Low-stress Route Learning Using the Lashley III Maze in Mice
Summary
The Lashley III maze is a route-learning task that does not rely on aversive stimuli or visual cues. It is thus a highly attractive option for evaluating learning and memory, especially in aging mice or otherwise where stress is a consideration.
Abstract
Protocol
1. Preparation:
- The Lashley III maze is constructed of Plexiglas and is shown in Figure 1. The start box, maze arms, and goal box are modular to allow for cleaning and modifiability of the maze. The walls of the start box, maze arms, and goal box are constructed of black Plexiglas to eliminate visual cues from outside and inside the maze. Maze arms are 45 cm long, 7 cm high, and 5 cm wide. Doors leading to consecutive arms of the maze are 4 cm x 4 cm and are situated 11 cm from the outer walls of the maze. The goal box is 19.5 cm long x 7 cm high x 5 cm wide. The start box is 8 cm x 9.5 cm x 7 cm. Separate clear Plexiglas pieces are placed on top of the start box, maze, and goal box to allow visualization of each mouse as it progresses through the maze while preventing mice from escaping. The maze rests on a base constructed of red Plexiglas that is large enough to hold the three pieces of the maze plus a pseudo-home cage that is the same size as the animal’s standard home cage.
- To prepare pseudo-home cages prior to the first maze trial, fresh bedding material of the same kind as that used in the animals’ home cages is added to individual cage bottoms having doorways (2 cm x 2 cm) cut into the sides. Place wire tops on these cage bottoms and individually number them, one for each animal in a testing cohort. These cages will serve as individual pseudo-home cages for each animal for the duration of the testing timeframe. Because we are investigating the roles of stress and/or anxiety-related behaviors in learning and memory, we use pseudo-home cages to avoid individually housing mice in their home cages. However, if these factors are not of primary concern, animals can be singly housed in home cages modified with doorways and these cages can serve as the home cages for successful maze navigation.
2. Testing:
- Each day, animals are moved to the behavior testing room a minimum of one hour before testing to allow them to acclimate to the testing environment.
- Thirty min before the testing start time, food and water is removed from all home cages. Keep the food and water in the behavior testing room but out of reach of the mice. Food and water is typically removed 30 min after the change from the light phase to the dark phase, after peak feeding has occurred. This ensures that animals are mostly food- and water -satiated prior to testing; however, testing during this period provides a small additional motivating force to navigate the maze even though there is no overt food or water reward upon task completion.
- After the 30 min pretest period has elapsed, clean the maze with 70% ethanol. Leave the door leading from the start box into the maze arms closed. The doors at both ends of the goal box are left open.
- Place the pseudo-home cage corresponding to the first animal at the end of the goal box.
- Carry the first mouse to the maze, cupping it in your hand. Put the mouse in the start box and replace the lid.
- When in position to observe the maze without obstruction, open the start box door and immediately start three stopwatches.
- When all four paws of the mouse have left the start box, close the start box door and stop the first timer.
- Record by hand the pathway the mouse travels as it navigates the maze. A mouse enters a new zone or arm of the maze when all four paws have crossed into that area (See Fig. 1).
- When all four paws enter the goal box, close the door leading between the maze and the goal box and stop the second stopwatch.
- When the mouse has completely entered the pseudo-home cage, close the door between the goal box and the cage. Stop the third stopwatch.
- Record the times from the three stopwatches. Count the number of errors the mouse has made. An error is defined as an entry into a dead-end cul-de-sac zone (e.g., going from arm L to zone K; Fig. 1) or travelling back through an already-travelled arm of the maze (e.g., going from arm F to arm C; Fig. 1). While we score mouse performance as it is occurring, it is possible to videotape or otherwise record maze trials to allow for subsequent scoring either by eye or by automated software. Experimenter discretion should be used to choose an appropriate method.
- Leave the animal in the pseudo-home cage for 1 min before returning it to its home cage.
- Repeat steps 2.3 to 2.12 for all mice in the testing cohort.
- When the last mouse in the cohort has been returned to its home cage, start the 30 min timer. At the end of this 30 min period, return food and water to all mice in the cohort and then return the animals to their colony room.
- Repeat this procedure on consecutive nights, with one trial per testing day per animal, until all mice in the cohort have reached a defined learning criteria or until a predetermined number of test trials has occurred. A mouse is classified as having learned the maze when it can perform the task with 0 or 1 error(s) on two consecutive trials. Mice are tested in the same order on successive trials.
3. Representative Results:
Days to learn (reach criterion) can be analyzed and compared between different groups in a particular study (Figs. 2A and 3A). Additionally, a learning index (learning ratio), which characterizes maze acquisition, can be evaluated over the first four days of the testing period (Figs. 2B and 3B). The learning index is the ratio of the number of correct path segments travelled to the total number of path segments travelled for each trial. The average learning index should be approximately 0.5 for trial 1, when mice are first exposed to the maze and navigating by chance. The learning index increases and approaches 1 between trials 2 and 4 indicating that learning is occurring. In Figure 2, young (2 mo) and aging (24 mo) male C57Bl/6NCr mice were trained on the Lashley maze for up to 15 trials. The young mice learned the maze in 7.2 (± 1.5) d. By contrast, the aging animals took 11.7 (± 2.1) d to reach criterion. There was a strong trend for aging C57Bl/6NCr mice to take longer to learn the maze ([t(23)=1.59, P=0.056]; one-tailed t-test, a priori hypothesis that days to learn is longer in aging mice). When the learning index is calculated, both age groups show a steady increase in the learning index from trials 2 through 4 (significant main effect of time P<0.001). However, there were no statistically significant differences in the learning index between young C57 mice and old C57 mice (main effect of age P=0.58).
Figure 3 is representative of studies examining the effects of background strain on learning behavior. Data from the young male C57Bl/6NCr shown in Fig. 2 are now compared to data from young Crl:CD-1(ICR)BR mice [CD-1, 4-5 mo]. CD-1 mice learned the Lashley III maze in 4.5 (± 1.4) d; however, there was no significant difference between the strains in days to learn (Fig. 3A; [t(18)=1.328, P=0.20]; two-tailed t-test). By contrast, examination of the learning index shows that, while all animals were navigating the maze on the first trial by chance, CD-1 mice learned the task more quickly (main effect of strain P<0.001). This is illustrated by the statistically significant increase in the learning index in trials 2 through 4 in CD-1 mice compared to C57Bl/6NCr mice. Interestingly, if CD-1 mice continue to be trained in the maze beyond the point of reaching criterion, they exhibit behavior indicative of overtraining (Fig. 4) which is discussed in more detail below.
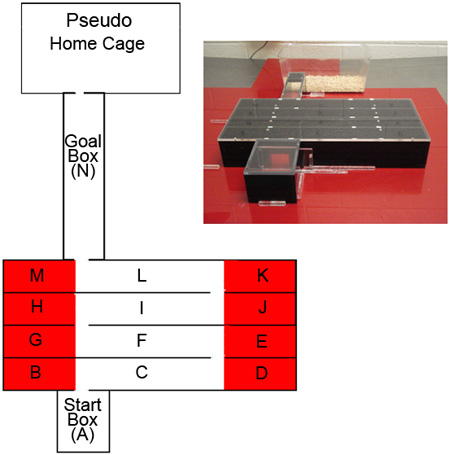
Figure 1: The Lashley III maze. The dead-end cul-de-sac zones of the maze are colored red in this diagram for illustrative purposes only. The base and arms of the actual maze are solid colors (inset). The maze is drawn to scale (dimensions in text).
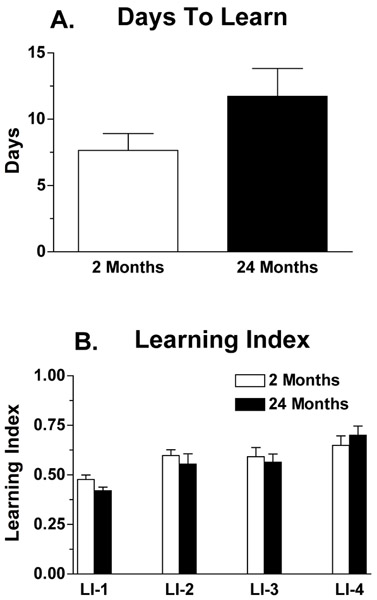
Figure 2: Lashley III maze performance in 2 and 24 month-old male C57Bl/6NCr mice. The Lashley III maze was used to assess route learning in young (2 mo) and aging (24 mo) male C57Bl/6NCr mice. The parameters measured were (a) days to criterion, the number of days required for a mouse to run the maze for two consecutive nights with 0 errors or 1 error; and (b) learning index, the number of correct four-paw arm entries made versus the total number of arm entries made on days 1-4 of Lashley III maze testing. Numbers of animals per group were n=10 for 2 month-old and n=15 for 24 month-old mice.
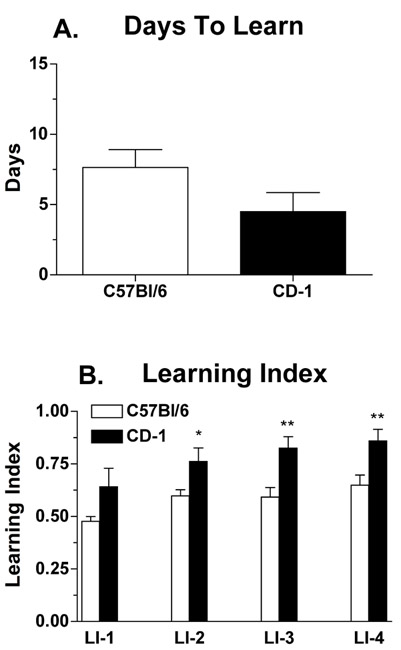
Figure 3: Lashley III maze performance in young male C57Bl/6NCr and CD-1 mice. The Lashley III maze was used to assess route learning in 2 month-old C57Bl/6NCr and 4-5 month-old CD-1 mice . The parameters measured were (a) days to criterion, the number of days required for a mouse to run the maze for two consecutive nights with 0 errors or 1 error; and (b) learning index, the number of correct four-paw arm entries made versus the total number of arm entries made on days 1-4 of Lashley III maze testing. Numbers of animals per group were n=10 for C57Bl/6NCr and n=10 for CD-1 mice. Statistical significances: *P<0.05, **P<0.01 versus C57Bl/6NCr mice.
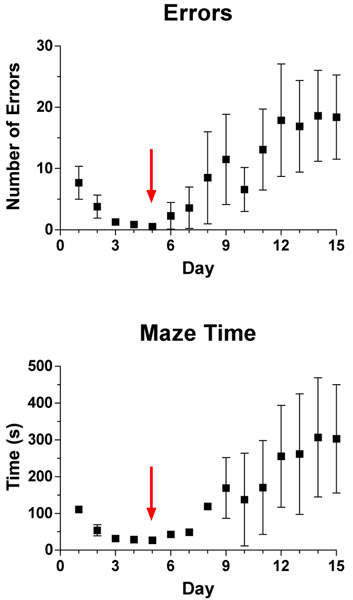
Figure 4: Lashley III maze performance in 4-5 month-old male CD-1 mice. The Lashley III maze was used to assess route learning in ten 4-5 month-old male CD-1 mice on 15 consecutive days. The parameters measured were (a) number of errors made per day, with an error defined as a four-paw entry into a dead-end cul-de-sac zone or travelling an already-travelled arm of the maze; and (b) time spent in the maze per day. The day at which criterion was reached is indicated with a red arrow.
Discussion
The most commonly used rodent behavioral tests to assess learning and memory are the Morris water maze and variants of fear conditioning2. These tests rely on visual or auditory cues for learning and aversive stimuli (e.g., water, footshock) to motivate animals to complete the tasks3. In aging rodents, as in aging humans, sensory modalities decline over time. Several background strains of mice lose their hearing or develop cataracts as they age, and mortality rates after 23.5 h of water deprivation have been shown to increase in mice as they age4. Thus, changes in the performance in these more widely used tests may be difficult to interpret in aging rodents.
The Lashley III maze was first described by Karl Lashley in 19295. Lashley used this paradigm to search for the memory engram in the cortex using cortical lesioning techniques. We have slightly modified the original maze to allow for its broad applicability to studies involving aging rodents. We have eliminated food-cued navigation of the maze and demonstrated that a pseudo-home cage environment provides sufficient motivation to both young and aging mice to navigate the maze1. Other investigators have also utilized a food pellet reward to facilitate learning of the Lashley III maze7. Visually-cued navigation, like that required in the Morris water maze and the Barnes circular maze, has been minimized2,6,8. Mice are not food deprived to 90% of their body weight, as is required for several variations of the radial arm maze, Y-maze and T-maze tasks2,9-10. Rather, food and water is available to animals ad libitum and is only removed 30 min before each testing trial with testing typically taking place during the active dark phase. Food is returned 30 min after the last mouse in a cohort has completed the task and is dissociated temporally from the return to the pseudo-home cage so as not to act as an overt reward for completing the task. Importantly, unavailability of food and water for the entire cohort should not extend beyond a period of 4-5 h per trial. Additionally, mice representing different experimental groups should be tested together on the same nights.
The modularity of the Lashley III maze allows for modifications designed to test different aspects of learning retention. For example, while acquisition occurs with the maze in a specific configuration, once acquisition is established, the maze can be rotated so that mice must turn in the opposite direction to complete the task, i.e., mice will turn right coming out of the start box to successfully navigate the maze during acquisition, but flexibility of learning (recall) can be measured by rotating the maze so that the mice must turn left out of the start box for successful navigation. Additional variations of the test procedure include establishing learning for a set period of time (usually 14 d) and then allowing a delay interval of several weeks after which retention of maze learning is measured. Retention intervals need to be established by pilot work and are study-/strain-dependent. Longer training sessions tend to be associated with longer retention intervals. Modifications of the Lashley III maze procedure itself include adding odor cues or noise stimuli to provide additional motivation for successful maze navigation. Stressors such as restraint stress can be applied at different times prior to, during, or after acquisition to investigate state-dependent learning and memory.
An important factor to consider when implementing the Lashley III maze is the duration of the training interval. Most available strains of inbred and outbred mice, including C57Bl/6, Swiss Webster, and BXD recombinant inbred mice, will reach the learning criterion within 14 days. Some strains, especially CD-1 mice, reach criterion after just 7 days. Overtraining of some strains of mice produces aberrant behavior in the maze, wherein mice will begin to show a steady and rapid increase in the number of errors made per trial and time spent exploring the maze. Figure 4 is representative of overtraining in 4-5 month-old male CD-1 mice. After the first exposure to the maze, we institute a 15 min cut-off period per trial for each mouse. After 15 min, the mouse is led through the maze to the pseudo-home cage and errors are not counted once the 15 min mark has been reached. If several mice in a cohort or in a larger study need to be led through the maze after reaching learning criteria, this can be indicative of overtraining. To circumvent problems with overtraining and to shorten the overall test duration, we have limited the acquisition phase to four trials in some studies and averaged the learning index from the third and fourth trials to determine a learning score. This learning score can be compared in place of days to learn.
Declarações
The authors have nothing to disclose.
Acknowledgements
The Lashley III maze used for these experiments was built by Tim Bowmaster, Paul Corman, Barry Dutrow, Ryan Jabco, and Tim Treaster from the Pennsylvania State University Physics Department Machine Shop. The authors would like to thank Ms. Shoba Belegundu, Ms. Tara Chrzanowski, and Ms. Alexandra Lewis for assistance in performing the experiments presented in Figures 2-4. Mr. Walter Jackson provided valuable feedback in the preparation of this protocol. This work was supported by funding from the National Institute of Mental Health (MH064756 and MH077978 to AMA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Lashley III maze | PSU Physics Machine Shop | N/A | Maze must be custom-built, not commercially available |
Referências
- Blizard, D. A. Return to home cage’ as a reward for maze learning in young and old genetically heterogeneous mice. Comparative Med. 56, 196-201 (2006).
- Crawley, J. N. . What’s wrong with my mouse?: Behavioral phenotyping of transgenic and knockout mice. , (2007).
- Blizard, D. A., Klein, L. C., Cohen, R., McClearn, G. E. A novel mouse-friendly cognitive task suitable for use in aging studies. Behav. Genet. 33, 181-189 (2003).
- Warren, J. Appetitive learning by old mice. Exp. Aging Res. 12, 99-105 (1986).
- Lashley, K. Brain mechanisms and intelligence: A quantitative study of injuries to the brain. , (1929).
- Barnes, C. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psych. 93, 74-104 (1979).
- Matzel, L. D. Individual differences in the expression of a general learning ability in mice. J. Neurosci. 23, 6423-6433 (2003).
- Brandeis, R., Brandys, Y., Yehuda, S. The use of the Morris water maze in the study of memory and learning. Int. J. Neurosci. 48, 29-69 (1989).
- Hepler, D. J., Wenk, G. L., Cribbs, B. L., Olton, D. S., Coyle, J. T. Memory impairments following basal forebrain lesions. Brain Res. 346, 8-14 (1985).
- Sci, B. e. h. a. v. B. r. a. i. n. . 2, 313-313 (1979).

