A Rapid and Specific Microplate Assay for the Determination of Intra- and Extracellular Ascorbate in Cultured Cells
PREPARAÇÃO DO INSTRUTOR
CONCEITOS
PROTOCOLO DO ALUNO
1. Determine Intracellular Ascorbate in Cultured Cells
- Cell culture and harvesting
- Grow suspension (e.g. human erythroleukemia, K562) or adherent cells (e.g. primary astrocytes) using standard culture procedures19-21,32,38. Note: to ensure cells contain ascorbate, load cultured cells with ascorbate either as ascorbate or DHA33,39.
- Create an ascorbate-containing cellular extract
- Incubate an appropriate number of phosphate-buffered saline (PBS)-washed suspension or adherent cells with 450 μl of ice-cold Cell Permeabilization Buffer [CPB; 0.1% (w/v) saponin in PBS; 4 °C]. Note: Determine the number of cells required to obtain an absorbance value within the linear range of the assay (see below) empirically by the end-user. Note: tissue extracts may also be used (see Discussion for further details).
- Agitate cells on ice for 10 min to ensure thorough cellular lysis.
- Create a clarified ascorbate-containing intracellular extract by removing cellular debris by centrifugation of the crude lysate at 16,000 × g for 5 min in a refrigerated microcentrifuge (4 °C).
- Carefully remove 4 x 100 μl aliquots from each sample and add to a horizontal sequence of wells in a 96-well flat bottom plate that contains either 25 μl/well of PBS ("-AO") only or 25 μl/well of 45.5 U/ml stock solution of L-ascorbate-oxidase (AO) in PBS ("+AO"). Note: this should be done in parallel with the preparation of the standards (see below).
- Ascorbate standard-curve construction (must be constructed anew for each assay)
- Prepare a stock solution of 10 mM ascorbic acid in ice-cold PBS. Note: Verify the concentration of ascorbate spectrophotometrically using a quartz cuvette with a 1 cm path-length at 265 nm (extinction coefficient = 14.5 mM-1 cm-1)40.
- Carefully prepare a series of ascorbate standards between 0 and 20 μM.
- In parallel with step 1.2.4, carefully remove 4 × 100 μl aliquots from each standard and add to a horizontal sequence of wells in a 96-well flat bottom plate that contains 25 μl/well of PBS ("-AO") or a 45.5 U/ml stock solution of AO in PBS ("+AO"). Note: 100 μl of the above ascorbate standards will contain 0-2 nmole of ascorbate. Please note, the purpose of the AO is to control for the contribution of non-ascorbate reductants to ferricyanide reduction.
- Determine intracellular ascorbate – Step 1: selectively remove ascorbate and quantitatively oxidize ascorbate with ferricyanide
- Orbitally mix the 96-well plate at 550 rpm at room temperature for 5 min in the dark by covering the plate in foil. Note: This step should oxidize all ascorbate in the "+ AO" wells to DHA. The "-AO" wells should be unaffected.
- Add 50 μl of 3.5 mM potassium ferricyanide (herein referred to as "ferricyanide") in PBS to all wells. The final [ferricyanide] = 1 mM. Note: Use a multipipettor.
- Orbitally mix the plate at 550 rpm at room temperature for another 5 min in the dark. Note: This step should result in the reduction of ferricyanide to ferrocyanide by ascorbate in a stoichiometric ratio of 2 molecules of ferricyanide reduced per 1 molecule of ascorbate oxidized.
- Immediately add 25 μl of a freshly constructed solution containing 50% (v/v) acetic acid and 30% (w/v) trichloroacetic acid (TCA). Note: Use a multipipettor.
- Determine intracellular ascorbate – Step 2: quantitate the amount of ferrocyanide formed37
- Add 100 μl of ferrocyanide determination solution37 to each well. Note: A working solution should be made immediately before use: 2 ml of 3 M Na-acetate (pH 6.0); 0.5 ml of glacial acetic acid (~17.4 M acetic acid); 2 ml of 0.2 M citric acid; 2 ml of 3.3 mM FeCl3 in 0.1 M acetic acid; 1 ml of 30 mM ferene-S. The final volume of this working solution should be 7.5 ml.
- Orbitally mix the plate in the dark at 550 rpm for 30 min at room temperature.
- Read the absorbance values of the wells at 593 nm (i.e. the absorbance maximum of the Fe(II)(ferene-S)3 complex).
- Calculate the amount of intracellular ascorbate as nmoles ascorbate per million cells by initially subtracting the A593 nm values for the '+ AO' wells from the corresponding '- AO' wells for each sample and then interpolating from the ascorbate standard curve (see step 1.3). When constructing the standard curve, plot this "difference value" for each standard against the amount of ascorbate per well.
2. Determination of Ascorbate-efflux from Cultured Cells
- Cell culture and harvesting
- Grow suspension or adherent cells as above (see step 1.1). Note: carry out the below assay in a 24-well plat format with suspension and adherent cells for best results.
- Resuspend suspension cells, or overlay adherent cells, with 400 μl of pre-warmed HEPES-buffered saline solution, with or without calcium and magnesium, and containing 5 mM D-glucose (HBS/D; pH 7.3, 37 °C) in wells of a 24-well plate. Add the same volume of HBS/D to specified cell-free wells on each 24-well plate to be examined. Note: the latter will serve as cell-free controls for the baseline reaction (see below).
3. Determine the Amount of Ascorbate Released
- The following stock solutions should be prepared beforehand: 120 U/ml AO in HBS/D (prepare fresh); 2.4 mM Ferene-S in HBS/D; 120 μM FeCl3 and 600 μM Na-citrate in HBS/D (prepare immediately from more concentrated stock solutions).
- To start the ferrireduction reaction (final volume per well should be 600 μl), add the following volumes of reagents to individual wells already containing 400 μl of HBS/D:
- Add 50 μl of AO (120 U/ml) or HBS/D to paired wells in triplicate. The final AO concentration should be 10 U/ml. Note: Label paired wells as "- AO" and "+ AO".
- Mix the plates with gentle orbital mixing for 5 min at 37 °C.
- Add 50 μl of 2.4 mM ferene-S to all wells. The final ferene-S concentration should be 200 μM. Mix the plates as above for 5 min at 37 °C.
- Add 50 μl of freshly prepared 120 μM ferric citrate. The final iron and citrate concentrations should be 10 μM iron and 50 μM citrate. Mix well.
- In three triplicate control wells, aspirate the overlying medium and add 600 μl HBS/D containing 0.1% saponin. Note: These will serve as "100% cell-lysis" controls for a lactate dehydrogenase (LDH) release assay to be conducted in parallel (see below).
- Incubate for 60 min in the dark at 37 °C.
- At the end of the ascorbate-efflux assay, rapidly aspirate 500 μl from each well and add to appropriately labelled wells in a 24-well plate. Note: for suspension cells, initially remove the cells by centrifugation at 4 °C.
- Add 300 μl aliquots of the supernatant to a 96-well plate and then read at 593 nm.
4. Determination of Extracellular Ascorbate
- Read the absorbance values of the wells at 593 nm.
- Calculate the amount of extracellular ascorbate as nmoles ascorbate per mg protein (or per million cells) as described for the determination of intracellular ascorbate in Step 1.5.4. Note: the end user should optimize conditions so that ascorbate release is not limited by intracellular ascorbate.
5. Determination of LDH Release
- With the remaining 200 μl of extracellular solution from each sample conduct an LDH release assay32,41 to determine the extent of cellular lysis. Note: calculate as a % of total releasable LDH. Determine releasable LDH from the samples that were treated with 0.1% saponin.
A Rapid and Specific Microplate Assay for the Determination of Intra- and Extracellular Ascorbate in Cultured Cells
Learning Objectives
Determination of Intracellular Ascorbate in Cultured Suspension Cells
In the first assay (Figure 1), intracellular ascorbate is determined, following ascorbate-specific (i.e. AO-sensitive) reduction of ferricyanide to ferrocyanide, using the highly sensitive determination of ferrocyanide by a previously published procedure37. The detection of ascorbate is based on the colorimetric chelation of ferrous iron that is generated by the ascorbate-dependent reduction of ferricyanide to ferrocyanide, followed by the reduction of ferric to ferrous iron by ferrocyanide. As supraphysiological concentrations of some divalent metal ions (e.g. Cu2+, Co2+ and Zn2+) can interfere, presumably by competing with ferrous iron for chelation by ferene-S, appropriate precautions should be taken under such conditions37.
Figure 2 shows a typical standard curve for a set of ascorbate standards 0-20 μM (or 0-2 nmole ascorbate per well of the 96-well plate; see step 6.5 in the "Protocol".). Although not shown in this figure, linearity is maintained up to 8 nmole ascorbate per well (i.e. a net A593 nm of ~1.6), which corresponds to a sample ascorbate concentration of ~80 μM. The assay can be used to successfully detect ascorbate levels below 0.25 nmole ascorbate per well (2.5 μM sample ascorbate concentration).
K562 cells rapidly accumulate intracellular ascorbate from extracellular DHA, but not ascorbate (see Figure 3; reproduced with permission from Lane and Lawen 200819). In this representative experiment, PBS-washed K562 cells (4 × 106 cells/ml) were incubated in PBS with 500 μM of either ascorbate (Asc), DHA, DHA + 50 μM cytochalasin B (DHA+CB), Asc + 5 mM ferricyanide (Asc/FIC) or Asc + 50 U/ml AO (Asc/AO) for 30 min at 37 °C. Intracellular ascorbate was determined as described in the "Protocol".
DHA uptake by K562 cells occurs by facilitative glucose transporter (GLUT)-mediated transport (see Figure 4; reproduced with permission from Lane and Lawen 200942). To assess the involvement of GLUTs in DHA uptake in this representative experiment, K562 cells were incubated with increasing concentrations of cytochalasin B (CB) or dihydrocytochalasin B (H2CB) dissolved in MBS containing 0.5% ethanol for 15 min at 37 °C prior to incubation with 400 μM DHA for 30 min at 37 °C in the same medium (Fig. 4A). Cells were then washed three times in 100 volumes of ice-cold MBS and their intracellular ascorbate determined as described in the “Protocol”. It is important to note while both CB and H2CB inhibit motile processes at micromolar concentrations (1-100 μM), only CB, which differs from H2CB by the presence of single double bond, inhibits GLUT-dependent transport at low micromolar concentrations (1-10 μM)43. Therefore, as DHA uptake was inhibitable by CB with an IC50 < 2.5 μM, but was not inhibitable by H2CB, DHA uptake probably occurs by GLUT-mediated transport38. Alternatively, in Figure 4B, washed cells were exposed to increasing concentrations of the GLUT-transportable, but non-metabolizable glucose analog 3-O-methyl-D-glucose (3-OMG) or the non-GLUT-transportable glucose stereoisomer L-glucose prior to incubation with DHA as in panel A. Again the results indicate GLUT involvement in DHA import as inhibition of intracellular ascorbate accumulation occurs only in the presence of the GLUT-transportable glucose analog.
Determination of Ascorbate-Efflux from Adherent Cells
In the second assay, the rate of ascorbate-efflux from cultured cells can be determined. This specific assay is important as the release of ascorbate from cells appears to be crucial for the ascorbate-regulated cellular uptake of non-transferrin-bound iron by cells19,20. The uptake of non-transferrin-bound iron is considered to be relevant to the pathophysiology of iron-overload disorders such as the hemochromatoses22, as well as to astrocyte-neuron iron exchange and homeostasis in the mammalian brain22. Indeed, we have recently shown that the major excitatory neurotransmitter, L-glutamate, triggers the release of ascorbate from astrocytes in a manner that depends on L-glutamate uptake by GLAST and subsequent cellular swelling that triggers the release of ascorbate by putative VSOACs32. In analogy, ascorbate release from ascorbate-loaded astrocytes can be stimulated by the excitatory amino acid, L-aspartate, but not the non-excitatory amino acid L-glutamine. This effect is depicted in Figure 5 (reproduced with permission from Lane and Lawen 201232), which shows dose-response curves for the stimulation of ascorbate (AA) release by aspartate (closed circles) and glutamine (open circles) from primary cultures of ascorbate-loaded mouse astrocytes.
It is important to discuss how this ascorbate-efflux assay differs from the assay provided for the determination of intracellular ascorbate. The major difference lies in the fact that the level of ascorbate remaining in solution is not determined by a chemical reaction conducted at the end of a given time point, as is the case for the intracellular ascorbate determination method. Instead, a "reductive signature" is accumulated during the period in which ascorbate is released from cells. This reductive signature is captured in the form of the ascorbate-dependent reduction of extracellular ferric citrate followed by the rapid chelation of the ferrous iron as a large extracellular and membrane-impermeant [Fe(II)(ferene-S)3]4- chelate. Ferene-S can be considered similarly membrane-impermeant over the short time course of the assay. The levels of this chromogenic complex can then be determined as an endpoint measurement. As only the AO-sensitive levels of this chelate are determined, the assay provides a high-degree of specificity for determination of extracellular L-ascorbate.
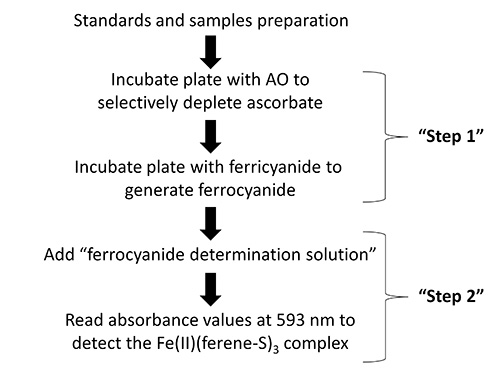
Figure 1. Flow diagram showing the key steps in the protocol for the determination of intracellular ascorbate.
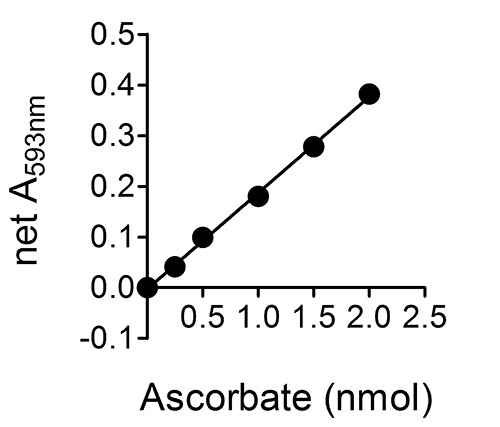
Figure 2. A typical standard curve for a set of ascorbate standards 0-20 μM (or 0-2 nmole ascorbate per well of the 96-well plate; see step 6.5 in the "Protocol".). Error bars are not shown as they are within the range occupied by the symbols.
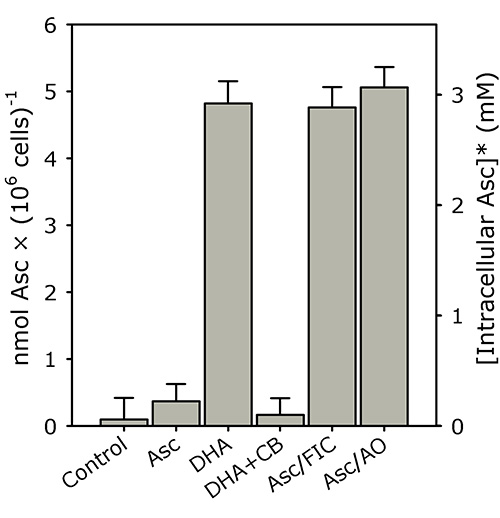
Figure 3. K562 cells rapidly accumulate intracellular ascorbate from extracellular DHA, but not ascorbate. PBS-washed K562 cells (4 × 106 cells/ml) were incubated in PBS with 500 μM of either ascorbate (Asc), DHA, DHA + 50 μM cytochalasin B (DHA+CB), Asc + 5 mM ferricyanide (Asc/FIC) or Asc + 50 U/ml AO (Asc/AO) for 30 min at 37 °C. Intracellular ascorbate was determined as described in the "Protocol". The results shown are means of three individual experiments (+ SD). *The intracellular ascorbate concentration was estimated using a pre-determined intracellular water space for K562 cells (i.e. ~1.6 μl/106 cells)19,20. This figure has been reproduced with permission from Lane and Lawen 200819.
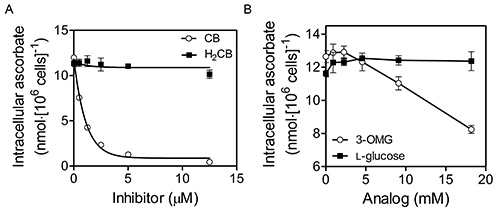
Figure 4. DHA uptake by K562 cells occurs by facilitative glucose transporter (GLUT)-mediated transport. K562 cells that had been grown to 6-8 × 106 cells/ml in RPMI + 10% (v/v) fetal bovine serum at 37 °C, 5% CO2 and 95% air were initially washed three times with MBS. Washed cells were then exposed to increasing concentrations of cytochalasin B (CB; Sigma) or dihydrocytochalasin B (H2CB) dissolved in Mops-buffered saline (MBS; 137 mM NaCl, 2.7 mM KCl, 15 mM MOPS-Na+, pH 7.3) containing 0.5% ethanol prior to incubation with 400 μM DHA for 30 min at 37 °C (A). Cells were then washed three times in 100 volumes of cold MBS and their intracellular ascorbate determined as described in the “Protocol”. As DHA uptake was inhibitable by CB, but not H2CB, DHA uptake occurs by GLUT-mediated transport. Alternatively, in (B), washed cells were exposed to increasing concentrations of the GLUT-transportable, but non-metabolizable glucose analog 3-O-methyl-D-glucose (3-OMG) or the non-GLUT-transportable glucose stereoisomer L-glucose during incubation with DHA as in (A). Again the results indicate GLUT involvement in DHA uptake. This figure has been reproduced with permission from Lane and Lawen 200842.
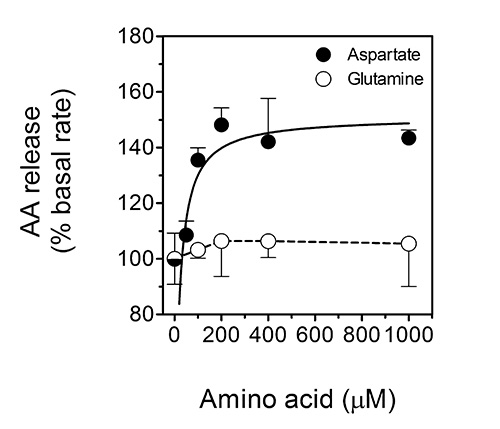
Figure 5. Ascorbate release from ascorbate-loaded astrocytes is stimulated by the excitatory amino acid L-aspartate, but not the non-excitatory amino acid L-glutamine. This figure shows dose-response curves for the stimulation of ascorbate (AA) release by aspartate (closed circles) and glutamine (open circles) from primary cultures of ascorbate-loaded mouse astrocytes. The data shown are means (± SD) of three experiments. P < 0.001 vs. the 'basal' condition. This figure has been reproduced with permission from Lane and Lawen 201232.
List of Materials
| Nunc 96-well flat-bottom plates | Thermo | 269620 | Any flat-bottom 96-well plate can be used |
| Refrigerated benchtop microcentrifuge | Eppendorf | 5415D | A non-refrigerated microcentrifuge that has been equilibrated to temperature in a cold room can also be used |
| Refrigerated bench-top centrifuge | Eppendorf | 5810R | Swing-bucket |
| Bio-Rad Benchmark Plus Microplate Spectrophotometer | Bio-Rad | Any microplate spectrophotometer capable of reading at 593 nm can be used and is recommended. If a filter-based plate reader is used, choose the closest wavelength possible and use the standard-curve method. | |
| Ependorf MixMate (microplate orbital mixer) | Eppendorf | This is a very versatile and reliable microplate mixer and works very well for these assays | |
| General-purpose buffers | |||
| Phosphate-buffered saline (PBS), pH 7.4 | |||
| MOPS-buffered saline (MBS); 137 mM NaCl, 2.7 mM KCl, 15 mM MOPS-Na+, pH 7.3 | |||
| MBS + 5 mM D-glucose (MBS/D) | |||
| HEPES-buffered saline + 5 mM D-glucose (HBS/D); 137 mM NaCl, 5.2 mM KCl, 1.8 mM CaCl2•2 H2O, 0.8 mM MgSO4•7 H2O, 5 mM D-glucose, 20 mM HEPES-Na+, pH 7.3) | |||
| Cell permeabilisation buffer (CPB; 0.1% saponin in PBS) | |||
| General chemicals | |||
| L-ascorbic acid or sodium L-ascorbate | Sigma-Aldrich | Highest purity preparations should be obtained | |
| Dehydro-L-ascorbic acid (DHA) dimer | Sigma-Aldrich | 30790 | Aqueous solutions theoretically yield 2 moles of DHA monomer per mole of DHA dimer |
| Cytochalasin B | Sigma-Aldrich | C6762 | Stock solutions prepared in DMSO or ethanol |
| Ascorbate oxidase (AO) | Sigma-Aldrich | A0157 | Stock solutions (120 U/ml) can be prepared in PBS or MBS and then frozen in aliquots |
| Potassium ferricyanide (FIC) | Sigma-Aldrich | 455989 | Trihydrate |
| Ferene-S (3-(2-Pyridyl)-5,6-di(2-furyl)-1,2,4-triazine-5′,5′′-disulfonic acid disodium salt) | Sigma-Aldrich | 92940 | |
| Sodium L-glutamate | Sigma-Aldrich | ||
| L-glutamine | Sigma-Aldrich | ||
| Saponin | Sigma-Aldrich | 47036 | Prepare a 0.1% stock solution |
| Stock solutions for intracellular ascorbate determination assay | |||
| 3 M sodium acetate (pH 6.0) | |||
| Glacial acetic acid | |||
| 0.2 M citric acid | |||
| 3.3 mM FeCl3 in 0.1 M acetic acid | |||
| 30 mM ferene-S | |||
| 50% (v/v) acetic acid + 30% (w/v) trichloroacetic acid (TCA) | |||
| Stock solutions for ascorbate-efflux assay | |||
| AO (120 U/ml) | |||
| 2.4 mM ferene-S | |||
| 0.12 mM FeCl3 in 0.6 mM sodium-citrate |
Preparação do Laboratório
Vitamin C (ascorbate) plays numerous important roles in cellular metabolism, many of which have only come to light in recent years. For instance, within the brain, ascorbate acts in a neuroprotective and neuromodulatory manner that involves ascorbate cycling between neurons and vicinal astrocytes – a relationship that appears to be crucial for brain ascorbate homeostasis. Additionally, emerging evidence strongly suggests that ascorbate has a greatly expanded role in regulating cellular and systemic iron metabolism than is classically recognized. The increasing recognition of the integral role of ascorbate in normal and deregulated cellular and organismal physiology demands a range of medium-throughput and high-sensitivity analytic techniques that can be executed without the need for highly expensive specialist equipment. Here we provide explicit instructions for a medium-throughput, specific and relatively inexpensive microplate assay for the determination of both intra- and extracellular ascorbate in cell culture.
Vitamin C (ascorbate) plays numerous important roles in cellular metabolism, many of which have only come to light in recent years. For instance, within the brain, ascorbate acts in a neuroprotective and neuromodulatory manner that involves ascorbate cycling between neurons and vicinal astrocytes – a relationship that appears to be crucial for brain ascorbate homeostasis. Additionally, emerging evidence strongly suggests that ascorbate has a greatly expanded role in regulating cellular and systemic iron metabolism than is classically recognized. The increasing recognition of the integral role of ascorbate in normal and deregulated cellular and organismal physiology demands a range of medium-throughput and high-sensitivity analytic techniques that can be executed without the need for highly expensive specialist equipment. Here we provide explicit instructions for a medium-throughput, specific and relatively inexpensive microplate assay for the determination of both intra- and extracellular ascorbate in cell culture.
Procedimento
Vitamin C (ascorbate) plays numerous important roles in cellular metabolism, many of which have only come to light in recent years. For instance, within the brain, ascorbate acts in a neuroprotective and neuromodulatory manner that involves ascorbate cycling between neurons and vicinal astrocytes – a relationship that appears to be crucial for brain ascorbate homeostasis. Additionally, emerging evidence strongly suggests that ascorbate has a greatly expanded role in regulating cellular and systemic iron metabolism than is classically recognized. The increasing recognition of the integral role of ascorbate in normal and deregulated cellular and organismal physiology demands a range of medium-throughput and high-sensitivity analytic techniques that can be executed without the need for highly expensive specialist equipment. Here we provide explicit instructions for a medium-throughput, specific and relatively inexpensive microplate assay for the determination of both intra- and extracellular ascorbate in cell culture.
