Montagem de um sistema de refluxo para reações químicas aquecidas
English
COMPARTILHAR
Visão Geral
Fonte: Laboratório do Dr. Philip Miller — Imperial College London
Muitos experimentos químicos requerem temperaturas elevadas antes de qualquer reação ser observada, no entanto, soluções de aquecimento de reagentes podem levar à perda de reagentes e/ou solvente via evaporação se seus pontos de ebulição forem suficientemente baixos. A fim de garantir a perda de reagentes ou solventes, um sistema de refluxo é usado para condensar quaisquer vapores produzidos no aquecimento e devolver esses condensados ao vaso de reação.
Princípios
Procedimento
Resultados
The outcome can be observed after spectroscopic characterization of the resultant solution, as the two reagents should now have reacted to form a new product. Typically, various purification strategies will be required to separate the desired product from undesired side reactions.
In this example, a transesterification reaction between dimethyl terephthalate (DMT) and ethylene glycol has occurred to afford bis(2-hydroxyethyl) terephthalate and methanol (Scheme 1). The refluxing solvent will be the methanol that is being produced (b.p. 65 °C). After heating the starting material (Figure 1) under reflux for 45 min, nuclear magnetic resonance (NMR) spectroscopy can be used to ensure product formation, as shown in Figure 2.

Scheme 1. Transesterification reaction between dimethyl terephthalate and ethylene glycol.
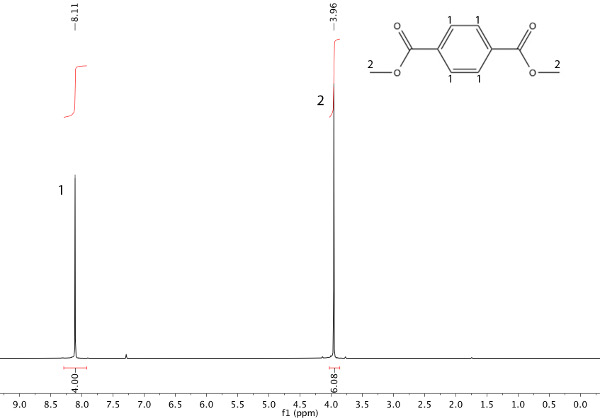
Figure 1. 1H NMR spectrum of starting material: dimethyl terephthalate (DMT).
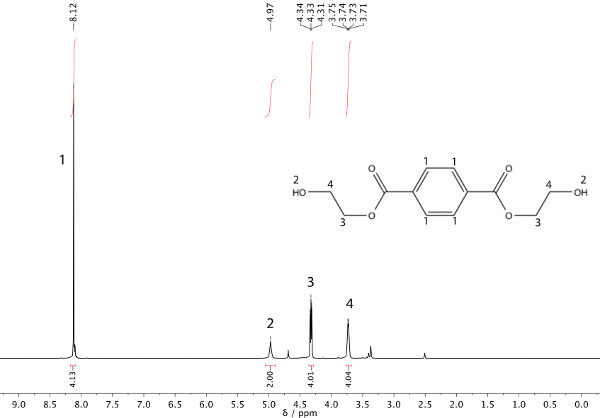
Figure 2. 1H NMR spectrum of product: bis(2-hydroxyethyl) terephthalate.
Applications and Summary
Performing reactions under reflux is an important technique to understand. Apart from providing a system whereby solvent and volatile reagents are recycled, it also allows fine control of reaction temperature, as this will be held constant at the boiling point of the chosen solvent. By careful choice of solvent, one can control the temperature within a very narrow range.
More advanced techniques can utilize refluxing solvents to perform sophisticated purification techniques such as Soxhlet extractions or fractional distillation. The later of which is used industrially on a huge scale, for instance in petroleum refineries in order to separate crude oil into various petrol fractions of different boiling points.
Referências
- Krell, E. Handbook of Laboratory Distillation. Berlin: Elsevier (1982).
Transcrição
A reflux condenser is an apparatus commonly used in organic chemistry to prevent reactant or solvent loss in a heated chemical reaction.
For chemical reactions that need to be carried out at elevated temperatures over long periods of time a reflux system can be used to prevent the loss of solvent through evaporation. Here, a cool water condenser is used to cool and return vaporized solvent and reactant back to the reaction vessel resulting in their conservation over time. This also ensures the reaction will be held at a constant temperature, as the chosen solvent will have a known, stable boiling point.
This video will explain the basics of a reflux experiment and demonstrate how to perform the technique in the laboratory with appropriate glassware and equipment.
The Arrhenius equation states that by increasing the temperature of a reaction, the reaction rate increases.
A reflux system operates under the dynamic balance between the evaporation and condensation rates of the solvent, reactant, and product molecules within the flask. The condenser is continually flushed with cold water and the round bottom flask is then placed into a heated bath. Upon heating, the solution evaporates and the condenser column cools the vapor molecules.
The vapor is condensed on the internal glass sidewall and then returns back down to the reaction flask as liquid condensate. If the vapor condenses too high in the condenser loss of solvent can occur and the flow rate of cold water must be increased. As time progresses and the reaction proceeds, all vaporized species are recovered and no loss occurs among the reactants, solvents, or products within the flask. For this protocol the entire reaction setup should be performed in a well-ventilated chemical hood with access to a nearby cold water source.
Now that you understand the basics of reflux let’s see how to setup and perform a simple transesterification reaction under heat and reflux conditions with the appropriate glassware.
Before performing the procedure inspect all glassware for signs of possible chemical contaminants from previous reactions. Eliminate all moisture by drying the glassware in an oven for 30 min. Remove the glassware once it has cooled to room temperature.
Next, apply a small amount of acetone to a clean lab tissue and wipe all ground-glass joints to remove chemical- and particle-contaminants. The clean flask and condenser column are now ready to be assembled into a reflux system. With a suitable solvent dissolve the chemical reagents inside the round-bottom flask. After adding a magnetic stir bar to the flask, connect the reflux condenser by joining the ground-glass ports of the glassware. Attach a Keck clip to the joint. Connect a tube between the cold-water source and the bottom port of the condenser column. Then, make another tube connection between the top of the condenser column and the lab sink. Finally, turn on the water slowly and fill the condenser column with circulating cold water. Adjust the water flow to prevent over-pressurizing the tube connections.
To complete the reflux setup, submerge the reaction vessel into a heating bath. Depending on the desired temperature range, these are filled with water or oil. For optimal heating, the level of the bath should be just above the meniscus of the reactants inside the flask.
Secure the condenser and flask combination in place using a ring stand and clamps with bosses. Begin the reaction by turning on the stirrer and hotplate. Heat the bath to approximately 15 °C above the boiling point of the solvent. Once equilibrium between evaporation and condensation has been reached a steady drip of condensed solvent will start falling back into the reaction vessel from the condenser column. When the chemical reaction is complete turn off the hot plate and re-clamp the apparatus higher up the ring stand. Allow cold water to continue circulating throughout the condenser until the setup has cooled to room temperature.
Then, turn off the cold-water source and disconnect the condenser from the reaction flask. To complete the disassembly empty any remaining water in the condenser into the sink, and remove all tubing from the glass column.
In this example, dimethyl terephthalate and ethylene glycol were refluxed to produce bis(2-hydroxyethyl) terephthalate and methanol as a byproduct. Due to its low boiling point the methanol acted as the refluxing solvent. In this transesterification reaction heating the mixture at 65 °C for 45 min ensured visible product formation upon NMR spectroscopy. For more information, see this collection’s video on NMR.
Applying controlled heat is a common requirement in a wide range of chemical reactions.
In this example, precise control over the composition, size, and electrical conductivity of semiconductor nanocrystals required precise chemical synthesis conditions. For the desired crystal conditions, the synthesis was performed at 370 °C. The condenser column prevented loss due to evaporation. By tailoring the reaction conditions, a collection of semiconductor nanocrystals exhibiting different symmetries were synthesized and placed in proximity with each other to create heterostructures that can manipulate photons at a nanoscale level. In another example, magnetic nanocluster particles were also synthesized using heated chemical reactions under reflux conditions. These nanoparticles’ magnetic and plasmonic properties aid in biomedical imaging.
The harsh reaction conditions were mitigated through a reflux setup.
Finally, reflux condensers can be used in a wide range of chemical reactions. In the Heck reaction, an unsaturated halide and an alkene are heated to form a substituted alkene.
Once again, the setup for the Heck reaction was similar to the previous examples, where the condenser – round-bottom flask combination was placed into a heated bath.
When combined with a palladium-containing organic catalyst, the Heck reaction can be useful in the syntheses of many pharmaceutical compounds.
You’ve just watched JoVE’s introduction to setting up a reflux system to be used in heated chemical reactions. You should now understand the underlying theory between the balance of evaporation and condensation and how to choose and assemble the appropriate glassware for your reflux reaction.
Thanks for watching!
