Bronchoalveolar Lavage (BAL) for Research; Obtaining Adequate Sample Yield
Summary
We describe a research technique for fiberoptic bronchoscopy and bronchoalveolar lavage using low pressure suction. The technique is used to harvest immune cells from the lung bronchoalveolar surfaces. Local anesthetic and mild conscious sedation (midazolam) is used. Subjects tolerate the procedure well and experience minimal side effects.
Abstract
We describe a research technique for fiberoptic bronchoscopy with bronchoalveolar lavage (BAL) using manual hand held suction in order to remove nonadherent cells and lung lining fluid from the mucosal surface. In research environments, BAL allows sampling of innate (lung macrophage), cellular (B- and T- cells), and humoral (immunoglobulin) responses within the lung.
BAL is internationally accepted for research purposes and since 1999 the technique has been performed in > 1,000 subjects in the UK and Malawi by our group.
Our technique uses gentle hand-held suction of instilled fluid; this is designed to maximize BAL volume returned and apply minimum shear force on ciliated epithelia in order to preserve the structure and function of cells within the BAL fluid and to preserve viability to facilitate the growth of cells in ex vivo culture. The research technique therefore uses a larger volume instillate (typically in the order of 200 ml) and employs manual suction to reduce cell damage.
Patients are given local anesthetic, offered conscious sedation (midazolam), and tolerate the procedure well with minimal side effects. Verbal and written subject information improves tolerance and written informed consent is mandatory. Safety of the subject is paramount. Subjects are carefully selected using clear inclusion and exclusion criteria.
This protocol includes a description of the potential risks, and the steps taken to mitigate them, a list of contraindications, pre- and post-procedure checks, as well as precise bronchoscopy and laboratory techniques.
Introduction
Background
Fiberoptic bronchoscopy was developed for clinical practice; it is widely used both diagnostically and therapeutically1,2. Bronchoalveolar lavage (BAL) removes nonadherent cells and lung lining fluid from the mucosal surface; biopsy is used to sample mucosal and submucosal tissues. In research environments, BAL allows sampling of innate (lung macrophages3-5), cellular (B- and T- cells6), and humoral (immunoglobulin7) responses within the lung.
BAL is internationally accepted for research purposes8 and since 1999 the technique has been performed in >1,000 subjects in the UK and Malawi by our group. We use this technique in studies of innate, cellular, and humoral immune responses to pneumococcal antigen including experimental human pneumococcal carriage9,17, biomass smoke5, HIV and vaccination, and adjunct treatment studies into recovery from pneumonia. Our technique uses gentle hand-held suction of instilled fluid; this is designed to maximize BAL volume returned and apply minimum shear force on ciliated epithelia in order to preserve the structure and function of cells within the BAL fluid.
In a research context BAL utilizes a different technique from that practiced by respiratory and intensive care physicians (often termed bronchial wash, washings, lavage, or BAL) who are aiming to gain diagnostic or therapeutic benefit. The research technique is designed to harvest cells and preserve viability to facilitate the growth of cells in ex vivo culture. For these reasons the research technique uses a larger volume instillate (typically in the order of 200 ml) and employs manual suction to reduce cell damage. Patients are given local anesthetic, offered conscious sedation (midazolam), and tolerate the procedure well with minimal side effects. Verbal and written subject information improves tolerance and written informed consent is mandatory1.
Goal
The overall goal is that the procedure should be safe and effective. Subjects should not experience any physiological disturbance and operators should consistently collect in excess of 100 ml of BAL from subjects. After the procedure, subjects should experience minimal side effects.
Safety
Safety of the subject is paramount. Subjects are carefully selected using clear inclusion and exclusion criteria. This protocol includes a description of the potential risks and the steps taken to mitigate them.
Contraindications to research bronchoscopy may be expressed as absolute or relative, and are included in our study protocol as exclusion and inclusion criteria. Our subjects are all screened to ensure full health.
Absolute contraindications include unstable cervical spine, unresponsive hypoxia, unstable angina, bleeding diathesis, and malignant cardiac arrhythmia.
Relative contraindications, including those conditions associated with increased complication rates, include:
General: poorly cooperative subject1, any significant general medical problem e.g. epilepsy, previous poorly tolerated bronchoscopy, known adverse reactions to lidocaine or midazolam, pregnancy, poor nutrition.
Respiratory: hypoxia [saturations (sats) on air <94%], hypercapnia, unstable asthma1, significantly impaired respiratory function1 (FEV1< 1 L), pulmonary hypertension.
Cardiovascular: uremia, within 6 weeks of myocardial infarction1, superior vena cava obstruction.
Other: immunosuppression (our group regularly performs this procedure in HIV positive subjects).
See Table 1 – Risks associated with BAL for research.
Protocol
1. The Subjects are Met on Arrival by the Respiratory Research Nurse
- The subject has been fasting for >4 hr for solid food and 2 hr for clear fluids1.
- The procedure is performed as a day case with appropriate pre- and post-procedure checks (as per standard hospital policy).
- The subject wears a hospital gown.
- A small gauge cannula is inserted (ideally into the left hand) and remains in situ until the end of the post-procedure recovery period1.
- Preprocedure observations are taken (blood pressure, heart rate, oxygen saturations [sats]).
- The subject is transferred to the bronchoscopy suite.
2. The Subject is Prepared for the Procedure in the Suite by a Respiratory Research Clinician Experienced in Bronchoscopy
- Monitoring equipment is attached including; 3-lead ECG, pulse oximeter1,10 and sphygmomanometer. Anesthetic support is not needed including written informed consent.
- Oxygen may be delivered via nasal cannula at up to 4 L/min, to achieve sats >90%1.
- The operator checks the bronchoscope, this includes ensuring effective suction by aspirating sterile normal saline.
- Topical anesthesia with lidocaine is achieved in the nasal passages (using Instillagel1) and the oral mucosa (using xylocaine)
- If sedation is appropriate and requested by the subject, intravenous midazolam is administered1. An appropriate reversal agent (flumazenil) is immediately available.
- Resuscitation equipment should be readily available1,10.
3. The Bronchoscope is Inserted and Positioned
- Intubation is usually via the nose. If this is not possible due to nasal polyps, inflamed turbinates, or any discomfort then the subject is intubated via the mouth (a mouth-guard is used to prevent damage to the scope or the subject’s teeth).
- Topical anesthesia at the larynx is completed using 4% lidocaine, usually a total of 4-6 ml is used. Commonly this causes coughing on instillation.
- The vocal cords are passed, and further mucosal anesthesia using 2 ml aliquots of 2% lidocaine at the carina, at the division of the right lower lobe (RLL) and right middle lobe (RML) and at the RML entrance.
- The bronchoscope is positioned within the RML, ideally in the medial segment, in a position where it is distal enough to be in a secure position but not too distal so that the airway collapses when suction is applied (‘Wink test’ using the suction button).
- Good positioning is indicated during the wink test by a bronchoscope that can be fully maintained in position by the bronchoscopist and an airway that does not fully close immediately on gentle suction.
4. The BAL is Performed
- Four 60 ml syringes are prefilled with warmed normal saline – 60 ml, 50 ml, 50 ml, and 40 ml in successive syringes. When the subject and bronchoscopist are ready the first syringe of saline is instilled by the bronchoscopy assistant whilst the bronchoscopist maintains the position in the RML.
- Gentle hand suction is then performed by the assistant using the same port and 50 ml syringe.
- This procedure is then repeated a further 3x, with a maximal volume of 200 ml used in our specific technique.
- The retrieved BAL fluid is expelled gently into labelled containers already held on melting ice. Glass containers may be presiliconized in order to inhibit cell attachment and maximize cell return.
- The BAL fluid appears hazy against the light with surface soap bubbles formed by surfactants.
- The bronchoscope is slowly fully withdrawn.
- The BAL fluid is transported without delay on ice for immediate processing.
5. Subject Recovery
- The subject is recovered for 2-4 hr by the respiratory research nurse on a ward.
- During this time they are allowed to rest and then only eat and drink >60 min1 after the procedure (when their swallow is assessed as safe), in order reduce the risk of aspiration.
- Post procedural observations are monitored and recorded. A clinical examination occurs prior to discharge.
- The subject is briefed predischarge about the common side effects (fever, mild right submammary chest discomfort, sore throat) and given a contact number in case significant side effects occur1.
- The subject is followed-up at 1-5 days post-procedure with a clinical contact. This may be either over the telephone or in person.
6. Cells are Isolated in the Laboratory – Perform on Melting Ice Where Possible
- The volume of BAL fluid is recorded and filtered through a double layer of sterile gauze swab to remove mucus plugs into prechilled, sterile 50 ml centrifuge tubes.
- Cells are pelleted by centrifugation at 500 x g at 4 °C for 5-10 min and washed by vortexing in 50 ml cold normal saline. Centrifugation is repeated once.
- Cells are resuspended in culture medium (RPMI-1640 + 10% fetal bovine serum + 2 mM L-glutamine + penicillin [40 IU/ml], streptomycin [75 IU/ml], and amphotericin B [0.5 IU/ml]) and differential counting performed using an equal volume of trypan blue and a hemocytometer.
- The cell suspension is normalized to the required density (we use 1 x 106 cells/ml), introduced by pipetting into appropriate tissue culture plates, and incubated at 37 °C for 3 hr to allow macrophage adherence. 1 ml of this cell suspension per well is appropriate for 24-well culture plates.
- For lymphocyte work, after 3 hr of incubation, the culture medium is gently pipetted up and down 3x, and collected. This medium contains lymphocytes and nonadherent macrophages and may be used to assess lymphocyte function; it may also be used in purification steps e.g. with CD14 magnetic beads to remove macrophages.
- For macrophage work, after 3 hr of incubation, the medium is carefully removed with minimal pipetting and replaced with fresh, warm medium without antibiotics (RPMI 1640 + 10% fetal bovine serum + 2 mM L-glutamine). The macrophages are adherent to the tissue culture plate (see Figure 1).
Representative Results
Good clinical results are a BAL volume of over 100 ml and a subject who has experienced at most, minor discomfort (see Table 1). This requires a relaxed and cooperative subject. The clinician must be confident, fully prepared (Materials), and understanding towards the volunteer.
Inter- and intra-subject variability in cellular yield (including differential counts) has been described. Differential cell counts from healthy subjects are typically >90% macrophages with small numbers of neutrophils (<2%) and lymphocytes (5-10%). BAL cells centrifuged onto microscope slides can be stained with a differential nuclear stain to obtain this information (Figure 1). In Figure 1, BAL cells have been fixed and stained using Hemacolor staining according to the manufacturer’s instructions. At least 300 cells should be counted to obtain reliable results for macrophages, neutrophils and lymphocytes; 500 cells for rarer cell types.
| Hazard | Risk | Reduced in this protocol by: |
| Effect of bronchoscopy and BAL1,14 | ||
| Mild discomfort | <25%14,# | Appropriate analgesia and sedation. Confident and caring approach to the subject. |
| Epistaxis | <1%# | Mild pressure for nasal intubation only. If subject discomfort or narrowed aperture – oral intubation instead. |
| Endobronchial hemorrhage (hemoptysis) | <0.1%*15 | No biopsies taken. Careful control during bronchoscopy – avoiding respiratory mucosa. |
| Nausea, vomiting and aspiration pneumonia | <0.02%14,# | Fasting for solid food >4 hr preprocedure1. Adequate topical anesthesia. Semirecumbent position during procedure. Careful post-procedure observation. |
| Fever | 0.0114 – 1%#,16 | Relates to inflammation, and may minimized by maximal collection of BAL. Single lobe BAL only.# |
| Sore nose/throat and hoarseness | <25%14,# | Adequate topical anesthesia of the nose, throat and larynx (minimising coughing). |
| Infection | <0.1% in HIV negative, 1% in HIV positive14,# |
Standard bronchoscope washing procedure1. Single lobe BAL only. # Early recognition of infection: clinical examination within 1 hr and clinical contact 1-5 days after bronchoscopy. |
| Chest pain/ cough | <0.2%14,# | Single lobe BAL only. Maximal BAL collection.# |
| Effects of drugs: lidocaine, midazolam | ||
| Arrhythmia | Very rare* | Maximum of 5 mg/kg14 lidocaine used. Warmed normal saline instilled. # Pulse oximetry (sats >90% with oxygen supplementation), cardiac monitoring throughout procedure. Benzodiazepine antagonist (flumazenil) immediately available. |
| Disorientation / agitation | <0.1%†# | |
| Cardio respiratory depression | Very rare* | |
* Not experienced in our group in over 1,500 research procedures.
† Idiosyncratic rather than dose-dependent reaction.
# Our group’s experience in over 1,500 research procedures.
Table 1. Risks associated with BAL for research. This includes the hazard or risk and how this is reduced by using the described technique. Effects of the bronchoscopy/BAL and also the effects of any medicinal drugs used are included.
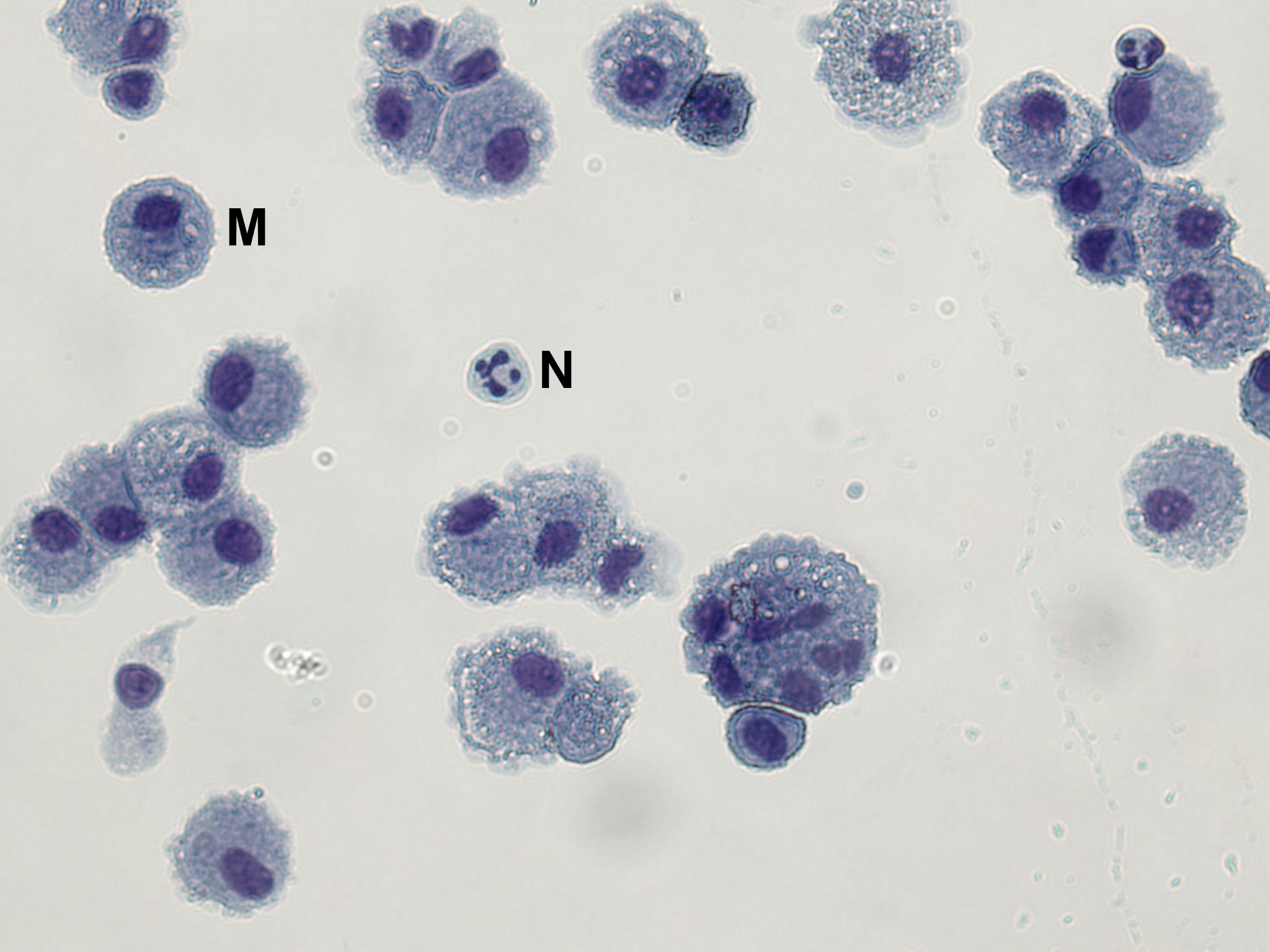
Figure 1. A Microscope Slide Showing BAL Macrophages and Occasional Neutrophils. BAL cells have been fixed in methanol and stained with Hemacolor red (eosin Y) and blue (azure B).
Discussion
In order to achieve good results the following are helpful:
- Good anesthetisation at the base of the tongue and posterior oropharynx. This is important because it improves the subject’s procedural tolerance and overall comfort.
- Either a pediatric (outer diameter 3.4-3.6 mm) or adult bronchoscope (outer diameter 4.8-5.9 mm) can be used.
- If there is any discomfort during nasal intubation, oral intubation is preferred. In general nasal intubation is either a straightforward technique or should be abandoned.
- After initial instillation of lidocaine to the oropharynx, the subject may prefer the bronchoscope to be withdrawn pending the onset of anesthesia, especially if the bronchoscope is inserted orally.
- Ensure at least 10 x 10 ml syringes are prefilled with only 2-4 ml of lidocaine. Air should be left in the syringes allowing improved delivery of the anesthesia directly onto cords and endobronchial mucosa.
- Since coughing during the procedure significantly reduces BAL yield; using warmed saline and having good mucosal anesthesia are important to reduce this.
- The 50 ml syringes must be prefilled with warmed saline. It is common for the first 50 ml of normal saline instilled to yield between 10-20 ml due to dead-space loss and the remainder running into the terminal airways. Further aliquots return a greater proportion of fluid.
- The operator should always check the bronchoscope prior to intubation, including ensuring effective suction by aspirating sterile normal saline. Loss of suction can commonly occur due to kinking of the tube or leaks around the connections with the bronchoscope or suction device.
- During manual suction, ideally the airway should not collapse. If suction recurrently collapses the airway, BAL returns are reduced to a valve mechanism, and local inflammation and bleeding is more likely: this in turn makes the airways more irritable and cough more likely. Choosing the most appropriate RML subsegmental branch and using low suction pressures are therefore both important.
- When air is constantly aspirated recheck the connection of the syringe to the bronchoscope and also the position of the bronchoscope in the distal airway. If this is a recurrent problem, post-procedure ensure that there are no holes in the bronchoscope itself.
- BAL from smokers is often darker due to the presence of intracellular particulates. Smokers may tolerate the bronchoscopy less well, and have increased cough.
- The preferable BAL volume yield is >100 ml. A yield of >150 ml is excellent. We have not experienced any yields >170 ml. There is no linear relationship between the cell yield and BAL volume yield.
- Yields of <100 ml are, in our experience, more likely to lead to side effects such as cough, chest pain and fever. We advise that subjects with yields of <100 ml be recovered in the left lateral position, this may aid drainage of any remaining lung fluid.
- Clinical contact should be made with the subject within 1-5 days to ensure no side effects.
- Midazolam sedation is used for <50% of subjects in the UK and <5% in Malawi. Midazolam does not appear to directly improve the tolerability; some operators feel that sedation may sometimes be counterproductive in maintaining a coherent (and reassuring) dialogue with the subject.
- In our experience, there is a significant learning curve with this technique. For example coordination between doctor and nurse takes time and yields are often lower at the beginning of the learning process.
- In HIV positive subjects, we use antibiotics prophylactically prebronchoscopy.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Thank you to Elena Mitsi (Research assistant) and Sr. Carole Hancock (Research nurse) and theatre staff for their contribution to filming, and to David Shaw (research nurse) for his support with our research bronchoscopies. Thanks also to our volunteer Rebecca Dunphy for allowing this procedure to be filmed.
Materials
|
Material |
Company |
Catalogue Number |
Comments |
|
Fibreoptic bronchoscope, light and suction source |
unspecified |
||
|
Surgical gown, sterile gloves |
unspecified |
||
|
Sphygnomanometer |
unspecified |
For continuous patient monitoring |
|
|
Pulse oximeter |
unspecified |
For continuous patient monitoring |
|
|
3 lead ECG monitor |
unspecified |
For continuous patient monitoring |
|
|
Nasal oxygen delivery 2-4l/min |
unspecified |
||
|
Lidocaine 10% spray (Xylocaine) |
AstraZeneca |
For topical anaesthesia |
|
|
Lidocaine hydrochloride 2% |
unspecified |
For topical anaesthesia |
|
|
Lidocaine gel (Instillagel) |
Farco-Pharma, Germany |
Alternative products available from other suppliers |
|
|
Normal saline, sterile 200ml |
unspecified |
Warm to 30oC |
|
|
10ml taper-end syringe (x10) |
unspecified |
For administration of lidocaine |
|
|
Intravenous cannula 18G |
unspecified |
For administration of midazolam |
|
|
Midazolam |
unspecified |
For sedation |
|
|
Flumazenil |
unspecified |
Reversal of benzodiazepine sedation (emergency use only) |
|
|
60ml taper-end syringes (x4) |
unspecified |
For normal saline injection and BAL fluid withdrawal. May require connector to attach to the injection port of the bronchoscope |
|
|
Sterile gauze swab |
Vernaid, UK |
Alternative products available from other suppliers |
|
|
Sterile container for BAL fluid |
See text and comments |
Siliconised glass bottles reduce macrophage adherence. Alternatively, 50ml centrifuge tubes may be used (total capacity 200ml) |
|
|
Sigmacote |
Sigma, UK |
SL2 |
Only if siliconised glass bottles used. NB: Harmful and flammable. If used, follow precautions detailed in manufacturer’s MSDS |
|
Centrifuge |
unspecified |
At least 4x50ml tube capacity. Refrigeration to 4oC preferred. |
|
|
Shandon Cytospin centrifuge |
Thermo Scientific, UK |
For differential count only |
|
|
RPMI 1640 medium with L-glutamine |
Sigma, UK |
R8758 |
Alternative products available from other suppliers |
|
Fetal bovine serum |
Sigma, UK |
F6178 |
Alternative products available from other suppliers |
|
Penicillin-Streptomycin |
Sigma, UK |
P4333 |
Alternative products available from other suppliers |
|
Amphotericin B |
Sigma, UK |
A2942 |
Alternative products available from other suppliers |
|
Tissue culture plates |
Greiner Bio-One, UK |
662160 |
Alternative products available from other suppliers |
|
Glass microscope slides |
unspecified |
For differential count only |
|
|
Shandon cytofunnel |
Thermo Scientific, UK |
A78710003 |
For differential count only |
|
Shandon cytoclip |
Thermo Scientific, UK |
59910052 |
For differential count only |
|
Hemacolor staining set (fixative, red and blue reagents) |
Merck, Germany |
111661 |
Use according to manufacturer’s instructions |
References
- BTS, The British Thoracic Society Bronchoscopy Guideline Committee – A sub-Committee of the Standards of Care Committee of the British Thoracic Society. Thorax. 56 (Suppl I), 1-21 (2001).
- . Technical recommendations and guidelines for bronchoalveolar lavage (BAL) report of the European Society for Pneumology Task Group. Eur. Respir. , 561-585 (1989).
- Gordon, S. B., Irving, G. R., Lawson, R. A., Lee, M. E., Read, R. C. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect. Immun. 68, 2286-2293 (2000).
- Gordon, S. B., et al. Pulmonary immunoglobulin responses to Streptococcus pneumoniae are altered but not reduced in human immunodeficiency virus-infected Malawian adults. J. Infect. Dis. 188, 666-670 (2003).
- Fullerton, D. G., et al. Domestic smoke exposure is associated with alveolar macrophage particulate load. Trop. Med. Int. Health. 14, 349-354 (2009).
- Jambo, K. C., et al. Bronchoalveolar CD4+ T cell responses to respiratory antigens are impaired in HIV-infected adults. Thorax. 66, 375-382 (2011).
- Eagan, R., et al. Lung fluid immunoglobulin from HIV-infected subjects has impaired opsonic function against pneumococci. Clin. Infect. Dis. 44, 1632-1638 (2007).
- Rose, A. S., Knox, K. S. Bronchoalveolar lavage as a research tool. Sem. Respir. Crit. Care Med. 28, 561-573 (2007).
- Wright, A. K. A., et al. Human Nasal Challenge with Streptococcus pneumoniae is Immunising in the Absence of Carriage. PLoS Pathog. , (2012).
- Bolliger, C. T., et al. ERS/ATS statement on interventional pulmonology European Respiratory Society/American Thoracic Society. Eur. Respir. J. 19, 356-373 (2002).
- Ettensohn, D. B., Jankowski, M. J., Duncan, P. G., Lalor, P. A. Bronchoalveolar lavage in the normal volunteer subject. I. Technical aspects and intersubject variability. Chest. 94, 275-280 (1988).
- Ettensohn, D. B., Jankowski, M. J., Redondo, A. A., Duncan, P. G. Bronchoalveolar lavage in the normal volunteer subject. 2. Safety and results of repeated BAL, and use in the assessment of intrasubject variability. Chest. 94, 281-285 (1988).
- De Brauwer, E. I., Jacobs, J. A., Nieman, F., Bruggeman, C. A., Drent, M. Bronchoalveolar lavage fluid differential cell count. How many cells should be counted. Anal. Quant. Cytol. Histol. 24, 337-341 (2002).
- Mtunthama, N., et al. Malawians permit research bronchoscopy due to perceived need for healthcare. J. Med. Ethics. 34, 303-307 (2008).
- Pereira, W., Kovnat, D. M., Snider, G. L. A prospective cooperative study of complications following flexible fiberoptic bronchoscopy. Chest. 73, 813-816 (1978).
- Huang, Y. C., Bassett, M. A., Levin, D., Montilla, T., Ghio, A. J. Acute phase reaction in healthy volunteers after bronchoscopy with lavage. Chest. 129, 1565-1569 (2006).
- Gritzfeld, J. F., et al. Experimental Human Pneumococcal Carriage. J. Vis. Exp. (72), (2013).

