Author Spotlight: Exploring Microglial Interaction and Stress-Response Circuitry Using the Limited Bedding and Nesting Model
Summary
This protocol describes an animal model for studying how early-life adversity, provoked by an impoverished environment and unpredictable maternal care during the early postnatal period, affects brain development and the future risk of mental disorders.
Abstract
Early-life adversity (ELA), such as abuse, neglect, lack of resources, and an unpredictable home environment, is a known risk factor for developing neuropsychiatric disorders such as depression. Animal models for ELA have been used to study the impact of chronic stress on brain development, and typically rely on manipulating the quality and/or quantity of maternal care, as this is the major source of early-life experiences in mammals, including humans. Here, a detailed protocol for employing the Limited Bedding and Nesting (LBN) model in mice is provided. This model mimics a low-resource environment, which provokes fragmented and unpredictable patterns of maternal care during a critical developmental window (postnatal days 2-9) by limiting the amount of nesting materials given to the dam to build a nest for her pups and separating the mice from the bedding via a mesh platform in the cage. Representative data are provided to illustrate the changes in maternal behavior, as well as the diminished pup weights and long-term changes in basal corticosterone levels, that result from the LBN model. As adults, offspring reared in the LBN environment have been shown to exhibit an aberrant stress response, cognitive deficits, and anhedonia-like behavior. Therefore, this model is an important tool to define how the maturation of stress-sensitive brain circuits is altered by ELA and results in long-term behavioral changes that confer vulnerability to mental disorders.
Introduction
The early postnatal period is a critical developmental window in which environmental influences can shift the trajectory of development. For example, early-life adversity (ELA) can alter brain development to provoke long-term changes in cognitive and emotional function. Examples of ELA include physical or emotional abuse, neglect, inadequate resources, and an unpredictable home environment occurring during childhood or adolescence1. It is known that ELA is a risk factor for developing disorders such as depression, substance use disorder, post-traumatic stress disorder (PTSD), and anxiety2,3,4,5. This is important given that the levels of childhood poverty in the US have more than doubled recently, from 5.2% in 2021 to 12.4% in 20226, and although poverty itself is not necessarily ELA, it does increase the probability of various types of ELA7.
Animal models have long been essential for understanding the effects of early-life stress on brain development and adult outcomes. The two main animal models used in recent years to dissect this phenomenon are maternal separation (MS) and an impoverished environment induced by limited bedding and nesting materials (LBN). MS was developed as a model of parental deprivation8. In it, rodent dams are taken away from their pups, usually for several hours, every day until weaning8. The MS paradigm has been found to result in depressive- and anxiety-like behaviors in adulthood9, as well as an aberrant response to chronic stress10,11. On the other hand, the LBN model, first developed in the Baram laboratory12, does not separate the dam from the pups, but rather modifies the environment in which the pups are reared, mimicking a low-resource environment12,13. Decreasing the amount of nesting material and preventing direct access to the bedding in this model results in disrupted maternal care from the dams3. Since robust and predictable maternal care is required for the proper development of cognitive and emotional brain circuits14, fragmented maternal care from LBN can result in a range of outcomes, including an over-active Hypothalamic-Pituitary-Adrenal (HPA) axis, shifted excitatory-inhibitory balance in multiple brain regions, increased corticotropin-releasing hormone (CRH) levels, and depressive-like behavior in the offspring13,15,16,17,18,19.
The exact mechanism by which ELA results in increased risk for neuropsychiatric disorders is not completely understood. It is thought to be related to alterations in the HPA axis circuitry19,20, and recent evidence shows that this may be caused by changes in microglial synaptic pruning19. The LBN model has been shown to be a crucial tool for understanding the perinatal environment's impact on brain development and long-term behavioral outcomes. Although this model was initially developed for rats, it has also been adapted for mice in order to take advantage of the existing transgenic tools12,13. Notably, the model is very similar in both species and provokes highly convergent outcomes, such as alterations in the HPA axis, cognitive deficits, and depressive-like behavior, thus highlighting its cross-species utility and translational potential. This article will provide a detailed description of how to employ the limited bedding and nesting model in mice, collecting and analyzing maternal behavior and offspring outcomes to validate the model's efficacy and the expected results.
Protocol
All of the procedures involving animals were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the Institutional Animal Care and Use Committee from Georgia State University (approval number A24011). The mice were bred and maintained in the Animal Facilities at Georgia State University. The experiments were performed on a C57BL/6J strain during the perinatal period (postnatal day [P] 2-10) and included males and females. The reagents and equipment used for this study are listed in the Table of Materials.
1. Material setup
- Cut the mesh divider according to the dimensions of the cage, leaving an excess of 3 cm on the longer sides.
NOTE: The mesh provided in the table of materials is cut by the manufacturer. - Fold the edges, including the excess, in a way that creates a platform fitted precisely to the edges of the cage and with ~2.5 cm height above the floor. This will allow urine and feces to go through the mesh divider without the animals being able to retrieve the corn cob bedding. Finally, ensure that all of the sharp edges are folded down to prevent any harm to the animals.
NOTE: The mesh dividers are reusable and should be thoroughly cleaned between uses with hot water and soap, followed by spraying with 70% ethanol. - Set up the cameras on tripods and prepare the recording system.
NOTE: It is recommended to use video management software. These recordings result in .mp4 files that are automatically binned into 1 h segments. - Adjust the settings to continuous recording.
NOTE: Additional recommended settings are 1920 x 1080 resolution and 30 frames/s. - Set the resulting .mp4 files to divide into 1 h segments if not automatically done by video management software.
- If analyzing the lights-off period, use infrared lighting.
NOTE: To reduce recording glare on the side of the cage, it is recommended to disable the infrared (IR) from the camera. Instead, use IR floodlights in the room during dark phase recording.
2. Limited bedding and nesting (LBN) paradigm
- Pair 1-4 females with a single-housed male breeder.
NOTE: The recommended age for the first pairing in experimental females is P75. Using nulliparous females is ideal, but in situations where this is not feasible, such as with valuable transgenic mouse lines, multiparous females may continue breeding for approximately four months afterward. It is recommended, although not required, to check for vaginal plugs daily to confirm the day of mating (embryonic day [E]0).- Once the dams are pregnant, keep the disturbances minimal.
NOTE: Multiparous females can generally be reused to produce up to five litters before fertility declines. If the dams are reused for the experiment, they should preferentially be used for the same condition each time or only switched from Control to LBN, but never the other way around, in order to avoid any residual long-term effects of LBN on the dam.
- Once the dams are pregnant, keep the disturbances minimal.
- On E17 (or if plugs were not checked, whenever the females look evidently pregnant), separate females into their own standard plexiglass cage and give them one cotton nestlet (5 x 5 cm) to build a nest.
NOTE: It is optimal to move separated females to a quiet, small room away from the main colony room with a 12 h light/dark cycle and minimal disruptions from personnel for the Control and LBN setup. - Record the date of birth (around E19).
- At P2, count and sex the pups. Randomly assign litters to LBN or Control conditions (if it is the dam's first litter). This interaction with the pups should be completed between 1 and 4 h after the lights turn on.
- Place pups carefully into a new, clean cage to be sorted by sex. The anogenital distance between the anus and genitals is greater in males than in females and can be used as an identifier.
- Separate males and females into groups to count them.
- Cull litters with more than 8 pups and discard litters with less than 4. The optimal litter size is 4-8 pups, as anything outside of this range can interfere with the distribution of maternal care during the study.
NOTE: The variables that can be affected due to litter size are pup weight, feeding opportunity, and maternal-pup interactions, which could confound the experiment. Ideally, mice will not be cross-fostered since they are less likely to survive than rats.
- Set up the Control and LBN conditions as shown in Figure 1.
- Control: Use a standard-size mouse shoebox cage (19.4 cm x 13.0 cm x 38.1 cm) with ~220 g of corn cob bedding (depending on the cage dimensions, aiming for ~1 cm height) and one standard square cotton nestlet (5 x 5 cm).
- To help the mice acclimate to this unfamiliar environment, place one of their fecal pellets from their old cage in each corner of the new cage. In addition, place a small (dime-sized) amount of used cotton nestlet from the previous cage over the new nestlet.
- LBN: Place the previously prepared mesh divider into a standard shoebox cage with ~110 g of corn cob bedding (aiming for ~0.5 cm height). The mesh should prevent direct contact between the mice and corn cob bedding.
- Finally, add half of a cotton nestlet square (2.5 x 5 cm). Repeat all of the steps above to help with acclimation.
NOTE: It is recommended to maintain shoebox cages on standard, non-ventilated racks because it has been shown that this setup is quiet and does not induce hypothermia in pups reared in LBN cages21. However, this may not be the case with the increased airflow in ventilated cages; therefore, researchers should measure noise levels and pup core temperatures in their system if they are using ventilated cages.
- Finally, add half of a cotton nestlet square (2.5 x 5 cm). Repeat all of the steps above to help with acclimation.
- Control: Use a standard-size mouse shoebox cage (19.4 cm x 13.0 cm x 38.1 cm) with ~220 g of corn cob bedding (depending on the cage dimensions, aiming for ~1 cm height) and one standard square cotton nestlet (5 x 5 cm).
- Add the pups to their designated cage and place them on top of the nestlet. Next, place the dam in the cage facing the pups, as this will help her notice that they are present more quickly. Ensure that enough food and water is included for at least 8 days to avoid disturbing the cage until the end of the experiment at P10.
- Place a camera on a tripod in front of the cage, allowing for a clear side view of the dam and her pups. Optionally, place mirrors around the cage to better capture all of the angles. Although only 1 h of video recording per day needs to be analyzed, the camera is set up to continuously record 24/7 during the experiment so that disturbances can be monitored.
NOTE: Mice can also be observed in person, and maternal behavior can be hand-scored, although it is preferable to video-record to prevent any disruptions due to the presence of the experimenter in the room. If ventilated racks are used, it also may be necessary to use a different procedure to arrange cameras for maternal care recording than that described here. - On the morning of P10, return all of the animals to standard caging conditions (ideally identical to the control condition above) and weigh the pups.
- Treat all of the pups the same and wean at P21 into groups of 2-5 same-sex littermates.
NOTE: Aim to house same-sex littermates together whenever possible, but if necessary, house same-sex offspring from different litters in the same condition together. It is recommended that housing control and LBN offspring together be avoided (see Yang et al. for the effect of cagemate composition on behavioral phenotypes22).
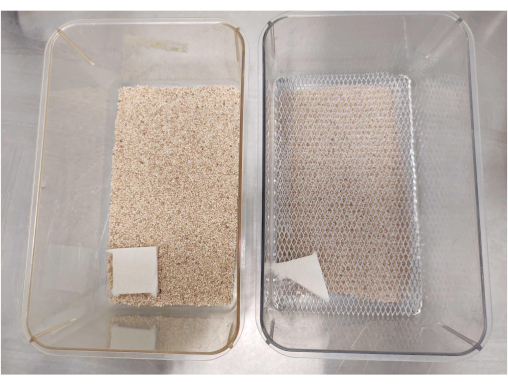
Figure 1: Example of cage setup. The cage on the left side of the image shows a standard control (CTL) cage containing a full amount of bedding and a full nestlet. The cage on the right side shows a limited bedding and nesting (LBN) setup with half the amount of bedding, half a nestlet, and a mesh divider for separating the animals from the bedding. Please click here to view a larger version of this figure.
3. Maternal behavior scoring
- Although continuous recordings are collected in this setup, analyze only 1 h from P3 to P6. It is recommended to analyze videos recorded no earlier than 1 h after the lights change to allow for habituation and to analyze videos from a consistent time each day.
NOTE: The period from P3 to P6 typically contains the largest differences in maternal care due to LBN; therefore, it is only necessary to analyze these days. The suggested observation/recording time allows for the capture of activity after transitioning from the active to inactive cycle or vice versa. However, the inactive phase is when the dam is most likely to engage in maternal care and has previously been shown to contain the largest group differences in maternal behavior3.
NOTE: Capture P2 recordings later than on other days because mice need at least 1 h to habituate to the cage change. Unless this is a variable of interest, discard this day from the analysis. - Score all of the behaviors as presented in Table 1. Typically, behaviors are scored for the first 50 min after the data collection time has begun.
NOTE: This can be done by hand or electronically using the Behavioral Observation Research Interactive Software (BORIS, an open-source software), or a similar type of software. Instructions below are for hand scoring and can be adapted for whatever software is choosen. - In a printed table, record the observed behavior using the abbreviation, the start time and duration of the bout, and any descriptive notes. For example, if the dam is actively nursing and then leaves the nest but pups are still attached to her, note this as AN, followed by O. The description of the pups (and how many) still attached should be kept as a note on the side in case this is needed later.
NOTE: Any behavior under 3 s long is not analyzed. This rule helps filter out momentary behaviors due to outside disturbances, such as environmental noise. An exception is made when there is a visible interruption to an AN bout (such as by a large movement or stretch) that is quickly resumed. In this case, the behavior noted is AN, followed by N-AN, as described in Table 1. - In the event of multiple behaviors happening at once, record the most active one. An example of this is if the dam is low nursing but later begins licking and grooming, low nursing is noted to stop, and the next behavior should be marked as LG with a note that this occurred during LN.
| Type of behavior | Abbreviation | Description | ||||
| Licking / grooming | LG | The dam is engaged in licking/grooming her pups. | ||||
| Active nursing | AN | The dam is nursing her pups standing up, while her back is arched. | ||||
| New active nursing | N-AN | This behavior is used specifically when the dam interrupted nursing but quickly resumes. This is an exception to the 3s rule. | ||||
| Low nursing | LN | The dam is actively nursing her pups, but her back is low or almost flat. This behavior commonly follows AN after a period of time. | ||||
| Side Nursing | SN | The dam is lying on her side when nursing (also known as passive nursing). | ||||
| Off nest | O | The dam is not on the nest, and she is not eating/drinking. This can be observed in her walking around the cage or exploring. | ||||
| Eating/drinking | E | The dam is off the nest eating or drinking. | ||||
| Self-grooming | SG | The dam is grooming herself. | ||||
| Carrying pups | C | The dam carries the pups, usually to relocate them back to the nest. | ||||
| Nest building | NB | The dam is actively constructing or relocating the nest. | ||||
| Move on nest | M | The dam is moving on the nest. This presents with the dam interacting with the pups in a way different than LG or any type of nursing, such as sniffing, rearing, or stepping on the pups. | ||||
Table 1: Description of the maternal care behaviors.
4. Maternal behavior data analysis
- Compile the scoring results into a spreadsheet.
- If the videos were analyzed electronically, delete the behaviors with bouts shorter than 3 s, as described in step 3.
- Calculate the average bout length, average frequency, and total duration of licking and grooming for each day of observation.
NOTE: Descriptive statistics can be performed on any of the scored behaviors, but licking and grooming will typically present the most evident disruption in terms of fragmentation by LBN (i.e., shorter, more frequent bouts). These variables can be analyzed as an average across P3-P6, or as repeated measures by day to look for any changes over time if this is of interest.
5. Calculation of entropy
NOTE: Entropy, or unpredictability, of maternal care behaviors is calculated based on the method proposed by Vegetabile et al.23. This method is based on the assumption that maternal care behaviors act as a Markov chain, which can be used to estimate the entropy rate of a behavioral sequence. Each dam's sequence of behaviors is characterized using the empirical transition matrix <pij> i,j = 1…7 of conditional probabilities of moving from one behavior (i) to another behavior (j), and the entropy rate is calculated from this as previously described3,23 and as follows:
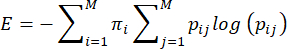
where pij is the conditional probability that behavior j is observed next after a dam is observed performing behavior i, πi is the frequency with which behavior i is observed, and M (=7) is the total number of different behaviors. The reader is referred to Vegetabile et al.23 for a discussion of the theoretical underpinnings of the equations; here, the focus is on how to apply the method in the LBN model.
- To compute this, arrange the format required for the analysis.
- Combine AN, LN, SN, and N-AN into a single variable named N by adding the times together, as they all involve nursing.
NOTE: Refer to Table 1 for the description of the maternal care behaviors. - Add the behaviors that are not related to self- or pup-directed behaviors (M and O) into O.
- Input LG, E, SG, C, and NB separately to result in 7 total behaviors.
- Combine AN, LN, SN, and N-AN into a single variable named N by adding the times together, as they all involve nursing.
- Create a spreadsheet with columns following this order: Mouse ID, Litter ID, Litter #, Treatment, Day, Time, Behavior, and Status.
- In the mouse ID, add the mouse line/genotype of the dam.
- In litter ID, use the dam's identifier followed by the litter number.
- In litter #, indicate the number of litters the dam has had.
- Ensure that the treatment is LBN or Control, according to the conditions in which the animals were placed.
- Indicate the day as the corresponding postnatal day analyzed.
- Indicate the time as the time stamp in the video when the behavior started and ended (relative to the start of recording).
- Select the behavior from the seven described earlier (N, O, LG, E, SG, C, and NB) (Table 1).
- Mark the two types of statuses, START and STOP. Therefore, each behavior will appear twice; the first notation marks the start, and the second marks the stop.
- Following this format, include the information of all the analyzed days.
- In the R environment, import the datasets that have been formatted as above.
- Install the packages available at https://github.com/bvegetabile/entropyRate.
NOTE: Running this code results in a folder named LBN; regardless of the condition, this folder contains the calculated entropy. Additionally, the entropy of each day can then be averaged by the subject for P3-P6 and compared between conditions.
Representative Results
The representative results demonstrate how ELA, imposed by an impoverished environment in LBN cages, affects maternal care from dams and offspring physiological outcomes. The daily entropy in maternal care behavior is higher in LBN across days P3-P6 (F1,58 = 7.21, p = 0.0094; Figure 2A), as well as the average entropy of each dam from this time period (t15 = 3.03, p = 0.0085; Figure 2B). Notably, there is no significant difference in average entropy rate across different litters from the same dam when maintained within the same treatment group (F1.699,4.247 = 0.57, p = 0.58; Figure 2C), suggesting that entropy rate may be a somewhat stable trait for each dam. Amongst all of the behaviors, licking and grooming is the one that has been shown to be most fragmented by LBN3. The LBN dams display a higher frequency of licking and grooming their pups (LG) (t16 = 4.04, p = 0.0010; Figure 2D) and in shorter bouts (t16 = 3.25, p = 0.0050; Figure 2E). However, there is no significant difference in the total duration of LG between the Control and LBN dams (t16 = 1.52, p = 0.15; Figure 2F).
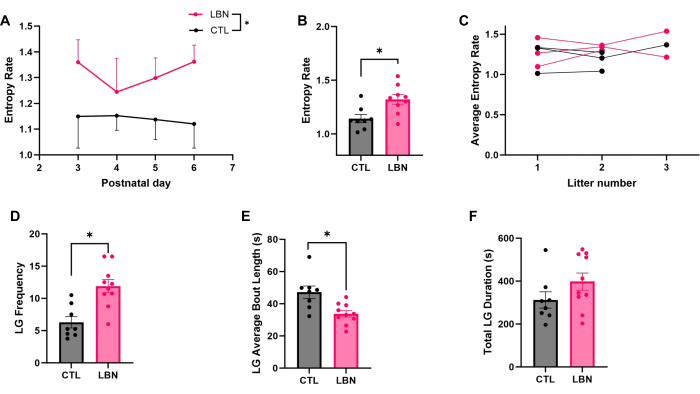
Figure 2: Maternal behavior analysis. (A) The daily entropy rate is higher in limited bedding and nesting (LBN) dams vs. control (CTL) as analyzed by the mixed-effects model (F1,58 = 7.21, p = 0.0094). (B) The average entropy rate is higher in LBN dams vs. CTL (t15 = 3.03, p = 0.0085). (C) The average entropy rate (P3-P6) is not significantly different across multiple litters within the same dam when maintained in the same treatment group (F1.699,4.247 = 0.57, p = 0.58). Each line represents a single dam. (D) The cumulative frequency of licking and grooming (LG) events is higher for LG dams vs. CTL (t16 = 4.04, p = 0.0010). (E) The LG average bout length is shorter for LG dams vs. CTL (t16 = 3.25, p = 0.0050). (F) The cumulative time spent on LG is not significantly different due to LBN (t16 = 1.52, p = 0.15). Data are mean ± SEM, * p < 0.05. Please click here to view a larger version of this figure.
Pups that were reared in LBN conditions are significantly smaller at P10 (t61 = 6.30, p < 0.0001; Figure 3A). This difference typically persists at weaning age (t62 = 6.29, p = <0.0001; Figure 3B) but is no longer observed by adulthood (t38 = 1.08, p = 0.29; Figure 3C). However, corticosterone levels are increased at baseline in adulthood (t18.79 = 2.23, p = 0.038; Figure 3D), suggesting enduring physiological effects of LBN. These are the expected differences in a successful experimental setup. In the case of a suboptimal setup, the values of LG bout length, entropy, and pup weights may present no differences, likely due to a stressed "Control" group. For the purposes of this paper, sex was collapsed when analyzing offspring outcomes because sex differences are not typically observed in the physiological outcomes described here; however, sex differences in other types of outcomes, such as cognitive and emotional behavior, are commonly reported in this model and should be investigated further4.
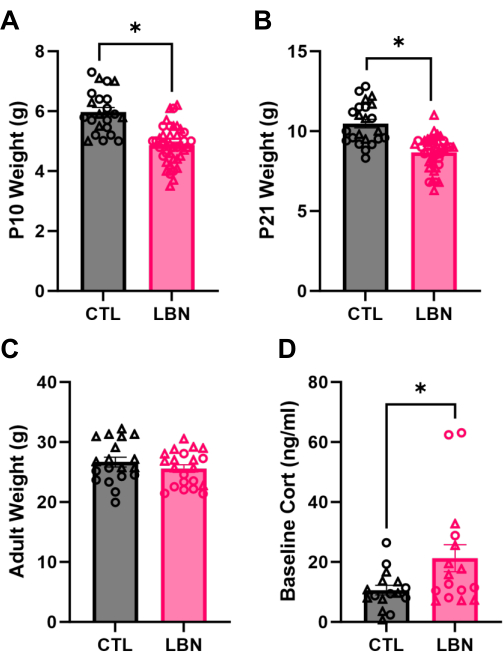
Figure 3: Offspring outcomes. (A) Limited bedding and nesting (LBN) decreases pup weight measured at P10, just before return to standard cages (t61 = 6.30, p < 0.0001). (B) Weight is still decreased by LBN at the weaning age (t62 = 6.29, p < 0.0001). (C) Adult weight no longer differs due to LBN (t38 = 1.08, p = 0.29). (D) The baseline concentration of corticosterone in adulthood is increased by LBN, as analyzed by Welch's t-test (t18.79 = 2.23, p = 0.038). For all graphs, females are shown with circles and males with triangles. Data are mean ± SEM, *p < 0.05. Please click here to view a larger version of this figure.
Discussion
This article provides a detailed protocol to apply the LBN model in mice. This model is an important tool for understanding how an ethologically and translationally relevant form of chronic stress in early life contributes to the development of neuropsychiatric disorders in the offspring13. It is also useful for studying maternal behavior and any changes in the dams' brain from a molecular, neuroendocrine, or circuit-based perspective24. For these types of questions, multiparity may be a more important variable to consider. We have observed that maternal behavior entropy scores remain consistent across multiple litters within the same dam (Figure 2C), suggesting that entropy may be a somewhat stable trait for each dam. This finding may justify the use of multiparous female mice when it is not feasible to use only nulliparous females, such as in the case of costly or rare transgenic mice. Due to the possible unintended effects of multiparity on other variables (e.g., intrauterine environment), it is recommended that the experimenter statistically control for the use of multiparous females during data analysis.
It is important to note that this model is highly sensitive to environmental disturbances. Multiple factors can disrupt the animals, causing problems and suboptimal results, such as cage flooding or loud noises such as from nearby construction. Usually, these disturbances will result in Control animals becoming stressed, and therefore, there will be no differences between the Control and LBN conditions. An indicator of these problems may be similar weights in Control and LBN pups at P10 and similar entropy values in maternal care behavior. Because of this, the ideal setup is a maternal care room that is only used for this purpose, quiet and away from the colony and other laboratory personnel. Disturbances can also lead to cannibalism of pups, especially in dams younger than P75. The risk of losing pups to cannibalism is lower in dams after P75 and often after the first litter. An alternative version of this method shifts the timing of the experimental manipulation to P4-P11 in order to decrease the risk of cannibalism even further25,26; although this is not necessary in the present laboratory conditions. Another version of LBN does not employ a mesh divider but still limits the amount of bedding and nesting material for the entire pre-weaning postnatal period27. It is important to note that changing the timeline may lead to different results, such as abusive maternal behavior, which may, in turn, result in different offspring outcomes, such as anxiety-like behaviors25,28. Other common problems include losing maternal behavior data due to the camera angle and large nest size; to help with this, it is advised to put a mirror in the back of the cage and be sure to only provide a single nestlet in the control condition in order to prevent the nest from becoming too large and concealing the pups from the camera. Finally, ensuring the mesh fits properly in the cage to prevent pups from getting stuck or hurt is essential.
An advantage of the LBN model is that it is easily customized and combined with other experimental factors. Examples of the variables that can be added to this protocol are diet29,30, immune challenges30,31, different transgenic lines32,33, and chemogenetics19. Moreover, some versions of this model employ a P10-P17 time frame and combine it with maternal separation (MS)34,35,36. This paradigm has also been adapted as a prenatal stressor, where dams are housed in the LBN environment from E14 to E1937,38, but the pups are never exposed to the stress directly. Finally, some studies use a two-hit model that involves ELA combined with different stress tests on the offspring when they reach adulthood19,39.
One of the limitations of this method is that it does not have a high throughput. The amount of data it produces is limited by the number of cameras and space available at one time, although the litters can easily be staggered over time. The manual scoring of maternal behavior is especially time-consuming and may introduce observer bias, but automated scoring is currently being developed40, thanks to the advent of machine learning-based approaches. If data are hand-scored, it is recommended that the same person scores all of the videos for one experiment in order to decrease inter-observer variability. Another limitation is that this model is designed for rodents that rely primarily on maternal care, making it difficult to study biparental care. However, some groups have adopted paternal deprivation as an alternative to MS in biparental species such as the California mouse41,42, so it is conceivable that LBN could also be adapted for these species.
Aside from LBN, maternal separation is the other most prominent model for ELA, because other forms of stress employed in adults (i.e., restraint stress) are not ethologically relevant and do not affect pups the same way. LBN differs from MS because it is a form of chronic stress that induces fragmented and unpredictable maternal behavior, whereas, in MS, deprivation is intermittent and usually occurs at predictable times each day43. Interestingly, both models can cause changes in the HPA axis and depressive-like behaviors in rodents, although LBN may be a more reproducible protocol with less experimenter interaction and higher consistency across laboratories1,11,13,16. Although this protocol is useful in mitigating some sources of variability (such as experimenter-related), other aspects like differences in animal facilities, origin of the animals, or strain can influence the results. For example, it is known that BALB/c mice have a more pronounced response to chronic restraint stress and may be differentially sensitive to ELA than C57BL/6J27,44.
In conclusion, the LBN model employs a low-resource environment with unpredictable maternal care and is a useful approach to understanding how early-life stress affects a wide variety of processes, such as physiological adaptations to stress, maternal behavior, and brain development. Notably, this model has been employed to understand how and why ELA is a risk factor for neuropsychiatric disorders1,5,9,12. In the future, the use of LBN will enhance our understanding of the biological basis of both mental and physical disorders and help elucidate novel therapeutic targets to treat the effects of stress during the sensitive perinatal period.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIMH K99/R00 Pathway to Independence Award #MH120327, Whitehall Foundation Grant #2022-08-051, and NARSAD Young Investigator Grant #31308 from the Brain & Behavior Research Foundation and The John and Polly Sparks Foundation. The authors would like to thank the Division of Animal Resources at Georgia State University for providing exceptional care to our animals., and Ryan Sleeth for his excellent technical support in setting up and maintaining our video management system. Dr. Bolton would also like to thank Dr. Tallie Z. Baram for excellent training in the proper implementation of the LBN model during her postdoctoral fellowship.
Materials
| 2-inch 4 MP 4x Zoom IR Mini PT Dome Network Camera | Hikvision | DS-2DE2A404IW-DE3(S6) | |
| Amazon Basics Aluminum Light Photography Tripod Stand with Case – Pack of 2, 2.8 – 6.7 Feet, 3.66 Pounds, Black | Amazon | From Amazon | |
| Blue Iris | Blue Iris Security | Optional video management software | |
| CAMVATE 1/4"-20 Mini Ball Head with Ceiling Mount for CCTV & Video Wall Monitors Mount – 1991 | Camvate | From Amazon | |
| Corn cob bedding | The Andersons | 4B | |
| Cotton nestlet | Ancare | NES3600 | |
| Mesh divider | McNICHOLS | 4700313244 | Standard, Aluminum, Alloy 3003-H14, 3/16" No. .032 Standard (Raised), 70% Open Area |
| Tendelux DI20 IR Illuminator | Tendelux | From Amazon |
References
- Warhaftig, G., Almeida, D., Turecki, G. Early life adversity across different cell- types in the brain. Neurosci Biobehav Rev. 148, 105113 (2023).
- Duffy, K. A., Mclaughlin, K. A., Green, P. A. Early life adversity and health-risk behaviors: Proposed psychological and neural mechanisms. Ann N Y AcadSci. 1428 (1), 151-169 (2018).
- Molet, J., et al. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry. 6 (1), e702 (2016).
- Garvin, M. M., Bolton, J. L. Sex-specific behavioral outcomes of early-life adversity and emerging microglia-dependent mechanisms. Front Behav Neurosci. 16, 1013865 (2022).
- Andersen, S. L. Neuroinflammation, early-life adversity, and brain development. Harv Rev Psychiatry. 30 (1), 24-39 (2022).
- Shrider, E. A., Creamer, J. . Poverty in the United States: 2022. , P60-P280 (2023).
- Roos, L. L., Wall-Wieler, E., Lee, J. B. Poverty and early childhood outcomes. Pediatrics. 143 (6), e20183426 (2019).
- Ader, R., Tatum, R., Beels, C. C. Social factors affecting emotionality and resistance to disease in animals: I. Age of separation from the mother and susceptibility to gastric ulcers in the rat. J Comp Physiol Psychol. 53 (5), 446-454 (1960).
- Nishi, M. Effects of early-life stress on the brain and behaviors: Implications of early maternal separation in rodents. Int J Mol Sci. 21 (19), 7212 (2020).
- Trujillo, V., Durando, P. E., Suárez, M. M. Maternal separation in early life modifies anxious behavior and fos and glucocorticoid receptor expression in limbic neurons after chronic stress in rats: Effects of tianeptine. Stress. 19 (1), 91-103 (2016).
- Yu, S., et al. Early life stress enhances the susceptibility to depression and interferes with neuroplasticity in the hippocampus of adolescent mice via regulating miR-34c-5p/SYT1 axis. J Psychiatr Res. 170, 262-276 (2023).
- Walker, C. D., et al. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: Critical considerations of methodology, outcomes and translational potential. Stress. 20 (5), 421-448 (2017).
- Rice, C. J., Sandman, C. A., Lenjavi, M. R., Baram, T. Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 149 (10), 4892-4900 (2008).
- Glynn, L. M., Baram, T. Z. The influence of unpredictable, fragmented parental signals on the developing brain. Front Neuroendocrinol. 53, 100736 (2019).
- Karst, H., et al. Acceleration of GABA-switch after early life stress changes mouse prefrontal glutamatergic transmission. Neuropharmacology. 234, 109543 (2023).
- Demaestri, C., et al. Resource scarcity but not maternal separation provokes unpredictable maternal care sequences in mice and both upregulate CRH-associated gene expression in the amygdala. Neurobiol Stress. 20, 100484 (2022).
- Breton, J. M., et al. Early life adversity reduces affiliative behavior with a stressed cagemate and leads to sex-specific alterations in corticosterone responses in adult mice. Horm Behav. 158, 105464 (2023).
- Bath, K. G., Manzano-Nieves, G., Goodwill, H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm Behav. 82, 64-71 (2016).
- Bolton, J. L., et al. Early stress-induced impaired microglial pruning of excitatory synapses on immature CRH-expressing neurons provokes aberrant adult stress responses. Cell Rep. 38 (13), 110600 (2022).
- Dahmen, B., et al. Effects of early-life adversity on hippocampal structures and associated HPA axis functions. Dev Neurosci. 40 (1), 13-22 (2018).
- Bolton, J. L., Short, A. K., Simeone, K. A., Daglian, J., Baram, T. Z. Programming of stress-sensitive neurons and circuits by early-life experiences. Front Behav Neurosci. 13, 30 (2019).
- Yang, M., Lewis, F., Foley, G., Crawley, J. N. In tribute to Bob Blanchard: Divergent behavioral phenotypes of 16p11.2 deletion mice reared in same-genotype versus mixed-genotype cages. Physiol Behav. 146, 16-27 (2015).
- Vegetabile, B. G., Stout-Oswald, S. A., Davis, E. P., Baram, T. Z., Stern, H. S. Estimating the entropy rate of finite Markov chains with application to behavior studies. J Educ Behav Stat. 44 (3), 282-308 (2019).
- Rincón-Cortés, M., Grace, A. Postpartum scarcity-adversity disrupts maternal behavior and induces a hypodopaminergic state in the rat dam and adult female offspring. Neuropsychopharmacology. 47 (2), 488-496 (2022).
- Gallo, M., et al. Limited bedding and nesting induces maternal behavior resembling both hypervigilance and abuse. Front behav neurosci. 13, 167 (2019).
- Manzano Nieves, G., Bravo, M., Baskoylu, S., Bath, K. G. Early life adversity decreases pre-adolescent fear expression by accelerating amygdala pv cell development. eLife. 9, e55263 (2020).
- Johnson, F. K., et al. Amygdala hyper-connectivity in a mouse model of unpredictable early life stress. Transl Psychiatry. 8 (1), 49 (2018).
- Demaestri, C., et al. Type of early life adversity confers differential, sex-dependent effects on early maturational milestones in mice. Horm Behav. 124, 104763 (2020).
- Reemst, K., et al. Molecular underpinnings of programming by early-life stress and the protective effects of early dietary ω6/ω3 ratio, basally and in response to LPS: Integrated mRNA-miRNAs approach. Brain Behav Immun. 117, 283-297 (2024).
- Reemst, K., et al. Early-life stress and dietary fatty acids impact the brain lipid/oxylipin profile into adulthood, basally and in response to LPS. Front Immunol. 13, 967437 (2022).
- Reemst, K., et al. Early-life stress lastingly impacts microglial transcriptome and function under basal and immune-challenged conditions. Transl Psychiatry. 12 (1), 507 (2022).
- Wang, T., et al. The nucleus accumbens CRH-CRHR1 system mediates early-life stress-induced sleep disturbance and dendritic atrophy in the adult mouse. Neurosci Bull. 39 (1), 41-56 (2023).
- Knop, J., Van, I. M. H., Bakermans-Kranenburg, M. J., Joëls, M., Van Der Veen, R. Maternal care of heterozygous dopamine receptor d4 knockout mice: Differential susceptibility to early-life rearing conditions. Genes Brain Behav. 19 (7), e12655 (2020).
- Bennett, S. N., Chang, A. B., Rogers, F. D., Jones, P., Peña, C. J. Thyroid hormones mediate the impact of early-life stress on ventral tegmental area gene expression and behavior. Horm Behav. 159, 105472 (2024).
- Parel, S. T., et al. Transcriptional signatures of early-life stress and antidepressant treatment efficacy. Proc Natl Acad Sci U S A. 120 (49), e2305776120 (2023).
- Julie-Anne, B., et al. Reactivation of early-life stress-sensitive neuronal ensembles contributes to lifelong stress hypersensitivity. J Neurosci. 43 (34), 5996 (2023).
- Bolton Jessica, L., et al. Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect. 121 (9), 1075-1082 (2013).
- Block, C. L., et al. Prenatal environmental stressors impair postnatal microglia function and adult behavior in males. Cell Rep. 40 (5), 111161 (2022).
- Peña, C. J., et al. Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat Commun. 10 (1), 5098 (2019).
- Lapp, H. E., Salazar, M. G., Champagne, F. A. Automated maternal behavior during early life in rodents (amber) pipeline. Sci Rep. 13 (1), 18277 (2023).
- Madison, F. N., Palin, N., Whitaker, A., Glasper, E. R. Sex-specific effects of neonatal paternal deprivation on microglial cell density in adult California mouse (Peromyscus californicus) dentate gyrus. Brain, Behav. Immun. 106, 1-10 (2022).
- Walker, S. L., Sud, N., Beyene, R., Palin, N., Glasper, E. R. Paternal deprivation induces vigilance-avoidant behavior and accompanies sex-specific alterations in stress reactivity and central proinflammatory cytokine response in California mice (Peromyscus californicus). Psychopharmacology. 240 (11), 2317-2334 (2023).
- Molet, J., Maras, P. M., Avishai-Eliner, S., Baram, T. Z. Naturalistic rodent models of chronic early-life stress. Dev Psychobiol. 56 (8), 1675-1688 (2014).
- Tsuchimine, S., et al. Comparison of physiological and behavioral responses to chronic restraint stress between C57BL/6J and balb/c mice. Biochem Biophys Res Commun. 525 (1), 33-38 (2020).

.
