Polarized Translocation of Fluorescent Proteins in Xenopus Ectoderm in Response to Wnt Signaling
PREPARAÇÃO DO INSTRUTOR
CONCEITOS
PROTOCOLO DO ALUNO
1. In Vitro Fertilization of Xenopus Eggs
- Obtain eggs from female frogs that were injected with human chorionic gonadotropin (400 unit/frog) 12 hours before the experiment.
- Place eggs into a small amount (0.5-1 ml) of 1 x Marc’s modified Ringer solution (MMR)10to the eggs and fertilize them in vitro with a small piece of dissected testis. After 2-3 min, add 0.1 x MMR to cover the whole surface of the eggs. In 20 minutes, egg jelly coat is removed by 3 % cysteine – HCL (adjusted to pH 8 with sodium hydroxide). Eggs are washed with 0.1 x MMR three times and left in a cold incubator (13° C) for injections.
- Fertilized eggs are allowed to develop to 2-4 cell stage. For injections, the embryos are transferred into the solution containing 3 % Ficoll, 0.5 x MMR.
2. RNA Microinjection
- RNAs are synthesized from linearized DNA templates using mMessage mMachine kit (Ambion) and diluted with RNase-free water at the stock concentration of 0.1-1 μg/μl. Optimal doses of RNAs for injections are determined in pilot experiments. RNAs for Diversin-RFP, the membrane marker GFP-CAAX and Frizzled 8 are used at 0.1-1 ng per injection.
- Injection needles are prepared with a needle puller from a capillary and then with a needle grinder. Before injection, each needle is calibrated with water to eject 10 nl of liquid per injection.
- For injections, embryos are placed on a plastic dish into a large droplet of 3 % Ficoll, 0.5 x MMR. One microliter of RNA solution is sucked into an injection needle with Narishige microinjector. 10 nl of RNA is injected into animal blastomeres of 8 cell embryos 2-3 times. The injected embryos are transferred into a well of the 12-well plate.
3. Preparing Ectodermal Explants
- When the injected embryos reach early gastrula stage, they are transferred into 0.6 x MMR solution in a 3 cm plastic dish coated with 1 % agarose. Vitelline membrane is removed manually with a pair of forceps. Ectodermal explants are excised from the embryos using a Tungsten needle and a hairloop.
- Ectodermal explants are transferred into a glass vial and fixed with 3.7 % formaldehyde in phosphate buffered saline (PBS) for 30 minutes. Fixed explants are washed with PBS three times (10 minutes each). DAPI is included into the third wash to stain nuclei.
- Explants are mounted on a slide glass. Two strips of scotch tape are attached to the slide and the explants are placed between the two strips. Since the outer surface of explants is pigmented, the inner side of the explants should face the microscope objective. Add two-three 20 μl drops of the mounting solution (70 % glycerol in PBS including 25 mg/ml DABCO, anti-fading reagent)11. Put a coverslip on top.
4. Imaging of Explants Under a Fluorescent Microscope
- Samples are viewed under Zeiss Axioplan fluorescent microscope with appropriate filters.
- Images are taken with the Apotome attachment to visualize a specific plane from several independent explants.
5. Cryosectioning
Cryosectioning is an alternative way to visualize the distribution of fluorescent proteins in the cell and more applicable for immunostaining. At stage 10, embryos are fixed for 1-2 hours with Dent’s fixative (20% DMSO, 80% methanol), washed with PBS, and embedded in 15 % fish gelatin/15 % sucrose solution11. The embedded embryos are quickly frozen on dry ice and cryosections are generated on Leica Cryostat. Cross sections would include ectodermal cells that inherit injected RNAs and their translated protein products. The sections retain fluorescence and can be immunostained with specific antibodies and then labeled with secondary antibodies conjugated with fluorescence. Nuclei are stained with DAPI. The mounting media are the same as described above. Imaging can be performed as described above.
6. Representative Results:
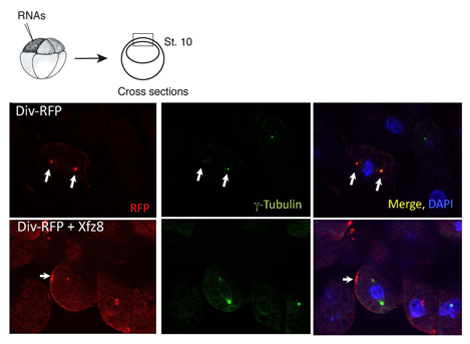
Figure 1. Frizzled receptor recruits Diversin to the cell membrane. Ectoderm cells expressing Frizzled 8 (Xfz8) and Div-RFP RNAs reveal Div-RFP at the cell membrane, instead of the centrosome (as revealed by g-tubulin co-staining). The scheme of the experiment is at the top; a typical cross-section is shown.
Polarized Translocation of Fluorescent Proteins in Xenopus Ectoderm in Response to Wnt Signaling
Learning Objectives
Preparação do Laboratório
Cell polarity is a fundamental property of eukaryotic cells that is dynamically regulated by both intrinsic and extrinsic factors during embryonic development 1, 2. One of the signaling pathways involved in this regulation is the Wnt pathway, which is used many times during embryogenesis and critical for human disease3, 4, 5. Multiple molecular components of this pathway coordinately regulate signaling in a spatially-restricted manner, but the underlying mechanisms are not fully understood. Xenopus embryonic epithelial cells is an excellent system to study subcellular localization of various signaling proteins. Fluorescent fusion proteins are expressed in Xenopus embryos by RNA microinjection, ectodermal explants are prepared and protein localization is evaluated by epifluorescence. In this experimental protocol we describe how subcellular localization of Diversin, a cytoplasmic protein that has been implicated in signaling and cell polarity determination6, 7 is visualized in Xenopus ectodermal cells to study Wnt signal transduction8. Coexpression of a Wnt ligand or a Frizzled receptor alters the distribution of Diversin fused with red fluorescent protein, RFP, and recruits it to the cell membrane in a polarized fashion 8, 9. This ex vivo protocol should be a useful addition to in vitro studies of cultured mammalian cells, in which spatial control of signaling differs from that of the intact tissue and is much more difficult to analyze.
Cell polarity is a fundamental property of eukaryotic cells that is dynamically regulated by both intrinsic and extrinsic factors during embryonic development 1, 2. One of the signaling pathways involved in this regulation is the Wnt pathway, which is used many times during embryogenesis and critical for human disease3, 4, 5. Multiple molecular components of this pathway coordinately regulate signaling in a spatially-restricted manner, but the underlying mechanisms are not fully understood. Xenopus embryonic epithelial cells is an excellent system to study subcellular localization of various signaling proteins. Fluorescent fusion proteins are expressed in Xenopus embryos by RNA microinjection, ectodermal explants are prepared and protein localization is evaluated by epifluorescence. In this experimental protocol we describe how subcellular localization of Diversin, a cytoplasmic protein that has been implicated in signaling and cell polarity determination6, 7 is visualized in Xenopus ectodermal cells to study Wnt signal transduction8. Coexpression of a Wnt ligand or a Frizzled receptor alters the distribution of Diversin fused with red fluorescent protein, RFP, and recruits it to the cell membrane in a polarized fashion 8, 9. This ex vivo protocol should be a useful addition to in vitro studies of cultured mammalian cells, in which spatial control of signaling differs from that of the intact tissue and is much more difficult to analyze.
Procedimento
Cell polarity is a fundamental property of eukaryotic cells that is dynamically regulated by both intrinsic and extrinsic factors during embryonic development 1, 2. One of the signaling pathways involved in this regulation is the Wnt pathway, which is used many times during embryogenesis and critical for human disease3, 4, 5. Multiple molecular components of this pathway coordinately regulate signaling in a spatially-restricted manner, but the underlying mechanisms are not fully understood. Xenopus embryonic epithelial cells is an excellent system to study subcellular localization of various signaling proteins. Fluorescent fusion proteins are expressed in Xenopus embryos by RNA microinjection, ectodermal explants are prepared and protein localization is evaluated by epifluorescence. In this experimental protocol we describe how subcellular localization of Diversin, a cytoplasmic protein that has been implicated in signaling and cell polarity determination6, 7 is visualized in Xenopus ectodermal cells to study Wnt signal transduction8. Coexpression of a Wnt ligand or a Frizzled receptor alters the distribution of Diversin fused with red fluorescent protein, RFP, and recruits it to the cell membrane in a polarized fashion 8, 9. This ex vivo protocol should be a useful addition to in vitro studies of cultured mammalian cells, in which spatial control of signaling differs from that of the intact tissue and is much more difficult to analyze.
