Assessing Human Spatial Navigation in a Virtual Space and its Sensitivity to Exercise
Summary
Here, we present a novel, brief, and active spatial navigation task that assesses both spatial navigation and episodic memory ability. Importantly, spatial navigation and episodic memory were associated with one another, and this task demonstrated sensitivity to exercise.
Abstract
Spatial navigation (SN) is the ability to locomote through the environment, which requires an understanding of where one is located in time and space. This capacity is known to rely on the sequential firing of place cells within the hippocampus. SN is an important behavior to investigate as this process deteriorates with age, especially in neurodegenerative disorders. However, the investigation of SN is limited by the lack of sophisticated behavioral techniques to assess this hippocampal-dependent task. Therefore, the goal of this protocol was to develop a novel, real-world approach to studying SN in humans. Specifically, an active virtual SN task was developed using a cross-platform game engine. During the encoding phase, participants navigated their way through a virtual city to locate landmarks. During the remembering phase, participants remembered where these reward locations were and delivered items to these locations. Time to find each location was captured and episodic memory was assessed by a free recall phase, including aspects of place, order, item, and association. Movement behavior (x, y, and z coordinates) was assessed through an asset available in the game engine. Importantly, results from this task demonstrate that it accurately captures both spatial learning and memory abilities as well as episodic memory. Further, findings indicate that this task is sensitive to exercise, which improves hippocampal functioning. Overall, the findings suggest a novel way to track human hippocampal functioning over the course of time, with this behavior being sensitive to physical activity training paradigms.
Introduction
Moving the body through time and space is critical for learning and remembering information about the environment. This ability is known as spatial navigation, and evolutionarily speaking, it is an essential survival tool for locating food, water, social counterparts, and other rewards in the environment1,2. Spatial navigation is dependent upon the hippocampus, a c-shaped limbic system structure in the medial temporal lobe. The hippocampus consists of the CA1, CA2, CA3, and dentate gyrus subregions. The hippocampus supports the encoding, consolidation, and retrieval of memories that help define the conscious experience. Specifically, spatial navigation supports episodic memory, a form of explicit memory that refers to the memory of personal experience, including aspects of time, place, and relevant details associated with the experience (e.g., sights, sounds, smells, emotions). As we spatially navigate through distinct environments, neurons known as place cells systematically fire, enabling us to understand where we are in both time and space. In fact, direct optical stimulation of these neurons has been shown to bias the behavior of rodents towards their physical location (i.e., place fields)3.
Assessing spatial navigation in rodents has traditionally been studied through such behavioral paradigms as the Morris Water Maze, Y maze, T maze, and radial arm maze4,5. Importantly, these behavioral tasks allow for the in vivo investigation of the neural correlates of spatial navigation using techniques such as electrophysiological depth recordings. However, assessing spatial navigation in humans has proven scientifically challenging because most scientific investigations happen in laboratories and not out in the real world. Previous studies in humans have assessed spatial abilities with traditional paper-based tasks such as bi-directional map learning tasks, mental rotation tasks, or spatial memory tasks6,7. Others have utilized computer-based tasks such as the Virtual Morris Water Task or other virtual maze tasks, which have been shown to be correlated with more traditional psychometric measures of spatial ability8,9. Additionally, with the accessibility of publicly available and free video game software packages, researchers have begun to develop 3-dimensional virtual environments that can either be presented on a computer screen or in virtual reality10,11,12,13,14,15. Scientific advances in mobile brain-body imaging (MoBI) have also allowed researchers to begin to explore spatial navigation in real-world settings16,17,18.
Importantly, spatial learning and memory is a cognitive ability that deteriorates with age, with elderly individuals being more likely to lose track of where they are or become lost when they try to return home. This deficit is most likely due to neurodegeneration that occurs at the level of the hippocampus – a highly plastic brain area that is one of the first to deteriorate with age19. Therefore, developing real-world methods to assess spatial navigational and episodic memory abilities is an important avenue of research. At the clinical level, these types of tasks may help determine the progression of memory decline or diagnose mild cognitive impairment, Alzheimer's disease, or other forms of dementia. Conversely, physical activity has been identified as one of the best mechanisms to improve spatial navigational abilities. Studies in rodents have shown that exercise enhances learning and memory on various spatial tasks, including the Morris Water Maze, Y maze, T maze, and radial arm maze20. Exercise-induced improvements in spatial abilities have also been demonstrated in humans, with this effect being significantly related to an increase in hippocampal volume7. However, this behavioral effect was demonstrated using a spatial memory task where participants were asked to remember the locations of dots on a screen – a task that may not hold much ecological validity to real-world spatial navigation. Little research has investigated the impact of exercise in humans on spatial navigational tasks presented in virtual environments.
Therefore, a cognitive task was designed to assess spatial learning and memory along with episodic memory using a virtual environment. Importantly, the task was designed using modern-day video game software to enable up-to-date graphic designs and realistic features (e.g., moving clouds in the sky). This task was tested in a group of healthy adults before and after they experienced long-term aerobic exercise practice. Results indicate that participants can encode and remember both spatial information as well as episodic memories regarding their virtual experience. Additionally, findings indicate that performance on this task is plastic, being affected by exercise.
Specifically, a virtual environment was developed through a cross-platform game engine21 that evaluated spatial navigation and episodic memory ability, unique cognitive skills supported by the hippocampus. The map used for this environment was derived from Miller et al. (2013)22. The game engine that was utilized allows developers to download assets to add unique features for the purposes of building virtual environments. An asset23 was utilized that allowed us to build a realistic city environment with roads and buildings through which participants could navigate. Additionally, an asset24 was used that allowed tracking of participants' x, y, and z coordinates and rotation while they traveled through the virtual environment. The aforementioned asset allowed the recording of these features on a millisecond timescale (~33 ms). The virtual environment was then compiled and administered as a spatial navigation task that participants could complete at home on a laptop or desktop computer. The below protocol details how to administer and engage with this spatial navigation task.
Protocol
All study documentation and data collection methods were approved by and in compliance with the New York University Committee on Activities Involving Human Subjects. Participants gave their informed consent prior to participating in any study-related activities.
1. Setting up gameplay
- Download the necessary files from the following public repository: https://github.com/embodiedbrainlab/BassoSpatialNavigationTask
- Download Unity Hub from unity.com/download and install Unity version 5.3.1f1.
- Open the file downloaded from the repository in step 1.1 as a Project on Unity.
- Once the project has been created with the downloaded files, select the File tab on top of the window and select Build and Run.
- The Build Settings window will first appear. Select SpatialNavigation > Scenes > Big City B Lures and Scenes/LeFin. Select the PC, Mac, & Linux Standalone and then click the Build and Run button.
NOTE: A window will appear asking the researcher to save a .exe (Application) file. Once the researcher has built the application, they can double-click the application to run future iterations of the protocol. If the researcher decides to run this file, its respective results will be saved in the same directory in which the application is located. - A window titled SpatialNavWeb Configuration will appear. Adjust the screen resolution and graphics quality under the Graphics tab. Change the controls of the game under the Input tab.
- Click Play! to begin the spatial navigation task.
2. Recording brain activity with electroencephalography (EEG) during the spatial navigation task
NOTE: EEG measures the activity of neurons in the cortex of the human brain in microvolts on a millisecond timescale through electrodes placed on the scalp. EEG is a noninvasive form of brain imaging that allows a participant's brain to be scanned while they conduct other activities, such as navigating virtual environments.
- Using a measuring tape, measure the participant's head from inion to nasion to ensure the proper fitting of the EEG cap.
- Place electrodes into the EEG cap (if necessary) and outfit the participant with the EEG cap, ensuring proper fitting and placement (Figure 1A).
- Start the EEG software. Fill each electrode with electrode gel to ensure that impedance measurements are below 25 kΩ.
- Once the EEG signal looks clean and without significant artifacts, begin recording.
- Have a member of the research team watch the participant as the participant performs the below steps.
- Send a trigger pulse to the EEG recording system at each of the following events (Figure 1B)
Start of the encoding phase
End of the encoding phase
Start of the remembering phase
End of the remembering phase
Start of the episodic memory phase
End of the episodic memory phase
Any other events that the researcher finds of interest
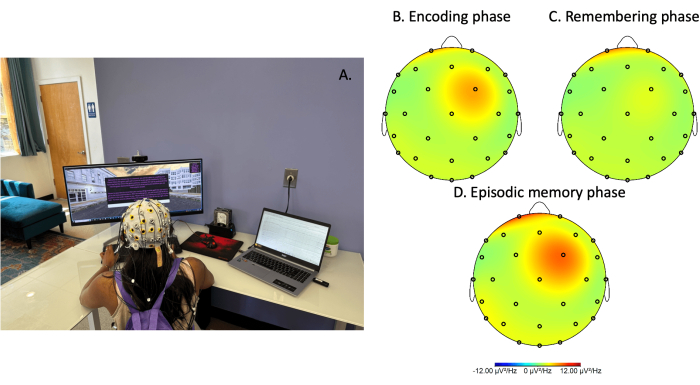
Figure 1: Electroencephalography recording during spatial navigation gameplay. (A) Image of a person equipped with a mobile electroencephalography (EEG) device while performing the spatial navigation task. Power spectral density plot of theta activity (4-8 Hz) during the (B) encoding phase, (C) remembering phase, and (D) episodic memory phase. All data was preprocessed and power normalized by frequency (uV2/Hz). Please click here to view a larger version of this figure.
NOTE: Researchers using Arduino technologies may also be able to send synchronized triggers between the EEG recording and the game-engine environment so that an exact pairing between the neurophysiological and behavioral data can occur on a millisecond timescale. With these markers, researchers will be able to refer back to the participant's brain activity before, during, and after critical interactions with the virtual environment. Researchers may also consider conducting a period of baseline brain activity before and/or after engagement with the virtual environment so that later comparisons can be made.
3. Instructions for the spatial navigation task (Figure 2)
- Instructions: Ensure that the participant is seated comfortably, ideally with their feet on the floor. Have the participant read the instructions on the screen, which will ask them to visit specific landmarks within a cityscape while trying to remember their surroundings and the paths they took (Figure 2A).
- Ensure that the participant is oriented to the mouse and the keyboard. Instruct the participant to use the mouse and left-click to begin the task (Figure 2A).
- Ensure the participant understands that they will need to navigate the environment with the W, A, S, and D keys on their keyboard.. The W key will move them forward, whereas the S key will move them backward. Alternatively, the up and down arrows will also move them forward and backward. The A key will move them to the left, and the D key will move them to the right.
- Ensure that the participant knows they can use the computer mouse to move the subject's point of view as if the participant were moving the head. Participants can look up, down, left, and right; no mouse clicks are necessary to move their point of view.
NOTE: Directions on navigating the virtual environment will appear in the top right corner of the participants' screen (Figure 2A-F).

Figure 2: Images of spatial navigation task. Screenshots of the spatial navigation and episodic memory task developed in a cross-platform game engine. Example screenshots are presented from left to right, starting at the top left corner: (A) overall instructions; (B) travel during encoding phase; (C) locating storefront during the encoding phase; (D) travel during encoding phase; (E) instructions for remembering phase; (F) delivery portion of remembering phase; (G) instructions for episodic memory phase; (H) episodic memory phase; (I) end of game. Please click here to view a larger version of this figure.
4. Encoding phase of the spatial navigation task
- Have the participant visit the first landmarks (Figure 3) by having them actively follow a green path with green arrows (Figure 2B).
- Once the participant arrives at the first landmark, have the participant walk through the green diamond at that location (Figure 2C).
- Once the participant collects the green diamond, have the participant visit the next landmark by following the green path. Once the participant arrives at the second landmark, have the participant walk through the green diamond at that location.
- Have the participant continue performing this task until the participant visits all five landmarks and collects all five diamonds (Figure 2D).
NOTE: Throughout the encoding phase of this task, participants will be asked to memorize the location of the five landmarks throughout the city (Figure 3). A bird's-eye view of the task is presented in Figure 4.

Figure 3: Images of storefronts. Participants visited five of the eighteen locations developed in the environment, each with a unique and detailed storefront. Examples of these locations included (A) a pizza parlor, (B) a vitamin shop, (C) a furniture store, (D) a wedding store, (E) a kiosk, and (F) a casino. Please click here to view a larger version of this figure.
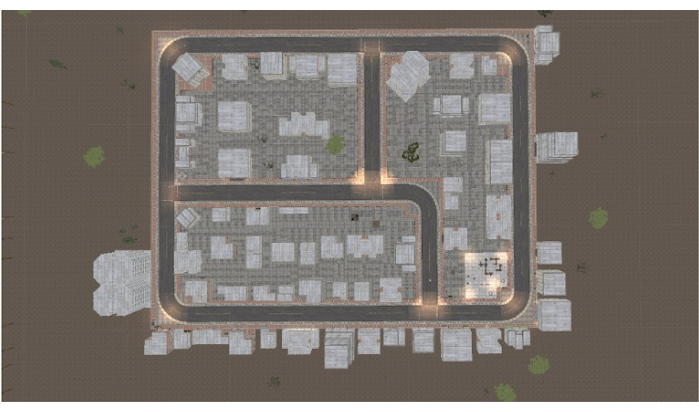
Figure 4: Map of spatial navigation task. Bird's eye view of the virtual environment through which participants navigated. Please click here to view a larger version of this figure.
5. Remembering phase of the spatial navigation task
- Next, have the participant revisit each landmark (i.e., remembering phase; Figure 2E).
NOTE: Participants will begin the remembering phase from the last location they visited during the encoding phase. - Have the participant use the mouse and left-click on top of Begin (Figure 2E).
- Have the participant visit the first landmark that the participant visited during the encoding phase.
- Have the participant "deliver" a unique item to this first landmark.
NOTE: No green path/arrows will be offered during this portion of the task (Figure 2F). - Once the participant delivers the item, have the participant navigate to the second landmark and deliver the next unique item. Have the participant continue performing this task until the participant visits all five landmarks and delivers all five items.
NOTE: This portion of the task will assess the participant's spatial learning and memory ability. To do this, the program will automatically calculate the time to find each landmark, the average seek time, and the total time of the task.
6. Episodic memory phase of the spatial navigation task
NOTE: Episodic memory testing will take place after the remembering phase is complete.
- To begin, have the participant use the mouse to left-click on top of Begin (Figure 2G).
- Have the participant recall the landmarks the participant visited and the items the participant delivered in the exact order as instructed earlier in the remembering phase (Figure 2G). Have the participant type the responses using the computer keyboard (Figure 2H).
7. Completing the task
- Have the participant read the final prompt to confirm the completion of the task and the submission of the data (Figure 2I).
8. Data collection and analysis
- Behavioral data
- Locate the Results.csv file in the application's directory (see Supplementary File 1 for example).
NOTE: If the researcher decides to click Build and Run under the File tab on the Unity Application, the Results file will be saved in the downloaded BassoSpatialNavigationTask-main folder. If the researcher instead ran the environment by double-clicking the built Application (step 1.5), the Results file will appear in the same directory as the application. The Results file is overwritten after each completion of the virtual environment. Thus, it is recommended to extract these results after each completion of the task and compile them in a separate file for multiple participants and trials. - Ensure that the data is clean and looks reasonable.
- Utilize Supplementary File 2 to calculate the appropriate scores, including start time, end time, average seek duration, place score, item score, order score, association score, and episodic memory score.
NOTE: Specifically, the place score is calculated by tallying the number of landmarks correctly recalled. Order score is calculated by determining the number of landmarks recalled in the correct sequence. Item score is calculated by tallying the number of items correctly recalled. The association score is calculated by tallying the correct pairing of place to item. Finally, the overall episodic memory score is calculated by summing the place, order, item, and association scores. Note that the raw output for the X/Z coordinates is not in the correct temporal sequence. To remedy this, sort the data in the Time column from the smallest to largest values. - Enter the data into a database of choice.
- Analyze the data using independent samples t-test, analysis of variance, or other appropriate statistical tests.
- Locate the Results.csv file in the application's directory (see Supplementary File 1 for example).
- EEG data
- Use a preprocessing pipeline to clean the EEG data25.
- Using an appropriate software package, conduct time-frequency analysis on EEG data over prolonged periods of time in which the participant navigated the virtual environment, such as during the encoding and remembering phases of the task.
- Conduct event-related potential analysis if interested in specific time periods that the participant interacted with the virtual environment.
- Conduct statistical analysis relevant to the EEG data and consider correlating the behavioral data with the EEG data.
Representative Results
Description of gameplay from a coding perspective: For the "encoding" phase, a series of eighteen waypoints were placed around the 3-dimensional space, with each having an associated "Delivery Item" (i.e., item to deliver to the location). References to these waypoints were stored in the player controller and ordered statically prior to beginning the task; that is, if the pizza shop was placed in position one, it would always be in position one at first. In order to provide some degree of randomness to the waypoints that participants encountered, the waypoint list was shuffled via the Fisher-Yates shuffle algorithm. The Fisher-Yates shuffle, as implemented for this study, generates a pseudorandom permutation of the original sequence in place. Any possible permutation can be generated with equal likeliness. The algorithm begins by selecting an element from the end of the list (n). A pseudorandom number is generated in the range of [0, n] and assigned to the value k. The nth value is then swapped with the kth value. Next, the value of n is decremented by one, and the process repeats until there is only a single index not yet considered.
After the list of waypoints was shuffled, the first five elements were selected. Optimal paths were generated via the game engine's navigation mesh system and built-in optimal path calculations. This series of paths began at the starting location of the participant and created a linked chain between each of the waypoints, terminating at the final waypoint. When the participants gained control, they were directed to follow these paths, designated by a green line and a moving arrow that provided intended direction information. Though this green line and moving arrow were provided, participants were able to actively navigate throughout the virtual environment. When the participant entered the bounds of the waypoint, the displayed path was swapped with the next path in the list.
Upon visiting the intended number of waypoint elements, the participant entered the "remembering" phase (termed RevisitIntermission in the code), where they were directed to revisit the landmarks in the order that they were previously shown. As the participant attempted to revisit the locations presented during the guided tour, they were presented with an image specified by the waypoints' associated "Delivery Item". They were not presented with a suggested path. Their movements were tracked with an object motion tracker component sourced from the asset store.
When the participants finished traveling to each presented waypoint, they were given instructions directing them to the next screen to recall the locations they had visited and the items delivered to each one. During the recall phase, the participants were presented with a prompt with two text entries. The first dictated the waypoint the participant was asked to travel to. The second dictated the "Delivery Item" associated with this waypoint. Response and response time were recorded for each prompt.
At the end of the task, the data were collected and stored in JSON representation. The first section recorded the revisit phase, where participants were asked to find locations without the aid of a guiding line. Recorded values included the waypoint name, the "Delivery Item" name, and the time it took to arrive at the waypoint. The second section recorded the responses presented during the recall phase. This section included participant responses for location, "Delivery Item", and the time it took to respond to the aforementioned prompts. All code can be found and downloaded at https://github.com/embodiedbrainlab/BassoSpatialNavigationTask.
Power analysis and statistics: A correlation point biserial model power analysis was conducted with G*Power 3.1 using a two-tailed test, an effect size of 0.3, alpha level of 0.05, and a power of 0.8 to determine a sample size of n = 8226. Descriptive statistics were used to assess participants' age, number of cycling classes, and general measures, including both spatial navigation and episodic memory abilities. An independent samples t-test was utilized to test significant differences between the total number of workouts between experimental and control groups. Considering that not all data were normally distributed, as assessed by Shapiro-Wilk's test (p<0.05), we utilized the non-parametric Spearman's rho correlation coefficient to assess relationships between spatial navigation and episodic memory abilities as well as age and spatial navigation abilities. An alpha value of 0.05 was utilized to determine statistical significance. Bonferroni corrections were used in a family of statistical tests where appropriate. IBM SPSS Statistics Version 26 was utilized for all statistical analyses. Pearson's product-moment correlation was utilized to assess the relationship between the total number of cycling workouts and spatial navigation abilities, as this was the procedure conducted by Basso et al. (2022)27.
Participants: N = 130 participants were recruited from Austin, TX, through various techniques, including online and flyer advertisements. Inclusion criteria included having English as their primary language and being between the ages of 25-55 years of age (average 30.16 ± 0.49). Additionally, all participants needed to report being physically healthy and having a moderate and regular exercise regimen (defined as exercising one or two times per week for 20 min or more for the past 3 months). Exclusion criteria included being a current smoker or preexisting physical health conditions that made exercise difficult or unsafe. Exclusion criteria also included having a current diagnosis of and/or taking medication for psychiatric or neurological conditions, including anxiety, depression, bipolar disorder, schizophrenia, or epilepsy.
For pre-intervention data, n = 11 participants were missing data due to technical issues, and n = 1 participant was excluded due to non-task adherence, leaving a total of n = 117 participants for analysis. Of the n = 80 participants that completed the three-month exercise regimen, n = 11 participants did not complete the final spatial navigation task, leaving a total of n = 69 participants for analysis of post-intervention and repeated measures data. This smaller sample size was utilized to examine the relationship between the number of cycling sessions and spatial navigation abilities. The control group engaged in 20.73 (± 0.72) workouts over the course of the intervention, whereas the experimental group engaged in 47.87 (± 2.24) workouts, which represented a statistically significant difference (t[45.76] = −11.554, p < 0.001).
General measures and their relationships: This new virtual environment task measures both spatial navigation and episodic memory capacity. During the initial pre-intervention testing period, the task took an average of 318.69 (±21.56) s to complete, with the average seek time for each of the five sites being 82.88 (±5.19) s (Figure 5A); these data points represent spatial navigation ability (i.e., spatial learning and memory). Additionally, participants were able to encode place, item, order, and association aspects of the virtual experience, with participants remembering 14.84 (±0.37) out of 20 novel experiences in their environment (Figure 5B); these data points represent episodic memory ability. Importantly, the total time (Figure 6A; r = -0.314, p < 0.001) and average seek time (Figure 6B; r = -0.286, p < 0.001) were significantly correlated with the episodic memory score, indicating that spatial navigation ability is associated with episodic memory in this task.
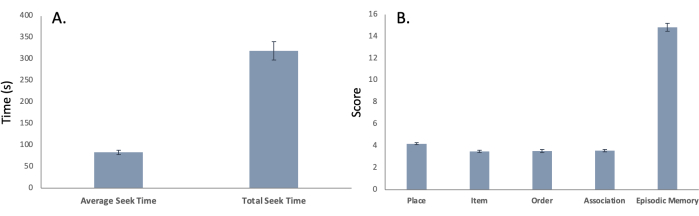
Figure 5: Time of task. Mean (± SEM) for both (A) spatial navigational ability represented in both average seek time and total seek time (provided in seconds) and (B) episodic memory ability represented in the encoding and remembering of place, item, order, association and overall episodic memory score. Please click here to view a larger version of this figure.
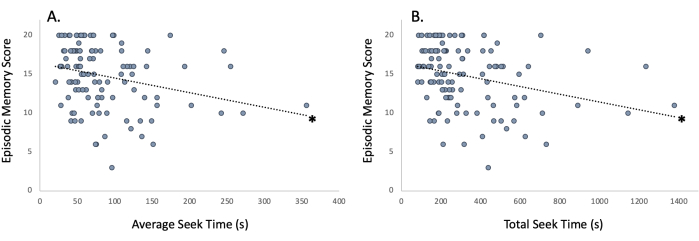
Figure 6: Relationship of spatial navigation ability to episodic memory. Improved spatial navigation ability as represented by shorter (A) average seek time and (B) total seek time is associated with enhanced episodic memory as represented by the episodic memory score. *p < 0.001. Please click here to view a larger version of this figure.
X and z coordinates represented in virtual space: Using an object motion tracker asset, x and z coordinates were tracked in this 3-dimensional virtual space (Supplementary File 1). As moving up and down in the game (i.e., jumping) is not enabled in this spatial navigation task, y coordinates did not provide useful information. However, the x and z coordinates enabled us to assess how the participant moved throughout the game. Based on this data, computer code was designed to visually display a heat map of where the participant traveled throughout the map. Figure 7 displays a heat map from one representative participant, which highlights the route the participant took during the remembering phase. The spots that are highlighted in yellow/red correspond to the delivery (i.e., reward) locations on the map.
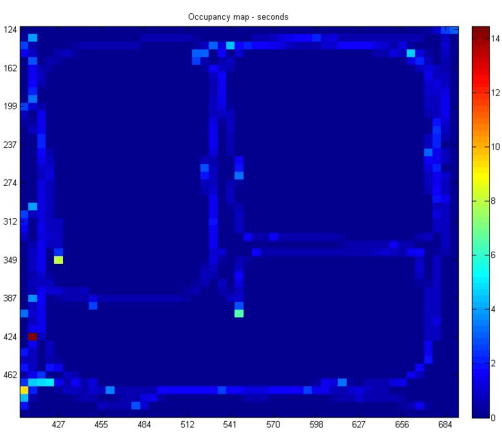
Figure 7: Occupancy heat map. Occupancy heat map demonstrating the route of the participant. Yellow/red sections of the graph represent those locations where the participant frequented and correspond to places in the spatial navigation task where participants had to deliver items (i.e., reward locations). Please click here to view a larger version of this figure.
Relationship between age and spatial navigation abilities: Initial investigations indicated that spatial navigation ability as assessed by total seek time was significantly associated with age (Figure 8; r = 0.157, p = 0.045). As age increases, spatial navigation ability decreases, as evidenced by an increased total seek time. However, when the Bonferroni correction was applied, with statistical significance being assessed at p = 0.025 for two correlations (i.e., total seek time and average seek duration), the correlation was no longer significant.
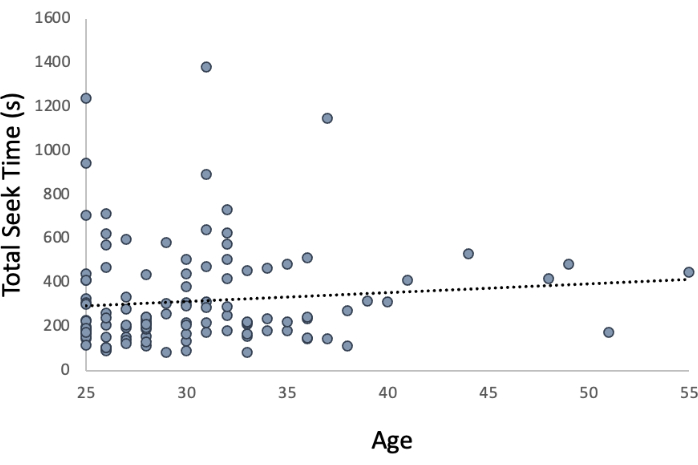
Figure 8: Relationship of spatial navigation ability to age. When evaluated using a Bonferonni correction (p < 0.025), age was not significantly associated with spatial navigation ability as represented by total seek time. Please click here to view a larger version of this figure.
Relationship between aerobic exercise training and spatial navigation abilities: Aerobic exercise training took place at an indoor cycling studio28. All classes were 45 min in duration and included cycling at moderate to vigorous intensities throughout the duration of the class. Participants underwent random assignment to either maintain their existing exercise regimen or increase their exercise regimen. Participants who maintained their exercise regimen engaged in 1 to 2 classes per week, whereas participants who increased their exercise regimen engaged in 4 to 7 classes per week. Participants engaged in their assigned exercise regimen for a period of 3 months. Spatial navigation and episodic memory ability were tested before and after exercise training. Additional details of the intervention can be found in Basso et al. (2022)27. The total number of cycling classes over the course of three months was significantly associated with average seek duration (Figure 9A; r = -0.321, p = 0.007) and total seek time (Figure 9B; r = -0.242, p = 0.045). However, when the Bonferroni correction was applied, with statistical significance being assessed at p = 0.025 for two correlations (i.e., total seek time and average seek duration), the correlation for total seek time was no longer significant. Additional findings from the intervention can be found in Basso et al. (2022)27.
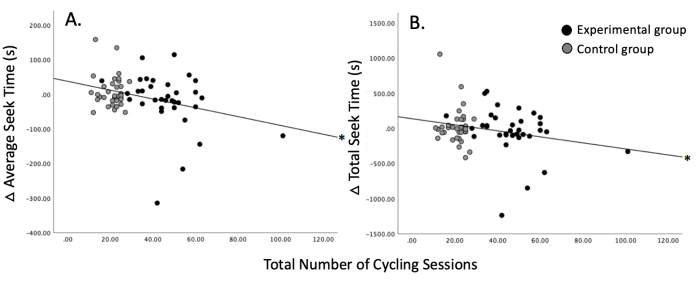
Figure 9: Relationship of spatial navigation ability to exercise. An increased number of cycling sessions is associated with improved spatial navigation ability as represented by (A) average seek time and (B) total seek time. *p < 0.05. This figure has been modified with permission from Basso et al.27. Please click here to view a larger version of this figure.
Supplementary File 1: Raw data 1. Raw data, including information regarding the remembering (revisit) and episodic memory (recall) phase of the spatial navigation task. Data regarding the participant's x and z coordinates from traveling around the 3-dimensional virtual space during the encoding and remembering phases of the experiment is also presented. Please click here to download this File.
Supplementary File 2: Raw data 2. Raw data with calculations (presented in red) to determine start time, end time, average seek duration, place score, item score, order score, association score, and episodic memory score. Please click here to download this File.
Discussion
This study examined the effectiveness of a novel virtual reality task in assessing spatial navigation in humans. This cognitive task, which only takes about 10 min to complete, can be utilized to assess two unique types of hippocampal-dependent cognition – spatial navigation and episodic memory ability. Importantly, spatial navigational ability was significantly associated with episodic memory ability. Finally, this task was sensitive to a physical activity training paradigm. That is, increased exercise was associated with increased performance. This task was inspired by the work of Miller et al. (2013) who investigated virtual environments in patients with drug-resistant epilepsy and hippocampal depth electrodes placed for the purpose of seizure localization. They found that during the familiarization phase of the spatial navigation task (i.e., the encoding phase), place-responsive cells in the hippocampus and associated medial temporal lobe structures became activated22. Additionally, they found that when participants were engaged in a free recall component (i.e., a remembering phase that did not involve active navigation), the same place-responsive cells that were active during encoding became active once again. Extant studies in rodents utilizing open field and maze-like environments have shown the existence of such place cells, with Drs. John O'Keefe, May-Britt Moser, and Edvard Moser winning the 2014 Nobel Prize in Physiology or Medicine for this discovery2,29,30,31. Additionally, studies using virtual environments in humans have shown that similar cells in the human hippocampus encode travel through time and space22,32,33. Though the task is similar to the one presented in Miller et al. (2013) and others22,34,35,36,37,38, it was developed with the most current cross-platform game engine and technologies, utilizing real-world features such as moving clouds and clear city landmarks and storefront features. Other researchers have utilized other spatial navigation tasks in humans; however, these tasks are limited in their ecological validity. For instance, the virtual Starmaze task is used to assess navigation abilities but places participants in a star-shaped maze39,40,41,42,43,44. Moreover, NavWell is an accessible platform that hosts spatial navigation and memory experiments akin to the Morris Water Maze in rodents (placing participants in a circular arena), and provides developers with basic geometric shapes to build an environment45. Additionally, the Landmarks assets on cross-platform game engines are available for building and developing spatial navigation tasks that exist in a square setting12. The present task is unique in that it provides users with a setting and task similar to the real world – navigating a cityscape and memorizing landmarks and actions. The task is also different from the virtual Starmaze task and NavWell because it assesses episodic memory in addition to spatial navigation.
In this task, spatial navigation ability was significantly related to episodic memory ability. Others have shown that these two cognitive abilities are indeed distinct and that they rely on different regions of the hippocampus38,46. The popular "Cognitive Map Theory" states that the brain builds and stores a "map" of an individual's spatial environment so that it can later be used in the future to guide actions and behaviors47. Research has suggested that the hippocampus encodes spatial information while also supporting episodic memory formation. More specifically, it is thought that the right hippocampus encodes spatial memory while the left hippocampus stores episodic memories38. Results of the current novel spatial navigation task, which demonstrate a clear link between spatial and episodic memory, lend support to the Cognitive Map Theory and suggest that this task could potentially be used to examine the relationship between spatial navigation and episodic memory in non-clinical populations. Future studies should seek to examine this relationship in clinical populations, including those with neurodegenerative disorders such as mild cognitive impairment, Alzheimer's disease, or other types of dementia.
This task was sensitive to exercise or the total amount of cycling sessions engaged in over a 3-month period. Previous studies in rodents have shown that exercise is one of the most potent ways to increase hippocampal-dependent cognition, including long-term memory, pattern separation, spontaneous alternation, contextual fear conditioning, passive avoidance learning, and novel object recognition, with this effect being dependent on exercise-induced increases in hippocampal neurogenesis48,49,50. Additionally, the literature has shown that long-term exercise enhances hippocampal functioning in humans, with improvements seen in word list recall, story recall, and both spatial and non-spatial relational memory; this effect is thought to be driven by exercise-induced increases in hippocampal volume7,27,51,52,53,54,55. This new spatial navigation task complements the rodent findings and adds to the human literature, showing the importance of physical activity for spatial navigation abilities.
Though in initial investigations, age was negatively associated with spatial navigation ability, this effect was eliminated when applying a Bonferroni correction. This indicates that spatial navigation ability may be preserved through the age of 55. Other literature demonstrates that spatial navigation is a cognitive ability that declines with age56,57,58. Neuroimaging studies have revealed that age-related neurodegeneration in areas including the hippocampus, parahippocampal gyrus, posterior cingulate cortex (retrosplenial cortex), parietal lobes, and prefrontal cortex may be involved in such age-related cognitive decline58. Considering that the age range was limited (25-55 years of age), by including a larger age range, especially older adults (65+), future researchers may see a significant correlation between age and spatial navigation ability. Future studies should consider conducting this spatial navigation task in adults 65 years and older and even those with mild cognitive impairment or other dementia-like disorders.
One obvious missing link in virtual navigation tasks is the lack of the body-brain relationship. That is, in navigating through real-world environments, the activation occurs at the level of the peripheral and central nervous systems, including activation of the proprioceptors, exteroceptors, interoceptors, and vestibular system along with the sensori-motor cortices, basal ganglia, and cerebellum. Without this physical input, virtual navigation may be distinctly different from physical navigation. Despite this, studies have shown that virtual environments stimulate the same brain regions as real-world navigation22,32,33. Making the task more active, as was the design in the current task, may help convince the brain that it is physically moving through time and space, mimicking natural spatial navigation. Others have found support for this hypothesis. A study by Meade et al. (2019) examined the differences between active and passive encoding while using a similar virtual spatial navigation task59. Active navigation refers to participants being able to move on their own through the virtual space (akin to the present study), while passive navigation consists of a guided tour where participants do not move but are rather shown the navigational route. The authors suggested that active navigation may be more beneficial for older populations due to the involvement of physical (e.g., locomotion and proprioception) and cognitive components (e.g., decision-making and attention), and may serve to enhance memory performance through direct involvement in the process of memory encoding. The active navigation utilized in the present study could explain the results, demonstrating that participants were able to accurately recall episodic memories of their experiences.
Active navigation may also help to engage multisensory integration areas such as the retrosplenial complex (RSC)60,61,62. A recent study found that actual ambulation during a virtual reality spatial navigation task requiring participants to travel between locations while remembering home and landmark locations resulted in RSC theta oscillations (i.e., 4-8 hertz neuronal oscillations recorded with EEG)16. This increased theta power was most prominent during head direction changes and rotations. In rodents, it has been shown that RSC theta activity is essential to spatial coding involving grid cells and head direction computation63,64. The RSC is also thought to be important for using cues from the environment to anchor a human's cognitive map47.
While virtual spatial navigation tasks provide many benefits, they preclude the individual from physically moving through time and space, causing limited activation of the proprioceptive, vestibular, and sensori-motor systems. An incongruence exists between sensory and motor processes, which may cause some participants to become dizzy or nauseous. In the present task, this was limited by controlling the speed at which participants were able to move through and look around the environment. To be able to encode all aspects of the environment, it was necessary to be able to look around (i.e., engage in virtual head rotation); however, this ability needed to be slow enough to ensure that participants did not get physically ill. Despite this, the ability to spatially navigate while sedentary is advantageous in that it allows researchers to study individuals who experience mobility issues, physical fatigue, or other disabilities that prevent an individual from being ambulatory. Another limitation is that this task has not yet been tested for reliability and validity, while other tasks are moving in this direction, including the virtual spatial navigation assessment (VSNA)65. Future research could examine associated neural activity through electroencephalography or functional magnetic resonance imaging while participants complete this spatial navigation task. Participants could also be fitted with devices measuring physiological variables such as heart rate variability and electrodermal activity. This would allow for an examination of both the peripheral and central mechanisms that occur while navigating virtual environments. Importantly, this task can be used to assess changes in spatial navigation ability over time. Future studies could utilize this task to investigate how aging or neurodegenerative conditions such as Alzheimer's or Parkinson's disease affect an individual's spatial navigation and episodic memory. Conversely, this task could be used to explore how additional mind-body-movement interventions affect spatial navigation and episodic memory, including dance, yoga, or meditation.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the iTHRIV Scholars Program, which is supported in part by the National Center for Advancing Translational Sciences of the NIH (UL1TR003015 and KL2TR003016). We would like to acknowledge Dr. Samuel McKenzie, Michael Astolfi, Meet Parekh, and Andrei Marks for their computer programming contributions.
Materials
| Unity Real-Time Development Platform | Unity | Unity Student / Unity Personal | https://unity.com/ |
References
- Maguire, E. A., Burgess, N., O’Keefe, J. Human spatial navigation: cognitive maps, sexual dimorphism, and neural substrates. Current Opinion in Neurobiology. 9 (2), 171-177 (1999).
- Buzsáki, G., Moser, E. I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neuroscience. 16 (2), 130-138 (2013).
- Robinson, N. T. M., et al. Targeted activation of hippocampal place cells drives memory-guided spatial behavior. Cell. 183 (7), 2041-2042 (2020).
- Fordyce, D. E., Farrar, R. P. Physical activity effects on hippocampal and parietal cortical cholinergic function and spatial learning in F344 rats. Behavioural Brain Research. 43 (2), 115-123 (1991).
- van Praag, H. Neurogenesis and exercise: past and future directions. Neuromolecular Medicine. 10 (2), 128-140 (2008).
- Heo, S., et al. Resting hippocampal blood flow, spatial memory and aging. Brain Research. 1315, 119-127 (2010).
- Erickson, K. I., et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 108 (7), 3017-3022 (2011).
- Dobbels, B., et al. The virtual Morris water task in 64 patients with bilateral vestibulopathy and the impact of hearing status. Frontiers in Neurology. 11, 710 (2020).
- Moffat, S. D., Hampson, E., Hatzipantelis, M. Navigation in a "Virtual" maze: Sex Differences and correlation with psychometric measures of spatial ability in humans. Evolution and Human Behavior. 19 (2), 73-87 (1998).
- Ijaz, K., Ahmadpour, N., Naismith, S. L., Calvo, R. A. An immersive virtual reality platform for assessing spatial navigation memory in predementia screening: Feasibility and usability study. JMIR Mental Health. 6 (9), 13887 (2019).
- Sakhare, A. R., Yang, V., Stradford, J., Tsang, I., Ravichandran, R., Pa, J. Cycling and spatial navigation in an enriched, immersive 3d virtual park environment: A feasibility study in younger and older adults. Frontiers in Aging Neuroscience. 11, 218 (2019).
- Starrett, M. J., et al. Landmarks: A solution for spatial navigation and memory experiments in virtual reality. Behavior Research Methods. 53 (3), 1046-1059 (2021).
- Diersch, N., Wolbers, T. The potential of virtual reality for spatial navigation research across the adult lifespan. The Journal of Experimental Biology. 222, 187252 (2019).
- Kuhrt, D., St John, N. R., Bellmund, J. L. S., Kaplan, R., Doeller, C. F. An immersive first-person navigation task for abstract knowledge acquisition). Scientific Reports. 11 (1), 5612 (2021).
- Castelli, L., Latini Corazzini, L., Geminiani, G. C. Spatial navigation in large-scale virtual environments: Gender differences in survey tasks. Computers in Human Behavior. 24 (4), 1643-1667 (2008).
- Do, T. -. T. N., Lin, C. -. T., Gramann, K. Human brain dynamics in active spatial navigation. Scientific Reports. 11 (1), 13036 (2021).
- Jungnickel, E., Gramann, K. Mobile brain/body imaging (MoBI) of physical interaction with dynamically moving objects. Frontiers in Human Neuroscience. 10, 306 (2016).
- Park, J. L., Dudchenko, P. A., Donaldson, D. I. Navigation in real-world environments: New opportunities afforded by advances in mobile brain imaging. Frontiers in Human Neuroscience. 12, 361 (2018).
- Bettio, L. E. B., Rajendran, L., Gil-Mohapel, J. The effects of aging in the hippocampus and cognitive decline. Neuroscience and Biobehavioral Reviews. 79, 66-86 (2017).
- Wang, Z., van Praag, H., Boecker, H., Hillman, C., Scheef, L., Struder, H. Exercise and the Brain: Neurogenesis, Synaptic Plasticity, Spine Density, and Angiogenesis. Functional Neuroimaging in Exercise and Sport Sciences. , (2012).
- . Unity Real-Time Development Platform. Unity Available from: https://unity.com/ (2023)
- Miller, J. F., et al. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science. 342 (6162), 1111-1114 (2013).
- Urban construction pack. Unity asset store. At Available from: https://assetstore.unity.com/packages/3d/environments/urban-construction-pack-8081#reviews (2018)
- . Object Motion Tracker. Unity Available from: https://forum.unity.com/threads/object-motion-traker-engine-trails-time-travel-game-mechanics-and-more.241544/ (2014)
- Makoto’s preprocessing pipeline. EEGLAB Available from: https://sccn.ucsd.edu/wiki/Makotos_preprocessing_pipeline (2023)
- Faul, F., Erdfelder, E., Buchner, A., Lang, A. -. G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior Research Methods. 41 (4), 1149-1160 (2009).
- Basso, J. C., et al. Examining the effect of increased aerobic exercise in moderately fit adults on psychological state and cognitive function. Frontiers in Human Neuroscience. 16, 833149 (2022).
- Keefe, J. O., Nadel, L. . The Hippocampus as a Cognitive Map. , (1978).
- Eichenbaum, H. The Hippocampus as a Cognitive Map . . . of Social Space. Neuron. 87 (1), 9-11 (2015).
- Moser, E. I., Kropff, E., Moser, M. -. B. Place cells, grid cells, and the brain’s spatial representation system. Annual Review of Neuroscience. 31, 69-89 (2008).
- Ekstrom, A. D., et al. Cellular networks underlying human spatial navigation. Nature. 425 (6954), 184-188 (2003).
- Jacobs, J., Kahana, M. J., Ekstrom, A. D., Mollison, M. V., Fried, I. A sense of direction in human entorhinal cortex. Proceedings of the National Academy of Sciences of the United States of America. 107 (14), 6487-6492 (2010).
- Spiers, H. J., Burgess, N., Hartley, T., Vargha-Khadem, F., O’Keefe, J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 11 (6), 715-725 (2001).
- Spiers, H. J., et al. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 124, 2476-2489 (2001).
- Maguire, E. A., Burgess, N., Donnett, J. G., Frackowiak, R. S., Frith, C. D., O’Keefe, J. Knowing where and getting there: a human navigation network). Science. 280 (5365), 921-924 (1998).
- King, J. A., Burgess, N., Hartley, T., Vargha-Khadem, F., O’Keefe, J. Human hippocampus and viewpoint dependence in spatial memory. Hippocampus. 12 (6), 811-820 (2002).
- Burgess, N., Maguire, E. A., O’Keefe, J. The human hippocampus and spatial and episodic memory. Neuron. 35 (4), 625-641 (2002).
- Laidi, C., et al. Preserved navigation abilities and spatio-temporal memory in individuals with autism spectrum disorder. Autism Research. 16 (2), 280-293 (2023).
- Iglói, K., Zaoui, M., Berthoz, A., Rondi-Reig, L. Sequential egocentric strategy is acquired as early as allocentric strategy: Parallel acquisition of these two navigation strategies. Hippocampus. 19 (12), 1199-1211 (2009).
- Bullens, J., Iglói, K., Berthoz, A., Postma, A., Rondi-Reig, L. Developmental time course of the acquisition of sequential egocentric and allocentric navigation strategies. Journal of Experimental Child Psychology. 107 (3), 337-350 (2010).
- Bellassen, V., Iglói, K., de Souza, L. C., Dubois, B., Rondi-Reig, L. Temporal order memory assessed during spatiotemporal navigation as a behavioral cognitive marker for differential Alzheimer’s disease diagnosis. The Journal of Neuroscience. 32 (6), 1942-1952 (2012).
- Iglói, K., et al. Interaction between hippocampus and cerebellum crus I in sequence-based but not place-based navigation. Cerebral Cortex. 25 (11), 4146-4154 (2015).
- Iglói, K., Doeller, C. F., Berthoz, A., Rondi-Reig, L., Burgess, N. Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proceedings of the National Academy of Sciences of the United States of America. 107 (32), 14466-14471 (2010).
- Commins, S., et al. NavWell: A simplified virtual-reality platform for spatial navigation and memory experiments. Behavior Research Methods. 52 (3), 1189-1207 (2020).
- Fan, C. L., Abdi, H., Levine, B. On the relationship between trait autobiographical episodic memory and spatial navigation. Memory & Cognition. 49 (2), 265-275 (2021).
- Epstein, R. A., Patai, E. Z., Julian, J. B., Spiers, H. J. The cognitive map in humans: spatial navigation and beyond. Nature Neuroscience. 20 (11), 1504-1513 (2017).
- van Praag, H., Kempermann, G., Gage, F. H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 2 (3), 266-270 (1999).
- van Praag, H., Christie, B. R., Sejnowski, T. J., Gage, F. H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 96 (23), 13427-13431 (1999).
- Voss, M. W., Soto, C., Yoo, S., Sodoma, M., Vivar, C., van Praag, H. Exercise and hippocampal memory systems. Trends in Cognitive Sciences. 23 (4), 318-333 (2019).
- Jennen, L., Mazereel, V., Lecei, A., Samaey, C., Vancampfort, D., van Winkel, R. Exercise to spot the differences: a framework for the effect of exercise on hippocampal pattern separation in humans. Reviews in the Neurosciences. 33 (5), 555-582 (2022).
- Griffin, &. #. 2. 0. 1. ;. W., Mullally, S., Foley, C., Warmington, S. A., O’Mara, S. M., Kelly, A. M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiology & Behavior. 104 (5), 934-941 (2011).
- Firth, J., et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage. 166, 230-238 (2018).
- Voss, M. W., Vivar, C., Kramer, A. F., van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences. 17 (10), 525-544 (2013).
- Pereira, A. C., et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 104 (13), 5638-5643 (2007).
- vander Ham, I. J. M., Claessen, M. H. G. How age relates to spatial navigation performance: Functional and methodological considerations. Ageing Research Reviews. 58, 101020 (2020).
- Zhong, J. Y., Moffat, S. D. Extrahippocampal contributions to age-related changes in spatial navigation ability. Frontiers in Human Neuroscience. 12, 272 (2018).
- Moffat, S. D. Aging and spatial navigation: what do we know and where do we go. Neuropsychology Review. 19 (4), 478-489 (2009).
- Meade, M. E., Meade, J. G., Sauzeon, H., Fernandes, M. A. Active navigation in virtual environments benefits spatial memory in older adults. Brain Sciences. 9 (3), 47 (2019).
- Powell, A., et al. Stable encoding of visual cues in the mouse retrosplenial cortex. Cerebral Cortex. 30 (8), 4424-4437 (2020).
- Fischer, L. F., Mojica Soto-Albors, R., Buck, F., Harnett, M. T. Representation of visual landmarks in retrosplenial cortex. eLife. 9, 51458 (2020).
- Stacho, M., Manahan-Vaughan, D. Mechanistic flexibility of the retrosplenial cortex enables its contribution to spatial cognition. Trends in Neurosciences. 45 (4), 284-296 (2022).
- Yoder, R. M., Clark, B. J., Taube, J. S. Origins of landmark encoding in the brain. Trends in Neurosciences. 34 (11), 561-571 (2011).
- Lozano, Y. R., Page, H., Jacob, P. -. Y., Lomi, E., Street, J., Jeffery, K. Retrosplenial and postsubicular head direction cells compared during visual landmark discrimination. Brain and Neuroscience Advances. 1, 2398212817721859 (2017).
- Ventura, M., Shute, V., Wright, T., Zhao, W. An investigation of the validity of the virtual spatial navigation assessment. Frontiers in Psychology. 4, 852 (2013).

