an organometallic compound, was first reported by Kealy and Pauson in 1951. They proposed a structure consisting of an iron atom with two single bonds to two carbon atoms on separate cyclopentadiene rings.
However, Wilkinson and Woodward suggested an alternative for the ferrocene structure, in which the iron atom is “sandwiched” between two cyclopentadiene rings, with equal binding to all 10 carbon atoms. The structure proposed by Wilkinson has since been confirmed by X-ray crystallography and proton NMR.
This video will illustrate the 18-electron rule to predict the structure of organometallic complexes, the synthesis of ferrocene, its spectroscopic and electrochemical analysis, and some of its applications.
When proposing molecular structures, always consider the amount of valence shell electrons. Main group elements can accommodate up to 8 electrons, while transition metals can contain up to 18 electrons in its valence shell. Transition metals have nine valence orbitals, 1 s, 3 p, and 5 d orbitals, with two electrons in each. With some exceptions, transition metal complexes with 18 valence electrons are highly stable compounds.
To determine the total electron count of a transition metal complex, two models can be used: the ionic, or the covalent method. Both methods utilize the same ligand classifications: X-type ligands include anionic groups such as halides, hydroxide, or alkoxide; L-type ligands include lone-pair donors such as amines and phosphines; and Z-type ligands are neutral Lewis acids, which are electron-pair acceptors. To demonstrate the two models, let’s use Co(NH3)3Cl3 as an example.
Consider the Co atom, which is in Group 9 of the periodic table and has 9 valence electrons. Since the oxidation state of the cobalt in this complex is +3, the total number of valence electrons contributed is 6.
The X-type ligands, being the 3 Cl, and L-type ligands, the 3 NH3, contribute a total of 12 electrons, while Z-type ligands are not available – yielding a total of 18 electrons.
In the covalent model the cobalt oxidation state is ignored, and the molecule is not ionic, resulting in 9 electrons total. X-type ligands donate one electron; L-type ligands donate two electrons; and Z-type ligands, if present, contribute none – also yielding a total of 18 electrons.
The counting of total electrons in ferrocene is more complex: the iron atom contributes 8 valence electrons, while the cyclopentadiene rings are classified as L2X-type ligands, providing 5 electrons each, which come from the two double bonds and a radical, resulting in a total of 18 electrons. Whereas Pauson’s original proposed structure would result in only 10 electrons, because of the single bonded cyclopentadienes.
Now that we have discussed the principles of structure determination, let’s synthesize ferrocene and identify which structure is correct.
In a fume hood, add a stir bar and 50 mL dicyclopentadiene to a clamped 100-mL round-bottom flask. Then attach the round-bottom flask to a distillation apparatus, and place it in an oil bath, with the receiving flask in an ice bath.
Set the hot plate to 160 °C and stir gently. Fractionally distill approximately 5 mL of the cyclopentadiene monomer, which must be kept cold.
Add to a 200-mL Schlenk flask labeled A, a stir bar and freshly ground KOH. Next, add 30 mL 1,2-dimethoxyethane, attach the flask to a N2 line, and fit with a rubber septum.
While stirring under a N2 atmosphere, add to the solution 2.75 mL cyclopentadiene via syringe and stir for at least 10 min.
In a separate 200-mL Schlenk flask labeled B, add ground FeCl2•4H2O and 12.5 mL DMSO. Then fit a rubber septum, attach to a N2 line, and stir under a N2 atmosphere until all of the iron has dissolved.
When this step is complete, insert either end of a double tipped needle into each Schlenk flask, and cannula transfer the iron solution to the cyclopentadiene solution dropwise over a 30-minute period.
When the reaction is complete, pour the mixture into a beaker containing a slurry of 6 M HCl and 50 g crushed ice and stir for a few minutes. Collect the resulting orange crystals by vacuum filtration on a fritted funnel, wash precipitate with ice-cold water, and then air-dry. Purify the crystals by sublimation.
Next, prepare an NMR sample of the purified ferrocene dissolved in chloroform-d. Use the ATR attachment of the infra-red spectrometer to obtain an IR spectrum. And, lastly, collect a cyclic voltammogram of the ferrocene in acetonitrile, at a scan rate of 100 mV/s.
The NMR analysis shows a single peak at 4.17 ppm, which confirms that all of the hydrogen atoms are magnetically equivalent. Furthermore, the IR spectrum shows a single sp2 C-H stretch at 3096 cm-1, confirming that the hydrogen atoms are equivalent, and that the proposed Wilkinson’s structure is correct.
Finally, let’s take a look at the CV of ferrocene, which shows a single oxidation event. The E1/2 half value can be calculated by taking the average of the cathodic peak potential and the anodic peak potential. In acetonitrile, the ferrocene/ferrocenium redox couple occurs at a potential of 90 mV.
Now that we have discussed a procedure for preparing ferrocene, let’s look at some of its applications.
Palladium-catalyzed cross-coupling reactions are a valuable synthetic tool in the pharmaceutical industry. However, a common undesired side reaction is beta-hydride elimination, which can be minimized with the use of 1,1′-bis(diphenylphosphino)ferrocene or dppf as a chelating agent with PdCl2, forming [1,1′-Bis(diphenylphosphino)ferrocene]palladium(II) dichloride abbreviated as (dppf)PdCl2.
The suppression of beta-hydride elimination, and the high product yield, has been attributed to the large bite angle of the dppf ligand. With the advent of the catalyst, reactions such as the Suzuki coupling, are possible and typically used to couple primary alkyl groups using 9-BBN reagents.
The ease with which ferrocene can undergo electrophilic aromatic substitution, as in the Friedel-Crafts acylation, or the formylation/Mannich reaction, has made it a promising source for organometallic drug candidates. These types of organometallic drugs have attracted interest due to their structural variety. For instance, M-arenes can support three functionalities, and M-carbenes can support two.
Currently ferroquine which contains elements of ferrocene and chloroquine, is undergoing evaluation as a commercial antimalarial drug. Furthermore, ferrocifen, which is based on the elements of ferrocene and tamoxifen, is currently undergoing clinical trials as a potential breast cancer drug. Additionally, efforts are being made in developing nucleoside analogues of ferrocene in the study of DNA/RNA pathways.
You’ve just watched JoVE’s introduction to the structure of ferrocene. You should now understand the 18-electron rule, the synthesis and characterization of ferrocene, and several of its applications. Thanks for watching!
