Sintesi di un complesso di cobalto (II) legato ad ossigeno
English
COMPARTILHAR
Visão Geral
Fonte: Deepika Das, Tamara M. Powers, Dipartimento di Chimica, Texas A & M University
La chimica bioinorganica è il campo di studio che indaga il ruolo che i metalli svolgono in biologia. Circa la metà di tutte le proteine contiene metalli e si stima che fino a un terzo di tutte le proteine si basi su siti attivi contenenti metalli per funzionare. Le proteine che presentano metalli, chiamate metalloproteine, svolgono un ruolo vitale in una varietà di funzioni cellulari necessarie per la vita. Le metalloproteine hanno incuriosito e ispirato i chimici inorganici sintetici per decenni e molti gruppi di ricerca hanno dedicato i loro programmi alla modellazione della chimica dei siti attivi contenenti metalli nelle proteine attraverso lo studio dei composti di coordinazione.
Il trasporto di O2 è un processo vitale per gli organismi viventi. O2-metalloproteine di trasporto sono responsabili del legame, del trasporto e del rilascio di ossigeno, che può quindi essere utilizzato per processi vitali come la respirazione. Il complesso di coordinazione del cobalto che trasporta ossigeno, [N,N‘-bis (salicilaldeide) etilendiaimino] cobalto (II) [Co (salen)]2 è stato ampiamente studiato per comprendere come i complessi metallici legano reversibilmente O2. 1
In questo esperimento, sintetizzaremo [Co(salen)]2 e studieremo la sua reazione reversibile con O2 in presenza di dimetilsolfossido (DMSO). In primo luogo, quantificheremo la quantità di O2 consumata all’esposizione di [Co(salen)]2 a DMSO. Osserveremo quindi visivamente il rilascio di O2 dall’addotto [Co(salen)]2-O2 esponendo il solido a CHCl3.
Princípios
Procedimento
Resultados
Characterization of Inactive [Co(salen)]2:
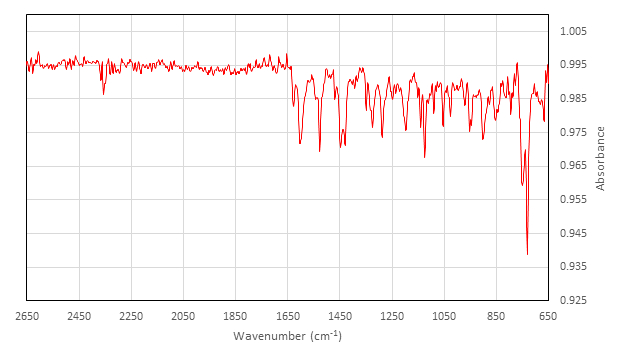
IR (cm-1) collected on ATR attachment: 2357 (w), 1626 (w), 1602 (m), 1542 (w), 1528 (m), 1454 (w), 1448 (m), 1429 (m), 1348 (w), 1327 (w), 1323 (m), 1288 (m), 1248 (w), 1236 (w), 1197 (m), 1140 (m), 1124 (m), 1089 (w), 1053 (m), 1026 (w), 970 (w), 952 (w), 947 (w), 902 (m), 878 (w), 845 (w), 813 (w), 794 (w), 750 (s), 730 (s).
O2 Uptake:
59.2 mg (0.090 mmol) of [Co(salen)]2 consumed 0.002 L of O2. Using standard pressure and the temperature recorded in step 3.6, the number of moles of O2 consumed was:

The calculated moles of Co in 0.090 mmol of [Co(salen)]2:
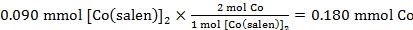
Therefore the Co:O2 ratio was:
0.180 mmol Co : 0.082 mmol O2
which is equivalent to a 2:0.91 ratio of Co to O2.
Addition of CHCl3 to [Co(salen)]2–O2 Adduct:
Upon addition of CHCl3, the CHCl3 solution turned red and a stream of bubbles was liberated from the solid, indicating release of O2 gas and formation of inactive [Co(salen)]2.
Applications and Summary
In this video, we explained the different ways that diatomic oxygen can coordinate to metal center(s). We synthesized the oxygen-carrying cobalt complex [Co(salen)]2 and studied its reversible binding with O2. Experimentally we demonstrated that inactive [Co(salen)]2 reversibly binds O2 and forms a 2:1 Co:O2 adduct in the presence of DMSO.
All vertebrates depend on hemoglobin, a metalloprotein found in red blood cells, to transport oxygen to respiratory organs as well as other tissues. In hemoglobin, oxygen reversibly binds to a heme group that features a single Fe center coordinated to a heterocyclic ring called a porphyrin (Figure 6a). Hemoglobin is not the only oxygen-carrying and storage metalloprotein. For example, mollusks possess a protein called hemocyanin, which features a dicopper active site that is responsible for oxygen transport (Figure 6b).
Using synthetic molecular species to model active sites in metalloproteins is challenging due to the distinct differences in electronic structure of a simple coordination compound compared to that of a metal surrounded by a protein superstructure. As a result, it is often difficult to precisely replicate the structure of the active site in metalloproteins. While there are examples of model complexes that structurally mimic metal active sites, there are fewer examples of structurally similar model complexes that exhibit reactivity inherent to the native metalloenzyme.
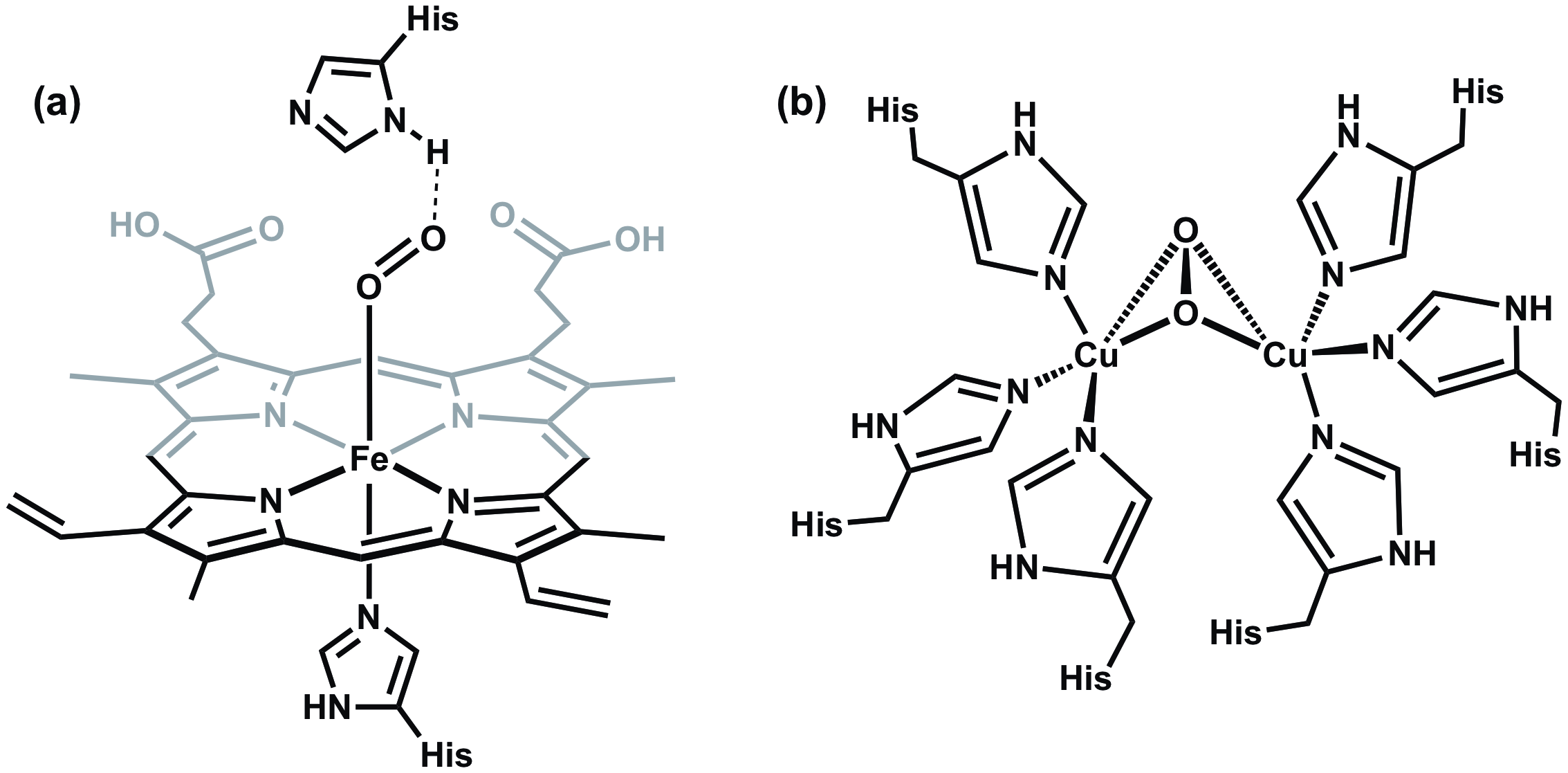
Figure 6. (a) The Fe center in hemoglobin binds to O2 in an end-on fashion, while (b) the copper containing active site in hemocyanin binds to O2 in a bridging side-on orientation.
Referências
- Niederhoffer, E. C., Timmons, J. H., Martell, A. E. Thermodynamics of Oxygen Binding in Natural and Synthetic Dioxygen Complexes. Chem Rev. 84, 137-203 (1984).
- Appleton, T. G. Oxygen uptake by cobalt(II) complex. An undergraduate experiment. J Chem Educ. 54 (7), 443 (1977).
- Ueno, K., Martell, A. E. Infrared Studies on Synthetic Oxygen Carriers. J Phys Chem.60, 1270–1275 (1956).
Transcrição
[N,N’-Bis(salicylaldehyde)ethylenediimino]cobalt(II), abbreviated [Co(salen)]2, is an organometallic complex, which is used to investigate oxygen-transporting metalloproteins.
Metalloproteins such as hemoglobin can reversibly bind O2 and to understand this mechanism, complexes such as [Co(salen)]2 are studied.
[Co(salen)]2 exists in two forms: active and inactive. The active form consists of a heterodimer, in which two cobalt centers form a very weak van-der-Waals interaction, providing enough space for insertion of molecular O2 in the solid state.
In the inactive form of [Co(salen)]2 the cobalt centers of each molecule form a dative bond with an oxygen atom on another molecule. This decreases the space between the units and molecular O2 cannot fit in anymore, unless a coordinating solvent, such as DMSO, is used, which facilitates the adduct’s stability.
This video will illustrate the principles of [Co(salen)]2, the synthesis of its inactive form, and the analysis of reversible binding to molecular O2.
Molecular O2 can coordinate to transition metal complexes in several ways: side-on, side-on bridging, end-on, and end-on bridging. In the inactive [Co(salen)]2, O2 coordinates to the two cobalt centers in an end-on bridging fashion and the coordinating DMSO completes the octahedral coordination sphere of each cobalt center generating a 2:1 complex, which can be explained by examining the molecular orbital diagram of O2 and the d-orbital splitting diagram of [Co(salen)]2.
Oxygen has two unpaired electrons in the π* molecular orbital, signifying a triplet ground state, while [Co(salen)]2 has one unpaired electron in its σ* molecular orbital.
The binding of O2 to [Co(salen)]2 is a redox reaction, in which two cobalt centers lose an electron each, and the O2 molecule gains two electrons, forming a peroxide (O22-).
The ratio of Co:O2 in a reaction can be determined by measuring the volume of O2 consumed in a closed system. Using the ideal gas law, the moles of consumed O2 can be calculated.
Furthermore, the reversibility of O2 binding can be studied by addition of CHCl3 to the product. CHCl3 is a non-coordinating solvent, which cannot stabilize the O2 adduct. Therefore, addition of CHCl3 to the [Co(salen)]2-O2 adduct leads to a decrease in concentration of DMSO and pushes the reaction in the reverse direction, resulting in liberation of O2.
Now that we have discussed the principles of [Co(salen)]2, let’s look at a procedure for the synthesis of its inactive form, and its use in consuming molecular O2.
In a fume hood, charge a clamped 250-mL three-necked flask with a stir bar, 95% ethanol and salicylaldehyde. Attach a condenser to the center neck and an addition funnel fitted with a septum on of the outer necks.
Fit the third neck of the 3-neck flask with a septum and attach a N2 line to the condenser. Under a N2 atmosphere, stir the reaction in a water bath at 80 °C, and add ethylene diamine by syringe.
In a separate 50-mL round bottom flask containing a stir bar, add Co(OAc)2·4H2O, and dissolve in 15 mL distilled water.
Once completely dissolved, transfer the cobalt acetate solution to the addition funnel, and degas by bubbling N2 through it for 10 minutes.
When degassing is complete, slowly add the cobalt acetate solution to the vigorously stirred salicylaldehyde mixture. Then stir at reflux for 1 hour.
When finished, remove the flask from the heating bath, and remove the condenser and addition funnel. Then submerge the flask in an ice-water bath to facilitate precipitation of [Co(salen)]2.
Vacuum filter the precipitate onto a Buchner funnel with filter paper, and wash the red solid with cold ethanol. Dry the solid completely, weigh it, and calculate the percent yield.
Connect a needle to an O2-gas cylinder with Tygon tubing. Then gently bubble O2 through 5 mL DMSO for 10 minutes.
Attach two 18-inch sections of Tygon tubing to either end of a graduated 10-mL glass pipette. Clamp the pipette to a ring stand with the lowest graduation facing up. Next, attach a long-stemmed glass funnel to the lower tubing piece, and clamp the funnel to the ring stand with the funnel facing up.
Make sure that the tubing connecting the pipette and the funnel form a U-shape. Add mineral oil to the funnel and tubing, until the funnel is about half-filled.
Attach a side-arm test tube to the tubing on the top of the pipette and add [Co(salen)]2 to it.
Transfer 2 mL of O2-saturated DMSO into a 3-mL test tube and, using a pair of tweezers, lower test tube B into test tube A without spilling.
Seal test tube A with a rubber septum tightened with copper wire. Insert a needle attached to the O2 tank into the septum and purge for 10 minutes. Then remove the needle and grease the top of the septum to prevent leaks.
Insert a free needle into the septum of test tube A to allow the mineral oil to reach the glass pipette, while covering the opening with a finger and slowly releasing pressure. Then remove the needle and re-cover the top of the septum with grease.
Adjust the heights of the funnel and pipette so that the oil levels line up in both pieces of glassware, and record the level of oil within the pipette.
Release the DMSO from test tube B by angling the side-arm of test tube A towards the ceiling. Once all of the DMSO has been added, hold the test tube upright and swirl it gently.
Continue to shake the test tubes until the oil level in the pipette stops rising, which means O2 is no longer being consumed. Then, adjust the height of the funnel so that the oil level in it is lined up with the oil level in the pipette. Record the new level of oil in the pipette and the temperature of the room.
Remove the septum from test tube A and transfer the contents to a 15-mL centrifuge tube. Place the tube in a centrifuge at a position opposite a tube carrying an equivalent amount of water.
Centrifuge the samples for at least 15 min, then gently remove the tube containing the [Co(salen)]2 pellet. Carefully decant the liquid without disturbing the pellet.
Hold the centrifuge tube containing the pellet at a 45º angle, and using a syringe slowly drip 1 mL of CHCl3 down the side of the tube. Observe any physical changes that occur.
Now let’s evaluate the results. The yield of the synthesized inactive [Co(salen)]2 is 2.4 g, which is 85%. The IR spectrum shows a peak at 1528 cm-1, which is indicative of the CN stretch. Furthermore, the absence of an O-H stretch indicates that no free ligand is present.
59.2 mg of [Co(salen)]2, which is equal to 0.090 mmol, consumed 2 mL of O2. Using the ideal gas law, standard pressure, and temperature recorded, the number of moles of 2 mL O2 was determined to be 0.082 mmol. Lastly, the number of mmol of Co in [Co(salen)]2 was determined, and divided by the number of mmol of O2 to obtain the ratio of Co:O2, which is 2:0.91.
Reversibility of O2 binding was demonstrated using CHCl3, where upon addition of the solvent the DMSO concentration decreased, and the reaction equilibrium shifted to the reactants, resulting in O2 release, as was observed in bubbling of the reaction and the color change to red.
Coordination complexes can be used in the field of chemistry and bioinorganic chemistry to study various metalloproteins.
For example, the metalloprotein hemoglobin is comprised of four globular protein sub units with the heme group embedded in each, making it difficult to study the protein’s active site. Synthetic inorganic chemists usemolecular species, such as [Co(salen)]2, to model active sites in metalloproteins, however, replication of structure and reactivity is often difficult, due to distinct differences in electronic structures between simple coordination compounds and metal surrounded protein superstructures.
Epichlorohydrin is a chemical reagent consisting of an epoxide and an alkyl chloride. It is used in the production of epoxy resins and other elastomers. However, despite its versatility, it is difficult to produce enantiopure epichlorohydrin.
To separate racemic mixtures of epichlorohydrin, chiral salen complexes can be used. For example, in a hydrolytic kinetic resolution of epoxides, the racemic epichlorohydrin is treated with a polystyrene-supported chiral salen ligand in the presence of water, which leads to the hydrolysis of one of the enantiomers. The enantiomer can be separated and the polymer-supported catalyst can be filtered off from the reaction mixture, and reused.
You’ve just watched JoVE’s introduction to [Co(salen)]2. You should now understand its principles, the procedure, and some of its applications. Thanks for watching!
