Activation and Measurement of NLRP3 Inflammasome Activity Using IL-1β in Human Monocyte-derived Dendritic Cells
Instructor Prep
concepts
Student Protocol
Ethics Statement: Research samples are obtained and stored for research with donors’ consent. All samples should be coded or anonymized prior to use. This protocol follows the guidelines of our Institutional Review Board.
1. Differentiation of Human Peripheral Blood Monocytes into Monocyte Derived Dendritic Cells.
Note: Human buffy coats serve as the source of human peripheral blood cells (PBMCs) and were obtained from the New York Blood Center (New York, NY). Blood donors are healthy volunteers. The 5 day procedure begins with the plating of human peripheral blood mononuclear cells (PBMCs) onto tissue culture flasks35,36. Notable differences from the published protocols are the following:
- Use 225 cm2 non pyrogenic polystyrene tissue culture flasks with a filter cap to adhere a total of 2×108 PBMCs per flask instead of 10 cm polystyrene tissue culture plates (58 cm2) (step 1).
- Prepare media with 5% pooled human serum (PHS; 30 ml) in 500 ml RPMI-1640 + L-glutamine, 5 ml 1 M HEPES buffer, and 1.4 ml 50 mg/ml Gentamicin (5% PHS media) followed by filtration through a 20 µM Filter. Add a total of 50 ml 5% PHS media per 225 cm2 tissue culture flask.
- Wash adhered cells three times with 25 ml fresh RPMI-1640. Shake vigorously for 5 sec during each wash and aspirate non adherent cells (step 4).
- Add 190 µl of 400 IU/µl IL-4 and 380 µl of 100 IU/µl GM-CSF per flask (step 3) on day 0, 2, and 4. Day 0 is defined as the day PBMCs are initially plated in Step 1 (Step 8).
- Harvest day 5 moDCs at a concentration of 1×106 cells/ml (step 15).
- Use moDCs immediately in their resting state. Steps 17-22 are never performed. Note: Samples should be processed as soon as possible after collection for best results.
- Aliquot moDCs at a concentration of 2×105 cells/well (200 µl/well of the 1×106 cells/ml) in a 96 well round bottomed plate (western blot, ELISA, FACs) or onto a poly-L-lysine treated chamberslide (microscopy) for experimentation. Note: There are at least 4 conditions: Completely unstimulated (negative control), priming only, activation only, and priming followed by activation. Conditions can be expanded to include diluent controls for R848 and nigericin if desired. If the downstream assay is ICS, duplicates are necessary for isotype controls.
2. Priming the Inflammasome – Signal 1
- Reconstitute lyophilized R848 in DMSO according to the manufacturer's instructions. Dilute working stock at RT with RPMI-1640.
- Add R848 at a 10 µM final concentration to appropriate wells for 18 hr. Place cells in an incubator at 37 °C and 5% CO2.
3. Activating the NLRP3 Inflammasome – Signal 2
- Reconstitute lyophilized nigericin in ethanol according to the manufacturer's instructions. Dilute working stock at RT with RPMI-1640 before adding to the appropriate conditions.
- Add nigericin at a 20 µM final concentration and return to the incubator at 37 °C and 5% CO2 for 6 hr. No washing steps occur between priming and during activation. Note: Secretion of IL-1β is increased by the presence of calcium ionophores, brefeldin A, monensin, dinitrophenol, or carbonyl cyanide chlorophenylhydrazone37,38. Therefore, addition of these secretory inhibitors are not recommended when analyzing inflammasome priming or activity since it will alter secretion of IL-1β.
4. IL-1ß Sample Collection
- Centrifuge the plate with cells and supernatants at 974 x g for 3 min.
- Without disturbing the cell pellet, aspirate the supernatant and transfer to a separate round bottomed plate in order to measure cytokine secretion from the supernatants. Note: Supernatants can be temporarily stored at -20 °C or -80 °C for long term storage.
- Wash the cellular pellets three times with 200 µl of 1x PBS at 974 x g for 3 min to remove any extracellular IL-1β from the cellular samples. Note: When washing cell pellets from a 96 well plate, remove the wash by swift plate inversion into a collection bin so as to not disturb the sample.
5. Measuring IL-1ß From Cellular and Supernatant Samples
- Intracellular Cytokine Staining (ICS): The ICS protocol is described below39,40. Note: do not suspend the cells if the downstream assay is microscopy. All washes and aspirations should be done without disturbing the cell layer/pellet (depending on the downstream assay).
- Add 100 µl of 5% PHS media into appropriate wells. Add appropriate volume (approximately 1 µl/2×105 cells, or 1 µl/well) of fluorescently labeled α-CD11c and α-CD14 (phenotype markers; clone B-ly6 and MφP9, respectively) or isotype control to the cells for 10 min at RT in the dark.
- Wash three times with 1x PBS at 974 x g for 3 min.
- Fix the cells by adding 100 µl of 4% PFA for 20 min at RT in the dark.
- Pause Point: Wash with 100 µl 1x PBS at 974 x g for 3 min and store at 4 °C O/N in 1x PBS.
- Add 100 µl of permeabilization buffer to cells for 30 min. Note: permeabilization buffer is composed of 1x PBS with 0.3% Triton X-100, and 1% bovine serum albumin (BSA) for permeabilization. Do not disturb adherent cells when downstream assay is microscopy.
- Add appropriate volume (approximately 62 ng antibody/2×105 cells, approximately or 2.5 µl/well) of α-IL-1β-FITC (clone 8516) or isotype control for 2 hr in an incubator at 37 °C.
- Wash cells three times with 200 µl of permeabilization buffer at 974 x g for 3 min in the dark.
- Optional: Stain with DAPI, add mountant, and place coverslip gently on top of a glass slide. Allow mountant to cure O/N before capturing microscopy images.
- Acquire the data by FACs or microscopy. Note: Compare the isotype for each condition to the appropriate staining when analyzing by FACs or microscopy.
- FACs: Acquire one sample at a time. Set the FSC/SSC gate on the live cells, followed by gating on CD11c+CD14– cells before analyzing the moDC population pro-IL-1β staining.
- Microscopy: Set the exposure time using the positive staining sample – R848 treated. Use DAPI+pro-IL-1β+ and DAPI+pro-IL-1β– to determine percentage of pro-IL-1β+ expressing moDCs.
- SDS-Page: Immunoblotting is performed to detect pro-IL-1β. Note: The technique described below combines standard immunoblotting technique, gradient 4-20% polyacrylamide gel, and fluorescent or chemiluminescent detection41,42 .
- Lyse cells directly in 10 µl denaturing lysis buffer (5 µl β-mercaptoethanol/950 µl Laemmli sample buffer followed by diluting 1:1 with cell lysis buffer). Transfer lysate to 1.5 ml Eppendorf tubes.
- Heat lysate on a dry plate for 10 min at 100 °C.
- Spin lysate at 20,800 x g on a minicentrifuge for 1 min to concentrate the liquid volume. Pause point: samples can be frozen at -20 °C for short term storage.
- Load the total volume onto the polyacrylamide gel. Run 140 V through the gel box for approximately 1 hr, or until the dye front runs off the bottom of the gel.
- Transfer the protein from the polyacrylamide gel onto a PVDF Immobilon-FL membrane for 90 min at 100 V. Block the membrane with 5% BSA in Tris Buffered Saline/ 0.1% Tween-20 (TBS-T) for 1 hr. Note: PVDF Immobilon-FL Is best for use with fluorescent detection. A membrane with a different composition is recommended for other detection techniques to increase the signal to background ratio.
- Dilute primary and secondary antibodies in 10 ml of 5% BSA/TBS-T. Perform primary antibody incubations at 4 °C O/N while shaking and secondary antibody at RT for 1 hr while shaking.
- Wash the membrane 3 times with TBS-T for 5 min while shaking between primary and secondary antibody incubations and again between secondary incubations and imaging. Note: Bands can be detected using fluorescent or chemiluminescent detection. Follow the manufacturer’s instructions for detection.
- ELISA: Samples that are frozen for storage need to equilibrate to RT before analysis. Spin down samples in a centrifuge to consolidate supernatant condensation. Follow the manufacturer’s instructions for measurement of IL-1β. Note: In this protocol IL-1β is specifically measured; however, simultaneous measurement of TNFα, IL-6, and the immunomodulatory cytokine IL-10 ensure the appropriate conditions were primed.
Activation and Measurement of NLRP3 Inflammasome Activity Using IL-1β in Human Monocyte-derived Dendritic Cells
Learning Objectives
These techniques measure TLR8 priming with R848. Intracellular cytokine staining for pro-IL-1β allows for microscopy and FACs readouts from CD14–CD11c+ moDCs. Both techniques can be quantified relative to a non primed, or resting, cell control as well as an isotype control (Figures 1 and 2). Percent of pro-IL-1β+ staining cells is multiplied by the geometric median of this population to provide the median fluorescent intensity (MFI). The MFI is comparable to the amount of pro-IL-1β present in the positive staining cells.
Immunoblotting measures pro-IL-1β from cell lysate, which is then quantified relative to an internal cellular control, such as β-tubulin or β-actin (Figure 3). Immunoblotting for pro-IL-1β in nigericin treated cells should reveal a decrease in pro-IL-1β. This is complemented by a concurrent increase in IL-1β in supernatants, measured by ELISA, only in R848 followed by nigericin conditions (Figures 3 and 4). All other conditions should result in no extracellular IL-1β present. Simultaneous measure of other inflammatory cytokines, such as TNFα, IL-10, and IL-6, ensure that nigericin is specific in causing the secretion of IL-1β. The level of priming is time (Figure 3) and dose (Figure 4) dependent, a response reflected in the degree of intracellular pro-IL-1β in R848 primed and extracellular IL-1β (as well as TNFα, IL-10, and IL-6) secretion in all R848 treated conditions (Figure 4).
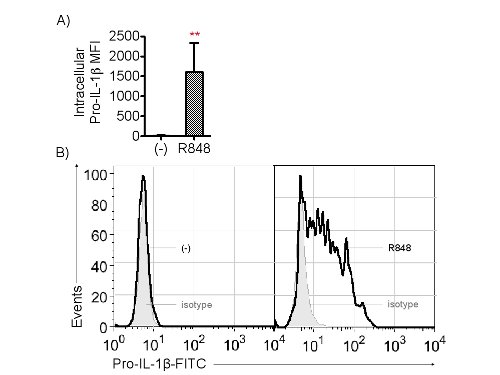
Figure 1. Cytoplasmic pro-IL-1β is detected by flow cytometry. Please click here to view a larger version of this figure.
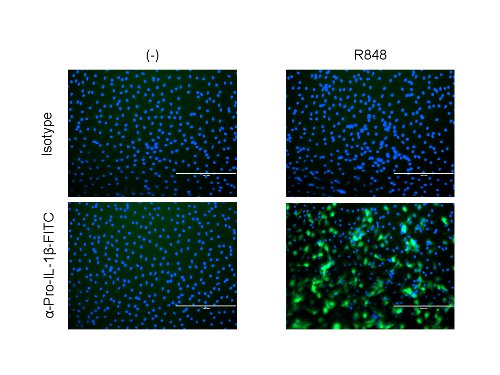
Figure 2. Cytoplasmic pro-IL-1β is detected by microscopy. Please click here to view a larger version of this figure.
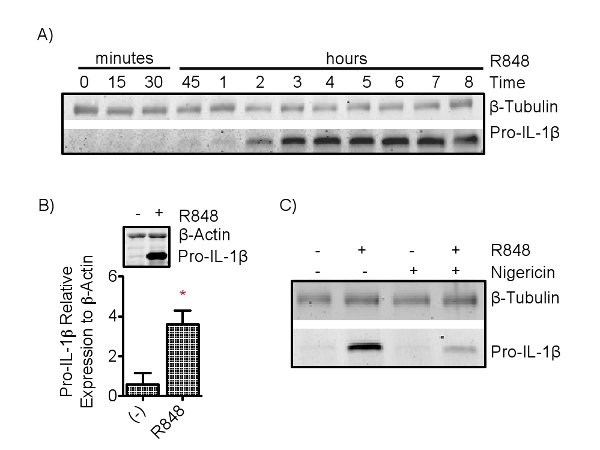
Figure 3. Cytoplasmic pro-IL-1β is detected by SDS-Page. Please click here to view a larger version of this figure.
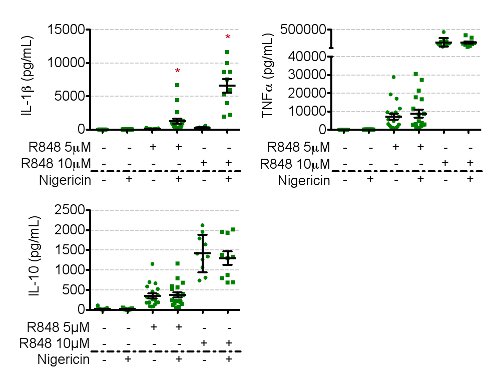
Figure 4. Secreted IL-1β is detected by ELISA. Please click here to view a larger version of this figure.
List of Materials
| Name of Reagent/ Equipment | Company | Catalog Number | Comments/Description |
| IL-4 | R&D | ||
| GM-CSF | Genzyme | NDC 58468-0180-2 | We acquire this item through our local pharmacy with a prescription |
| RPMI 1640 with L-glutamine | Cellgro | 10-040-CV | |
| Peripheral blood mononuclear cells | New York Blood Center | PBMCs were isolated from the blood of healthy donors | |
| 12-well tissue culture plates | Sigma-Aldrich | 3516 | |
| 96-well round bottom tissue culture plates | Sigma-Aldrich | 3799 | |
| α-IL-1β-FITC | R&D | IC201F | |
| FITC isotype control | Miltenyi Biotec | 130-092-213 | |
| α-β-Tubulin | Santa Cruz | SC-9014 | |
| α-IL-1β | R&D | mab201 | |
| PVDF Immobilon-FL membrane | Millipore | IPFL00010 | |
| gradient 4-12 % polycrylamide gel | Bio Rad | 161-1159 | |
| laemmli sample buffer | Bio Rad | 161-0737 | |
| BSA | Equitech Bio Inc | 30% solution sterile/filtered | |
| PFA | Electron Microscopy Sciences | 15710 | 16% solution |
| human inflammatory cytokine bead array kit | BD | 551811 | |
| nigericin | Invivogen | tlrl-nig | |
| R848 | 3M Corp. | ||
| α-CD14 | BD | 340436 | |
| α-CD11c | BD | 555392 | |
| β-mercaptoethanol | Sigma-Aldrich | M6250-10ML | |
| TBS | On site stock room | ||
| Tween-20 | Sigma-Aldrich | P2287-100mL | |
| Nunc EasYFlask 225cm2, Filter Cap, 70mL working volume, 30/Cs | Thermo Scientific | 159933 | |
| 20 μM Sterile Disposable Filter Units | Thermo Scientific | 569-0020 | |
| Gentamicin | Invitrogen | 15750060 | |
| Hepes | Invitrogen | 15630080 | |
| goat α-mouse IRDye 800CW | Licor | 926-32210 | |
| donkey α-rabbit IRDye 680RD | Licor | 926-68073 | |
| Spectra multicolor broad range protein ladder | Thermo Scientific | 26634 | |
| Tris Glycine SDS 10x | Bio Rad | 1610732 | |
| Tris Glycine 10x | Bio Rad | 161-0734 | |
| Methanol – 4L | Fisher Scientific | A433P-4 | |
| Prolong Gold antifade Reagent with DAPI | Life Technologies | P-36931 | |
| 8 chamber polystyrene vessel tissue culture treated glass slide | BD Falcon | 354108 | |
| Poly-L-Lysine | Sigma | P4707 |
Lab Prep
Inflammatory processes resulting from the secretion of Interleukin (IL)-1 family cytokines by immune cells lead to local or systemic inflammation, tissue remodeling and repair, and virologic control1,2 . Interleukin-1β is an essential element of the innate immune response and contributes to eliminate invading pathogens while preventing the establishment of persistent infection1-5.
Inflammasomes are the key signaling platform for the activation of interleukin 1 converting enzyme (ICE or Caspase-1). The NLRP3 inflammasome requires at least two signals in DCs to cause IL-1β secretion6. Pro-IL-1β protein expression is limited in resting cells; therefore a priming signal is required for IL-1β transcription and protein expression. A second signal sensed by NLRP3 results in the formation of the multi-protein NLRP3 inflammasome. The ability of dendritic cells to respond to the signals required for IL-1β secretion can be tested using a synthetic purine, R848, which is sensed by TLR8 in human monocyte derived dendritic cells (moDCs) to prime cells, followed by activation of the NLRP3 inflammasome with the bacterial toxin and potassium ionophore, nigericin.
Monocyte derived DCs are easily produced in culture and provide significantly more cells than purified human myeloid DCs. The method presented here differs from other inflammasome assays in that it uses in vitro human, instead of mouse derived, DCs thus allowing for the study of the inflammasome in human disease and infection.
Inflammatory processes resulting from the secretion of Interleukin (IL)-1 family cytokines by immune cells lead to local or systemic inflammation, tissue remodeling and repair, and virologic control1,2 . Interleukin-1β is an essential element of the innate immune response and contributes to eliminate invading pathogens while preventing the establishment of persistent infection1-5.
Inflammasomes are the key signaling platform for the activation of interleukin 1 converting enzyme (ICE or Caspase-1). The NLRP3 inflammasome requires at least two signals in DCs to cause IL-1β secretion6. Pro-IL-1β protein expression is limited in resting cells; therefore a priming signal is required for IL-1β transcription and protein expression. A second signal sensed by NLRP3 results in the formation of the multi-protein NLRP3 inflammasome. The ability of dendritic cells to respond to the signals required for IL-1β secretion can be tested using a synthetic purine, R848, which is sensed by TLR8 in human monocyte derived dendritic cells (moDCs) to prime cells, followed by activation of the NLRP3 inflammasome with the bacterial toxin and potassium ionophore, nigericin.
Monocyte derived DCs are easily produced in culture and provide significantly more cells than purified human myeloid DCs. The method presented here differs from other inflammasome assays in that it uses in vitro human, instead of mouse derived, DCs thus allowing for the study of the inflammasome in human disease and infection.
Procedure
Inflammatory processes resulting from the secretion of Interleukin (IL)-1 family cytokines by immune cells lead to local or systemic inflammation, tissue remodeling and repair, and virologic control1,2 . Interleukin-1β is an essential element of the innate immune response and contributes to eliminate invading pathogens while preventing the establishment of persistent infection1-5.
Inflammasomes are the key signaling platform for the activation of interleukin 1 converting enzyme (ICE or Caspase-1). The NLRP3 inflammasome requires at least two signals in DCs to cause IL-1β secretion6. Pro-IL-1β protein expression is limited in resting cells; therefore a priming signal is required for IL-1β transcription and protein expression. A second signal sensed by NLRP3 results in the formation of the multi-protein NLRP3 inflammasome. The ability of dendritic cells to respond to the signals required for IL-1β secretion can be tested using a synthetic purine, R848, which is sensed by TLR8 in human monocyte derived dendritic cells (moDCs) to prime cells, followed by activation of the NLRP3 inflammasome with the bacterial toxin and potassium ionophore, nigericin.
Monocyte derived DCs are easily produced in culture and provide significantly more cells than purified human myeloid DCs. The method presented here differs from other inflammasome assays in that it uses in vitro human, instead of mouse derived, DCs thus allowing for the study of the inflammasome in human disease and infection.
