Ex Vivo Infection of Live Tissue with Oncolytic Viruses
Summary
Oncolytic viruses are promising for cancer therapeutics. The ability to ascertain the infectability of live tissue specimens obtained from patients prior to treatment is a unique advantage of this therapeutic approach. This protocol describes how to process tissues for ex vivo infection with oncolytic virus and subsequent viral quantification.
Abstract
Oncolytic Viruses (OVs) are novel therapeutics that selectively replicate in and kill tumor cells1. Several clinical trials evaluating the effectiveness of a variety of oncolytic platforms including HSV, Reovirus, and Vaccinia OVs as treatment for cancer are currently underway2-5. One key characteristic of oncolytic viruses is that they can be genetically modified to express reporter transgenes which makes it possible to visualize the infection of tissues by microscopy or bio-luminescence imaging6,7. This offers a unique advantage since it is possible to infect tissues from patients ex vivo prior to therapy in order to ascertain the likelihood of successful oncolytic virotherapy8. To this end, it is critical to appropriately sample tissue to compensate for tissue heterogeneity and assess tissue viability, particularly prior to infection9. It is also important to follow viral replication using reporter transgenes if expressed by the oncolytic platform as well as by direct titration of tissues following homogenization in order to discriminate between abortive and productive infection. The object of this protocol is to address these issues and herein describes 1. The sampling and preparation of tumor tissue for cell culture 2. The assessment of tissue viability using the metabolic dye alamar blue 3. Ex vivo infection of cultured tissues with vaccinia virus expressing either GFP or firefly luciferase 4. Detection of transgene expression by fluorescence microscopy or using an In Vivo Imaging System (IVIS) 5. Quantification of virus by plaque assay. This comprehensive method presents several advantages including ease of tissue processing, compensation for tissue heterogeneity, control of tissue viability, and discrimination between abortive infection and bone fide viral replication.
Protocol
1. Tissue processing
- For best results, this protocol should be performed using freshly isolated tissue deposited in DMEM media containing 10% FBS, 1% Pennicilin /Streptomycin solution, and 0.1% Amphothericin B solution immediately following surgery prior to processing. If this is not possible, tissues can be left overnight in this medium at 4 degrees prior to processing.
- Prior to processing the sample, sterilize metal forceps and disposable blades by depositing them in a 70% ethanol solution for at least 5 minutes. Also prepare a 24-well plate with 2 ml of DMEM media containing 10% FBS, 1% Pennicilin /Streptomycin solution, and 0.1% Amphothericin B.
- Collect the tissue sample in a laminar flow cell culture hood using sterilized forceps and deposit the tissue in an empty 15 cm petri dish, keeping the lid on the side, sterile side up.
- In the cell culture hood, use a 2 mm biopsy punch to obtain different cores from a variety of regions within the tissue as in Figure 1. Deposit the cores in the lid of the 15 cm petri with the help of forceps, leaving enough space in between each core so that they can be easily cut along the horizontal axis.
- Split each core into 4 even quarters using a sterilized razor blade as in Figure 1.
- Using a pipet tip, put each core quarter from a given core in a different well from A1 to A4 as in figure 1, already containing 1.5 ml of DMEM containing 10% FBS, 1% Pennicilin /Streptomycin solution, and 0.1% Amphothericin B solution. Repeat for each core. This should allow representative sampling of the tumor, while minimizing bias in a given well/condition. For better representation, increase the number of cores.
2. Assessment of tissue viability
- Following tissue processing, add 25μl Alamar blue to well # A1 and # A2 as shown in figure 1 and incubate for 1 hour at 37 degrees in a humidified incubator with 5% CO2.
- Following incubation with alamar blue, remove 3 times 100 μl from each A1 and A2 well and transfer to 6 different wells of a 96-well plate. Read the signal using a fluorescence plate reader (530 excitation, 590 emission) and keep the data for your records.
- After Alamar blue signal has been read, transfer all pieces of tissue using a pipet tip, from well A1 to C1 that contains DMEM, 10% FBS + PS + AmphoB. Be careful not to transfer an excessive amount of media from well A1.
- Infect wells A3 and A4 respectively with 106 PFU of GFP-expressing and luciferase-expressing vaccinia virus diluted in 25 μl of media. Well A2 can be infected with a virus that that expresses any given transgene including none at all.
- 72 hours later, add 25 μl Alamar blue to wells C1 and D1 and repeat step 2.2
3. Visualization of GFP transgene expression by florescence microscopy
- Remove all cell culture media covering the tissue pieces in order to take good fluorescence pictures.
- Using a fluorescence-capable dissection microscope, first take a phase contrast image at an appropriate resolution
- Switch to fluorescence mode and take a picture using the appropriate filter to visualize the transgene of interest, in this case GFP.
- Use a fluorescence filter for another wavelength as far a possible from that of the transgene of interest (such as RFP) to take a picture of background fluorescence
4. Visualization of luciferase expression using an In Vivo imaging System (IVIS)
- Make sure that the IVIS is initialized before you begin.
- In wells A4 and B4, add 5 μl of luciferin substrate 10 mg/ml, mix well and incubate for 5 minutes at room temperature.
- Set the IVIS exposure time to 5 seconds and take a picture of your wells. If the image is saturated, repeat with lower exposure time. If there is no signal, increase the exposure time.
- Using the IVIS imaging software, you may remove background using the signal from well B4 and select the region of interest to quantify the luminescence signal.
5. Assessing viral titers by plaque assay
- Prior to quantifying viral titers, tissues must first be homogenized to release viral particles. This can be done several months later in the infected tissue samples are stored at -80
- Weigh the sample that needs to be homogenized using an analytical scale. In this case, we will use the sample collected from well A2.
- Place the tissue is in a 5 ml polystyrene round-bottom falcon tube and add 1 ml of PBS. Homogenize the tissue using a tissue homogenizer. If necessary, store the homogenate at -80 for assessment of viral titers at a later time.
- To titer vaccinia virus, first plate 1 million U2OS cells in a 6-well plate and incubate overnight in a 37 degree, 5% CO2 humidified incubator such that they have reached approximately 95 % confluency the following day.
- Use serum free media to do serial dilutions of virus, making sure to change tips between each dilution step. Typically we do 1 in 10 dilutions and use 100 μl to transfer into 900 μl. How many dilutions are made depends on the anticipated virus yield.
- Following making the dilutions, remove the media covering the plated U20S cells then add 500 μl of diluted virus stock (for each dilution) to infect the U2OS cells. Incubate the cells for 1 hour minutes at 37 degrees Celcius in a 5% CO2 humidified incubator.
- During this time, warm up the 2 X concentrated DMEM containing 20% FBS, and the 3% CMC solution in a 37 degree water bath.
- After the 1 hour incubation, remove the media covering infected U2OS cells. Mix 1:1 volumes of 3% CMC : 2X DMEM, 20% FBS together and use 2 ml of this mixture to cover each well of the infected U2OS cells.
- Put cells back in a 37 degree 5% CO2 humidified incubator for another 48 hours.
- After 48 hours, add 2 ml of methanol-acetic acid fixative on top of the CMC overlay in each well and incubate at room temperature for 10 minutes in a cell culture hood.
- Discard the fixed overlay and wash remainder off of the wells using tap water.
- Stain the fixed U2OS cells with 2ml of a coomassie blue solution per well and incubate for 30 minutes at room temperature at low speed on a plate shaker.
- Remove the coomassie stain from the wells and wash the plates using tap water. Allow to dry with lid off for about an hour.
- The Resulting viral plaques can be easily visualized in figure 2C. Plates can be stored indefinitely at this stage.
- Count plaques at the dilution step where between 10 and 100 plaques are visible.
- Multiply the number of plaques counted by the dilution used and multiply the resulting number by 2 to give a titer in PFU/ml. For example, if 25 plaques are counted in the well where a million-fold dilution was used, the titer of the initial undiluted sample is 25 multiplied by 1 million multiplied by 2 or 50 million PFU/ml. Further divide this number by the weight initially measure for the sample to report titers in PFU/g
6. Representative Results:
In order to accurately determine whether a surgically obtained normal/tumor tissue sample is or is not infectable with virus, one must first ensure that the tissue sample is at least viable. Fig 1A shows that viability can be assessed using a metabolic dye (Alamar blue) and that both normal and tumor tissue can remain metabolically active over a period of at least 72h. This suggests that tissues cultured ex vivo can support viral replication. A significant advantage of oncolytic viruses is that they can be engineered to express therapeutic or imaging transgenes. Fig 2D-E shows that GFP or Luciferase can be detected from tissues infected ex vivo, further supporting that these tissues are viable and also infectable. Although increased transgene expression is generally associated with viral replication, it does not necessarily equate to a productive viral life cycle that leads to self-amplification and spread, which is thought to be important for therapeutic activity. For this reason, it is necessary to determine whether more virus is produced than what was used to initially infect the tissue. Fig 2B shows that upon viral quantification by plaque assay, more virus is obtained after 72 hours infection then tissue collected immediately after infection. Overall, these data show that surgically excised tumor tissue can remain viable in cell culture for a period of at least 72 hours, during which time viral replication can be supported.
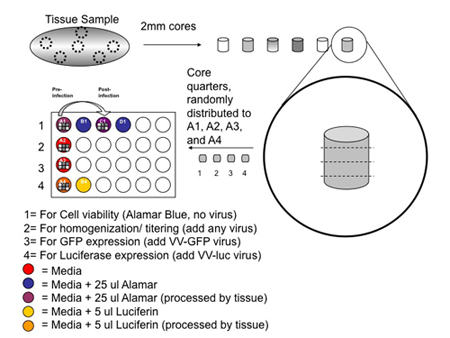
Figure 1. Overview of the tissue sampling / sectioning protocol. Tissue sample is processed by removing several 2 mm cores which are subsequently divided into four quarters which are randomly distributed into wells A1-A4. The color codes indicate the reagents used in each well. The grey-shaded squares within the wells represent each quarter from the 6 individual cores depicted. The tissue in well A1 is transferred to a new well (C1) with media after the initial alamar blue reading. A second reading is done at the end of the 72 hour incubation with viruses. Wells A4 and B4 are supplemented with Luciferin after the 72 hour incubation post-infection
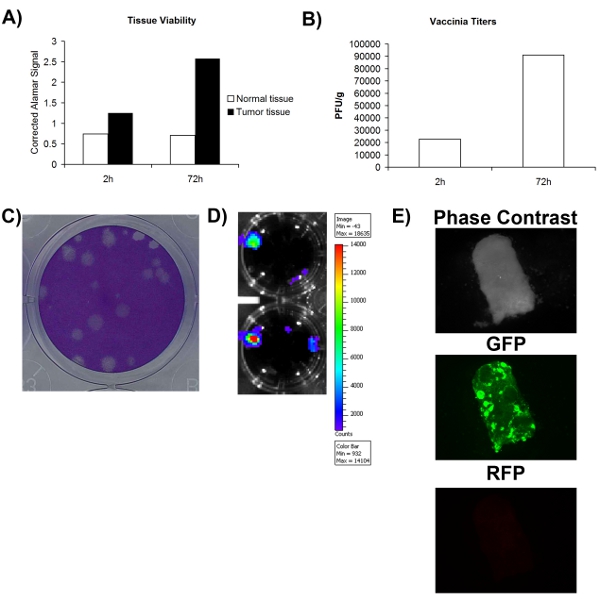
Figure 2. Viability and infectability of patient tissue samples. A) Tissue viability as measured by alamar blue signal at 2 hours and 72 hours post collection. Y-axis represents the blank corrected alamar blue signal. B) Titer of vaccinia virus collected per gram of tissue samples 2 and 72 hours post-infection. C) Example of vaccinia virus plaques on U2OS cells following coomassie staining. D) Luminescence signal obtained from patient VV-luciferase-infected tissue imaged using IVIS. E) Fluorescence microscopy pictures of patient tissue sample infected with VV-GFP.
Discussion
One of the critical steps in this protocol is obtaining fresh tissue sample. If the sample is put into cell culture following a long wait at the operating room in an inappropriate media (eg. PBS), this may compromise tissue viability and prevent infectability. Of note, normal tissue is inherently more prone to these effects than tumor tissues. Another critical point is how many cores are used to sample the tissue and the consistency of their size. Inconsistencies in size will lead to variability across patient samples since factors such as tissue hypoxia and infectectable surface will fluctuate depending on the size of the cores and core quarters. While this can be partially resolved by using tissue slicers, one advantage of the method presented here is that it is relatively easy, less prone to contamination, and widely applicable to a variety of tissue types, including soft or viscous tissues which are not easily amenable to tissue slicing. Notably, the number of cores can be increased in order to get better tissue representation. Also, the cores can be cut into more pieces (eg. 5-8) to accommodate other potential assays to be done in parallel such as DNA, RNA, or protein extraction. However, the number of pieces in which the cores can be cut to reproducible sizes will depend on the actual size of the cores, which can be modified by using different sized corers. Optionally, one way to help obtain reproducibly sized tissue pieces is to even out the length of the cores using a ruler prior to further subdividing them into smaller pieces. The protocol can naturally be modified to accommodate other viruses and we have found that tissues can be viable and support replication for up to 6 days. The protocol can further be extended to measurements of other virally-expressed transgenes including cytrokines. Following infection, tissues can also be embedded in paraffin for further sectioning and staining by immunohistochemical methods, which allows for further refinement of the tissue histology and how it relates to viral infection10-12.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Dr. Hesham Abdelbary for providing human surgical specimens for the data presented in Fig 2.
Materials
| Name of the reagent | Company | Catalogue number | Comments (optional) |
|---|---|---|---|
| High glucose DMEM | Hyclone | SH30243.01 | |
| Fetal Bovine Serum | NorthBio Inc. | NBSF-701 | |
| Amphothericin B solution | Sigma Aldrich | A2942 | Use at 0.1% |
| Pennicilin-Streptomycin | Sigma Aldrich | P4333 | Use at 1% |
| Alamar Blue | Invitrogen | DAL1025 | |
| D-Luciferin potassium salt | Molecular Imaging Products | D-Luciferin potassium salt 1g | Resuspend in PBS at 10 mg/ml and filter on 0.22 μm filter |
| MEM powder | Gibco | #41500018 | Make in half the suggested volume to make 2X MEM and filter on 0.22 μm filter prior to use |
| Carboxymethyl cellulose (CMC) | Sigma Aldrich | C9481 | Make a 3% solution in deionized water and autoclave. Note that powder can take some time to resuspend |
| Coomassie Brilliant Blue R | Sigma Aldrich | B7920 | |
| 2 mm Biopsy punch | Miltex | MX-33-31 | |
| Double Edge Prep Blades | Personna Medical Care | 74-0002 | |
| Fluorescence disection Microscope | Leica | model M205 FA | |
| In vivo imaging system (IVIS) | Caliper Life Sciences | IVIS® 200 series | |
| Tissue Tearor | Biospec products | model 985370-395 |
References
- Parato, K. A., Senger, D., Forsyth, P. A., Bell, J. C. Recent progress in the battle between oncolytic viruses and cancer. Nat Rev Cancer. 5, 965-976 (2005).
- Renouf, L. C., Thway, K., Sibtain, A., McNeish, I. A., Newbold, K. L., Goldsweig, H., Coffin, R., Nutting, C. M. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 16, 4005-4015 (2010).
- Lal, R., Harris, D., Postel-Vinay, S., de Bono, J. Reovirus: Rationale and clinical trial update. Curr Opin Mol Ther. 11, 532-539 (2009).
- Park, B. H., Hwang, T., Liu, T. C., Sze, D. Y., Kim, J. S., Kwon, H. C., Oh, S. Y., Han, S. Y., Yoon, J. H., Hong, S. H., Moon, A., Speth, K., Park, C., Ahn, Y. J., Daneshmand, M., Rhee, B. G., Pinedo, H. M., Bell, J. C., Kirn, D. H. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 9, 533-542 (2008).
- Breitbach, C. J., Reid, T., Burke, J., Bell, J. C., Kirn, D. H. Navigating the clinical development landscape for oncolytic viruses and other cancer therapeutics: no shortcuts on the road to approval. Cytokine Growth Factor Rev. 21, 85-89 (2010).
- Boeuf, F. L. e. Synergistic interaction between oncolytic viruses augments tumor killing. Mol Ther. 18, 888-895 (2010).
- Silva, N. D. e., Atkins, H., Kirn, D. H., Bell, J. C., Breitbach, C. J. Double trouble for tumours: exploiting the tumour microenvironment to enhance anticancer effect of oncolytic viruses. Cytokine Growth Factor Rev. 21, 135-141 (2010).
- Diallo, J. S. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol Ther. 18, 1123-1129 (2010).
- Cooke, S. L. Intra-tumour genetic heterogeneity and poor chemoradiotherapy response in cervical cancer. Br J Cancer. , (2010).
- Pennington, K., Chu, Q. D., Curiel, D. T., Li, B. D. L., Mathis, J. M. The Utility of a Tissue Slice Model System to Determine Breast Cancer Infectivity by Oncolytic Adenoviruses. J Surg Res. , 1-6 (2010).
- Hochberg, M. Tropism of herpes simplex virus type 1 to non-melanoma skin cancers. Br J Dermatol. , (2010).
- Kuip, H. v. a. n. d. e. r. Short term culture of breast cancer tissues to study the activity of the anticancer drug taxol in an intact tumor environment. BMC Cancer. 6, 86-86 (2006).

