Developing a Humanized Mouse Model with Human Liver Cells and Immune Cells
Abstract
Source: Dagur, R. S. et al., Establishment of the Dual Humanized TK-NOG Mouse Model for HIV-associated Liver Pathogenesis. J. Vis. Exp. (2019)
This video demonstrates a method for generating a dual humanized mouse model with a human immune system and liver cells. It involves transplanting human liver cells and hematopoietic stem and progenitor cells into a transgenic immunodeficient mouse via intrasplenic injection, leading to engraftment of liver cells and development of human immune cells.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Processing of Umbilical Cord Blood and the Isolation of Human Hematopoietic Stem/Progenitor Cells (HSPCs)
- Perform all steps of the protocol under sterile conditions in laminar flow cabinets.
- Take umbilical cord blood (CB) collected in heparinized tubes and make the volume up to 35 mL by adding phosphate-buffered saline (PBS). Layer the sample on top of the lymphocytes separation medium (LSM) as illustrated in Figure 1 and centrifuge the LSM with the layered CB at 400 x g for 35 min at 4 °C with no brakes.
NOTE: Dilute the blood carefully and gently to avoid mixing at the interface. - Remove the top LSM and plasma layer carefully and transfer the white buffy coat interface to a new tube using a transfer pipette.
- Resuspend the buffy coat in 30 – 40 mL of ice-cold buffer (PBS + 0.5% bovine serum albumin [BSA] + 2 mM ethylenediaminetetraacetic acid [EDTA]). Using a pipette, combine 20 µL of the cell suspension with 20 µL of 0.4% trypan blue and pipette 10 µL of the mixture into the outer opening of either of the two chambers of a counting slide and insert the slide in an automated cell counter to count the cells.
NOTE: Use ice-cold buffer in all steps, as it helps keep the cells viable. - Centrifuge the cells at 300 x g for 10 min at 4 °C and aspirate the supernatant carefully. Then, add 300 µL of ice-cold buffer.
- Add 100 µL of human Fc receptor blocking reagent and 100 µL of monoclonal mouse anti-human CD34 antibody-conjugated microbeads for up to 1 x 108 cells (see the Table of Materials). Incubate for 30 min at 4 °C, add 10 mL of ice-cold buffer to wash the cells, and centrifuge at 300 x g for 10 min at 4 °C.
NOTE: Scale this up according to the cell number if more than 1 x 108 cells are present. - Carefully remove the supernatant, resuspend the pellet in 500 µL of buffer, and proceed with the magnetic separation step to enrich HSPCs.
- Place a positive selection LS column (see the Table of Materials) in the magnetic-activated cell sorting field and pass it through with 3 mL of buffer.
- Load the sample to the LS column that can entrap microbeads bound to human CD34+ in samples and allow it to flow under the influence of gravity into the collection tube.
- Wash the column 3x with buffer and collect the elute in the same collection tube of the CD34– fraction of cells.
- Plunge the column with 5 mL of buffer to elute the CD34+ cells into a new collection tube. Repeat the procedure to achieve a purity of >90%.
- Count the eluted CD34+ cells using trypan blue dye in a hemocytometer. After counting, centrifuge the CD34+ cells at 300 x g for 5 min and discard the supernatant.
- Resuspend the CD34+ cells in 25 µL of PBS for an injection to be used immediately in transplantation or cryopreserve the cells at a concentration of 1-2 million/mL in freezing medium (Roswell Park Memorial Institute medium [RPMI 1640 medium] + 50% fetal bovine serum (FBS) + 10% dimethyl sulfoxide [DMSO]) for further use in transplantation.
NOTE: Always recount viable cells before using them in transplantation. - To check the purity of the CD34+ elute, take 50 µL of the suspension and incubate it with 10 µL of phycoerythrin or PE-conjugated anti-human CD34 antibody for 30 min at 4 °C. After the antibody incubation, wash the stained cells with PBS, resuspend them in 100 µL of PBS, and then, proceed to perform flow cytometry. Add an additional tube of cells with no antibody to design the gate in the flow cytometer.
- After acquisition, analyze the data by selecting the region of interest on a forward scatter (FSC) and side scatter (SSC) plot, followed by gating for single cells on FSC-area and FSC-height plots. Gate CD34-positive cells on single cells in the PE channel and SSC-area plot.
2. Preparation of Human Hepatocytes (HEPs) for Transplantation
- Remove the cryopreserved hepatocytes from the liquid nitrogen, quickly submerge the vial in the water bath, and thaw for approximately 90 – 120 s.
- Remove the vial cap and pour the thawed hepatocytes into the 50 mL conical tube of the warmed thawing medium.
- Suspend the cells by rocking the 50 mL tube by hand for a few seconds.
NOTE: Do not vortex the tube. - Pellet the cells at 100 x g for 8 min at room temperature.Wash the pelleted cells in PBS with 0.1% BSA and pool them with either fresh or thawed HSPCs (ratio 10:1) in PBS in a final volume of 80 µL/mouse.
3. Animal Handling, Screening, Genotyping, and Treatment for Human HSPC and Hepatocyte Transplantation
- Animal handling
- As a result of severe immunodeficiency, breed, house, and handle TK-NOG mice under aseptic conditions.
- Always wear a lab coat, gloves, shoe covers, and a face mask to prevent infection with potentially pathogenic microorganisms.
- Use sterile gloves and instruments for surgery and handle the animals aseptically throughout the surgery.
- Selecting TK-NOG mice for the experiment
- Maintain the TK-NOG strain colony by breeding female TK-NOG mice with male non-TK-NOG littermates and select transgenic offspring by genotyping.
NOTE: Perform genotyping (see step 3.3) to determine the presence or absence of the transgene in newborn male and female mice at the time of weaning. - Select males at 6 – 8 weeks of age for transplantation due to their high sensitivity to the GCV-mediated depletion of HSV-TK transgene-expressing hepatocytes.
- Ear-tag the mice at the time of weaning or surgery to ease identification. Note down the weight and health status of the animals.
- Maintain the TK-NOG strain colony by breeding female TK-NOG mice with male non-TK-NOG littermates and select transgenic offspring by genotyping.
- Genotyping for the presence of the HSV-TK transgene using quantitative real-time polymerase chain reaction
- Perform genotyping at the time of weaning (usually at 3 – 4 weeks of age). For genotyping, cut a piece of the mouse ear in a laminar flow biological safety cabinet to maintain sterility and extract genomic DNA by using a genomic DNA isolation kit.
- Amplify genomic DNA extracted from ear piece in a 20 µL reaction mixture to screen for HSV-TK transgene under control of human albumin promoter by adding 1 µL of forward primer 5'-CCATGCACGTCTTTATCCTGG-3', 1 µL of reverse primer 5'-TAAGTTGCAGCAGGGCGTC-3', 0.5 µL of FAM probe 5′-FAM-AATCGCCCGCCGGCTGC-MGB-3', and 10 µL of master mix on a real-time polymerase chain reaction (PCR) instrument.
- Set the real-time PCR settings as follows: 60 °C for 30 s (preread stage), 95 °C for 10 min (hold stage), 40 cycles of 95 °C for 15 s and 60 °C for 1 min (PCR stage), and 60 °C for 30 s (postread stage).
NOTE: A cycle of threshold (Ct) below 22 is considered positive for HSV-TK transgene.
- Treatment using ganciclovir and treosulfan
- Using 27 G needle, inject the TK-NOG mice with intraperitoneal GCV injections (6 mg/kg) 2x a day at day 7 and at day 5 in 100 µL of saline before surgery to deplete the mice's transgenic parenchymal cells (as shown in the experimental strategy in Figure 2).
- On days 3, 2, and 1 before the surgery, precondition mice with nonmyeloablative intraperitoneal doses of treosulfan (1.5 g/kg/day) in 100 µL of saline, using a 27 G needle.
- One day before the surgery, draw two to three drops (~100 µL) of blood from the submandibular vein by pricking it with a 5 mm lancet, and isolate the serum by centrifuging (1,500 x g for 10 min at 4 °C) for the alanine aminotransferase (ALT) assay to assess the degree of liver damage.
- Preparation for the surgery
- Use clippers to shave the mouse's fur surrounding the incision site (at the left of the peritoneal wall) before surgery.
- Adjust the oxygen flow to 1 L/min and the isoflurane flow to 3% – 5% in an induction chamber using a mouse anesthesia machine. Place one mouse at a time in the induction chamber for anesthesia.
- Attach the one end of a sterile extension tube (with a holding capacity of 550 µL of suspension; see the Table of Materials for specifications) to a 30 G needle and the other end to a 1 mL syringe.
- Fill the syringe with the suspension (80 µL/mouse) of pooled HEPs and HSPCs (see section 2), fit the syringe in the notch of a repetitive dispensing pipette, and adjust the dispenser to dispense 10 µL in each press.
- Once the mice are anesthetized (usually after 3 – 4 min), switch the isoflurane flow to the nose cone and reduce the isoflurane flow rate to 2% – 3%.
- Intrasplenic transplantation of human HSPCs and hepatocytes in mice
- Perform all surgery steps in a laminar flow cabinet under sterile conditions.
- Place a clean sterile drape over the working surface and scrub the left side of the body of each mouse with 10 % povidone-iodine followed by 70 % isopropyl alcohol, before making an incision.
- Make a small incision (~1 – 1.5 cm in length and 5 mm deep) in the skin, muscle, and peritoneum at the left of the peritoneal wall with Vanna's type scissors to enter the peritoneal cavity approximately 5 mm below the lower edge of the rib cage.
- Locate the spleen, pull it slightly with forceps to the operating area for easy access, and insert the 30 G needle into the lower pole of the spleen.
- Unlock the plunger of the dispensing pipette and dispense 10 µL of the volume at a time, with a limit of 60-80 µL per spleen. Retract the needle slowly and clip the spleen with ligating clips using a ligation applier.
- Push the spleen back into the body cavity with cotton-tipped applicators wetted with sterile PBS.
- Close the muscle layer of the abdominal wall using an absorbable suture interrupted suture pattern (not continuous). Accomplish skin closure with an interrupted suture pattern using non-absorbable sutures.
Representative Results
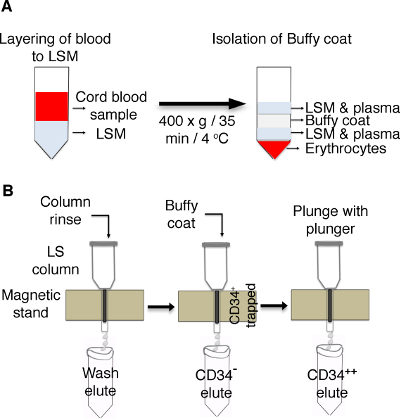
Figure 1: Schematic of the enrichment of CD34+ cells from cord blood. (A) Cord blood is layered on lymphocytes separation medium (LSM) and centrifuged to isolate the buffy coat. (B) LS columns are placed on a magnetic stand and rinsed with a BSA buffer, followed by adding a buffy coat. Cells positive for CD34 are trapped in the columns, and CD34-cells are eluted in separate tubes. Trapped CD34+ cells in column resins are plunged with a plunger, and the cells are collected in a new tube.
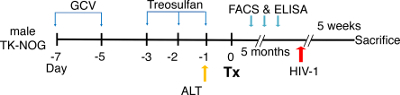
Figure 2: Schematic view of the experimental design for the dual reconstitution of humanized liver and immune system mice, followed by HIV-1 infection. TK-NOG mice are injected with ganciclovir (GCV) at a dose of 6 mg/kg, 2x a day, on day -7 and day -5, followed by a treosulfan injection on days -3, -2 and -1. To screen the mice for transplantation (Tx), an alanine aminotransferase (ALT) assay is performed one day before the surgery, and mice with ALT levels of >200 and <600 U/L are selected. After transplantation, the mice are checked for a reconstitution of the human immune system by flow cytometry (FACS) and for liver reconstitution by assessing their albumin level using ELISA. The mice are infected with HIV-1 5 weeks before they are sacrificed.
Disclosures
The authors have nothing to disclose.
Materials
| 27G1/2" needles | BD biosciences | 305109 | |
| 30G1/2" needles | BD biosciences | 305106 | |
| 5 mL polystyrene round-bottom tube 12 x 75 mm style | Corning | 352054 | |
| BD 1 mL Tuberculin Syringe Without Needle | BD biosciences | 309659 | |
| BD FACS array bioanalyzer | BD Biosciences | For purity check of eluted CD34+ cells | |
| BD FACS array software | BD Biosciences | Software to analysis acquired CD34+ cell on FACS array | |
| BD FACS lysing solution | BD Biosciences | 349202 | To lyse red blood cells |
| BD LSR II | BD Biosciences | Instrument for acquisiton of flow cytometry samples | |
| BD Vacutainer Plastic Blood Collection Tube | BD biosciences | BD 367874 | To collect Cord blood |
| Bovine Serum Albumin | Sigma-aldrich | A9576 | |
| CD34 MicroBead Kit, human | Miltenyi Biotec | 130-046-702 | For isoation of CD34+ HSPC |
| CD34-PE, human | Miltenyi Biotec | 130-081-002 | Antibody used for purity check of eluted CD34+ cells |
| Cell counting slides | Bio-rad | 1450015 | |
| ChargeSwitch gDNA Mini Tissue Kit | Thermofisher scientific | CS11204 | For extraction of genomic DNA from ear piece |
| Cotton-tipped applicators | McKesson | 24-106-2S | |
| DMSO (Dimethyl sulfoxide) | Sigma-aldrich | D2650-5X5ML | |
| Extension set Microbore Slide Clamp(s) Fixed Male Luer Lock. L: 60 in L: 152 cm PV: 0.55 mL Fluid Path Sterile | BD biosciences | 30914 | Attached to dispensing pippet and to load with HSPC and HEP suspesion |
| FACS Diva version 6 | BD Biosciences | Flow cytometer software required for acqusition of sample | |
| Fetal Bovine Serum (FBS) | Gibco | 10438026 | |
| FLOWJO analysis software v10.2 | FLOWJO, LLC | Flow cytometry analysis software | |
| Ganciclovir | APP Pharmaceuticals, Inc. | 315110 | Prescripition drug |
| Greiner MiniCollect EDTA Tubes | Greiner bio-one | 450475 | |
| Hepatocytes thawing medium | Triangle Research Labs | MCHT50 | |
| Horizon Open Ligating Clip Appliers | Teleflex | 537061 | To hold the ligating clips |
| Human hepatocyte | Triangle Research Labs | HUCP1 | Cryopreserved human hepatocytes, induction qualified |
| Iris Scissors, Straight | Ted Pella, Inc. | 13295 | |
| Lancet | MEDIpoint | Goldenrod 5 mm | |
| LS columns | Miltenyi Biotec | 130-042-401 | Used to entrap CD34+ microbeads (positive selection) |
| Lymphocyte Separation Medium (LSM) | MP Biomedicals | 50494 | For isoation of lymphocytes from peripheral blood |
| MACS MultiStand | Miltenyi Biotec | 130-042-303 | Holds Qudro MACS seperator and LS columns |
| McPherson-Vannas Micro Dissecting Spring Scissors | Roboz Surgical Instrument Co. | RS-5605 | Used to make an incision on skin to expose spleen |
| Micro Dissecting Forceps | Roboz Surgical Instrument Co. | RS-5157 | To hold and pull out spleen from peritoneal cavity |
| Mouse CD45-FITC | BD Biosciences | 553080 | Mouse-specific |
| PBS (Phosphate Buffered Saline) | Hyclone | SH30256.02 | |
| Qudro MACS separator | Miltenyi Biotec | 130-090-976 | holds four LS columns |
| RPMI 1640 medium | Gibco | 11875093 | |
| StepOne Plus Real Time PCR | Applied Biosystems | Instrument used to genotype | |
| Stepper Series Repetitive Dispensing Pipette 1ml | DYMAX CORP | T15469 | Used to dispense HSPC and HEP supension in controlled manner |
| TaqMan Gene Expression Master Mix | Thermofisher scientific | 4369016 | |
| TC20 automated cell counter | Bio-rad | 1450102 | |
| TK-NOG mice | Provided by the Central Institute for Experimental Animals (CIEA, Japan; Drs. Mamoru Ito and Hiroshi Suemizu) | ||
| Treosulfan | Medac GmbH | Provided by Dr. Joachim Baumgart (medac GmbH) | |
| Trypan Blue | Bio-rad | 1450022 | |
| Vannas-type Micro Scissors, Straight, 80mm L | Ted Pella, Inc. | 1346 | Used to make an incision on skin to expose spleen |
| Weck hemoclip traditional titanium ligating clips | Esutures | 523700 | To ligate the spleen post-injection |
Tags

.
