Consensus Brain-derived Protein, Extraction Protocol for the Study of Human and Murine Brain Proteome Using Both 2D-DIGE and Mini 2DE Immunoblotting
Summary
A common protein extraction protocol using urea/thiourea/SDS buffer for human and mice brain tissue allows indentification of proteins by 2D-DIGE and their subsequent characterization by mini 2DE immunoblotting. This method enables one to obtain more reproducible and reliable results from human biopsies and experimental models.
Abstract
Two-dimensional gel electrophoresis (2DE) is a powerful tool to uncover proteome modifications potentially related to different physiological or pathological conditions. Basically, this technique is based on the separation of proteins according to their isoelectric point in a first step, and secondly according to their molecular weights by SDS polyacrylamide gel electrophoresis (SDS-PAGE). In this report an optimized sample preparation protocol for little amount of human post-mortem and mouse brain tissue is described. This method enables to perform both two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) and mini 2DE immunoblotting. The combination of these approaches allows one to not only find new proteins and/or protein modifications in their expression thanks to its compatibility with mass spectrometry detection, but also a new insight into markers validation. Thus, mini-2DE coupled to western blotting permits to identify and validate post-translational modifications, proteins catabolism and provides a qualitative comparison among different conditions and/or treatments. Herein, we provide a method to study components of protein aggregates found in AD and Lewy body dementia such as the amyloid-beta peptide and the alpha-synuclein. Our method can thus be adapted for the analysis of the proteome and insoluble proteins extract from human brain tissue and mice models too. In parallel, it may provide useful information for the study of molecular and cellular pathways involved in neurodegenerative diseases as well as potential novel biomarkers and therapeutic targets.
Introduction
Mental and neurological disorders represent 13% of the global burden of disease, new challenges like pathophysiological mechanisms, risk factors and prodromal biomarkers must be explored1. In line with this objective, proteomics studies of the human brain become indispensable to uncover molecular pathways involved in processes like memory, behavior, emotions and neuronal plasticity for instance, not only for physiological but also for pathological conditions. Therefore, the use of animal models and more specifically the transgenic mice, brings a wide range of possibilities to mimic the etiology of human neurodegenerative disorders2.
Proteomics approaches are nowadays available in order to accomplish these new perspectives in the neuroscience field. Two-dimensional gel electrophoresis (2DE) is a potent and likely simple method that enables to compare the proteome of a wide range of samples. Moreover, it is also a powerful method to isolate a protein from a complex mixture in order to identify and further analyze by mass spectrometry. This technique essentially consists in two successive steps: 1) protein separation according to their isoelectric point (pI) by isoelectrofocusing (IEF). More precisely, an electric potential is applied across the immobiline acrylamide strips among a pH gradient and then proteins will migrate and focus on a determined pI in function of their global net charge. 2) Isoelectrically focused proteins are denatured and negatively charged by the addition of sodium dodecyl sulfate (SDS), thereby proteins in their first structure are separated depending on their apparent molecular weight (MW) by SDS-PAGE3. These two distinct properties allow us to tackle a double value to go further in the study of the proteome. On one hand this approach offers the possibility to perform a quantitative analysis using minimal dyes 2D-DIGE method, and on the other hand a qualitative analysis by mini-2DE coupled to western blotting.
Quantitative analysis by 2D-DIGE bestows protein expression changes all over the sample proteome. Briefly, samples are labeled with three cyanines (Cy2, Cy3 and Cy5) emitting at three distinct wavelengths (blue, green and red). These fluor minimal dyes containing N-hydroxy succinimidyl ester group react with the ε-amino group of lysines residues of proteins resulting in covalent amide bonds4. Lysine residues are labeled only between 1-3% and thus preventing multiple labels addition per protein and major net charge modifications5,6. Cy3 and Cy5 are often used to label two independent samples while Cy2 tags a mix of equal proportion of the samples to compare. The two main advantages are that all labeled samples are mixed and IEF and SDS-PAGE are carried out in one gel at once for each step, avoiding the inter variability among experiments due to gels comparison. Moreover, it presents a high detection threshold of around 1 femtomole of protein7. Gels are scanned and 2D software compares the 2D gel fluorescence images, where Cy2 serves as an internal standard allowing for identification of statistical differences among the spots for their posterior identification by mass spectrometry. The 2D analysis software using the internal standard achieves a fast detection of less than 10% of differences between samples with more than 95% of statistical confidence8.
Qualitative analysis by mini-2DE is a crucial step for protein characterization. The principle is the same as previously described for pI and MW separation, but in this case proteins are transferred from a small polyacrylamide gel to a membrane and immunoblotting is performed afterwards. While one dimension gel electrophoresis provides changes in protein expression for one or several protein epitopes in function of the antibody, the information of mini-2DE endows with two additional parameters. Firstly, the protein isovariants change in function of the pI, indicating that post-translational modifications might take place. Secondly, mass spectrometry identification may be indicative of plausible zymogens and catabolic products of proteins. Therefore, modifications observed by 2D-DIGE are likely indicative of the mechanisms underlying changes in global proteome profile. Alignments of immunoblots among several samples for the same protein epitope/s by mini-2DE may reflect acidity changes shedding light into post-translational variations slightly observed or even not by monodimensional immunoblotting9,10. Moreover, this analysis informs about the potential cleavage sites due to the knowledge of the pI and MW of the metabolic residues11,12.
The combination of these two techniques provides a complementary proteomics analysis. On one hand 2D-DIGE affords a type of differences allowing a precise isolation of polypeptides whose expression is different. These differences consist essentially into the appearance or disappearance of a spot or increase/decrease of intensity of a given one by software analysis of the fluorescent gels. However, these observations by themselves are unlikely to explain the nature of the modification observed. For these reasons, once the polypeptide is isolated and identified by mass spectrometry, the use of mini-2DE enables to precisely confirm 1) the identity of the protein isolated and 2) the nature of the difference: change in the isovariant/isoform level of expression, post-translational modifications and cleavage processes for instance. However, it is necessary to develop a starting lysis buffer both compatible with 2D-DIGE and with mini-2DE in order to limit the potential dispersions resulting from the use of extraction protocols that are very dissimilar.
In the present article, we described an adapted protocol for the preparation, extraction and performance of 2D-DIGE and mini-2DE techniques for brain proteins coming from human and mouse tissue.
Protocol
1. Homogenization and Total Protein Extraction from Human and Mouse Brain Tissue
- Brain tissue homogenization. Homogenize the brain tissue 10% (w/v) in 8 M urea, 2 M thiourea and 1% w/v SDS buffer (UTS) using a potter glass (for human samples) or Teflon homogenizer (for mice samples). Sonicate at 60 Hz 30 pulses with a ultrasound generator applying 0.5 sec for total tissue disintegration13,14.
- Determine protein concentration with Bradford assay, using BSA as standard. This UTS buffer extraction is totally compatible with one dimension SDS-PAGE as long as lysates are not over-heated to avoid carbamylation15.
- Add 1% (w/v) of Amberlite and incubate with gentle mixing during 10 min at room temperature. Thereafter filter onto 0.22 µm syringe filters and finally add the SDS to the urea/thiourea solution. This step reduces the amount of uric acid and isocyanic acid, which are in equilibrium with urea in solution and facilitate its degradation.
- Chloroform/methanol protein precipitation. Precipitate between 100-150 µg of protein for 11 cm IPG Strips and 1-1.5 mg for 18 cm ones (independently on the pH range selected to perform the IEF).
- Perform all the steps on ice or at 4 °C. Cool chloroform and methanol at 4 °C for 30 min before starting the precipitation procedure.
- Add three volumes of methanol and one volume of chloroform to one volume of UTS lysate. Shake manually the mixture and add three volumes of cold water. Vortex during 1 min and pellet the proteins by centrifugation at 12,000 x g for 30 min at 4 °C.
- Discard the supernatant using a Pasteur pipette and add three volumes of cold MeOH. Vortex again and centrifuge at 12,000 x g during 30 min at 4 °C. Finally discard supernatant and dry the pellet under N2.
- Preparation of proteins for 2D analysis. Resuspend the protein-dried pellet in 2D-buffer (8 M urea, 2 M thiourea supplemented with 4% CHAPS). Wash the solution with Amberlite as indicated above and try to keep the proportion of 500 µg of protein per 200 µl of 2D buffer for 2D-DIGE. Note: 2D buffer can be stored at -80 °C or kept at 4 °C during one week (to prevent urea degradation).
- Sonicate and store the samples at -80 °C until carrying out the following step. Noteworthy, this protocol can be used after formic acid solubilization of protein aggregates10. Briefly, evaporate formic acid under N2 and resuspend the pellet as described.
2. 2D-DIGE
- Samples test quality. Dose samples again using Bradford method. Dilute 15 µg of proteins in LDS sample buffer, heat at 37 °C15 and load onto 4-12% polyacrylamide gels.
- Coomassie staining. Stain polyacrylamide gel with 0.1% (w/v) Coomassie Blue G-250, 50% EtOH and 10% (v/v) acetic acid solution for at least 1 hr. Let background destain overnight in 7% acetic acid and 10% (v/v) ethanol solution.
- Pre-electrophoretic labeling of samples. Prior to performing cyanine dye labeling, verify the pH of the sample, which should be basic or even neutral since the N-hydroxysuccinimide substitution is more efficient at basic pH and given to be optimal at pH 8.6.
- Label minimally the two samples of study with Cy3 or Cy5 fluorescent dyes with a ratio of 50 μg protein/400 pmol dye and keep for 1 hr at 4 °C in the darkness to facilitate the NHS ester reactive group of the cyanines with the unprotonated amine groups of lysines.
- Reduce sample variations making a cross-labeling with Cy3 and Cy5 dyes, avoiding a preferential coupling of one sample to a particular cyanine. Note that the cyanine-dye minimal labeling can be adapted for small amount of proteins with keeping the proportion of 1 µg protein per 8 pmol cyanine-dye. If so, the mini-2DE system can be used instead of the large 2D gel system. This will enable the 2D-DIGE analysis for a small amount of brain tissue.
- Label a pool of both samples with the same amount of protein (50 µg in total) with Cy2 fluorescent dye and used as internal standard. Use the same conditions as previously indicated.
- Complete to a total volume 350 μl with 2D buffer containing 1.1% Destreak reagent, 1.2% of IPG buffer and traces of Bromophenol Blue the total 150 µg of labeled proteins (50 µg of Cy2, 50 µg of Cy3 and 50 µg of Cy2). Perform this step in quadruplicate using four independent strips. Moreover, prepare two additional non-labeled strips (one for each sample) with 350-450 µg of protein for the preparative gels.
- Leave the six loaded strips covered with mineral oil overnight for passive rehydration.
- Isoelectrofocusing. Carry out the IEF at 20 °C. For a pH 3-11NL, 18 cm strip the protocol is: maximum current setting is 50 μA/strip during 8 stages: 1) 150 V step for 1 hr, 2) 200 V step for 5 hr, 3) 500 V gradient for 2 hr, 4) 1,000 V gradient for 2 hr, 5) 2,000 V gradient for 2 hr, 6) 4,000 V gradient for 2 hr, 7) 8,000 V gradient for 2 hr and 8) for a total of 24,000 V·hr step. After IEF, dispatch the excess of mineral oil using chromatography paper and store the strips at -20 °C until the second dimension.
- IPG Strips equilibration. Equilibrate strips in 6 M urea, 2% SDS, 30% Glycerol, 50 mM Tris-HCl pH 8.6 buffer with 1% of DTT during 15 min and after with 4.7% of iodoacetamide in the same buffer but without DTT.
- Second dimension or SDS-PAGE electrophoresis. Make the six 12% SDS-PAGE gels (1.5 M Tris-HCl pH 8.6, 12% acrylamide/bisacrylamide, 0.1% (w/v) SDS, 0.072% (w/v) APS and 0.1% (v/v) TEMED) at the same time using a large 2D system, with four low-fluorescence glass plates for the strips with fluorescence samples and two other glass plates with 1.5 mm of thickness for preparative gels in order to excise the protein spots.
- Load the six IPG Strips on the top of the gels and over layered with 0.5% (w/v) of agarose. Running buffer is composed of 25 mM Tris, 192 mM glycine and 0.1% (w/v) SDS. Carry out the migration at 2.5 W/gel overnight with a refrigeration system set to 4 °C16,17.
- Fluorescence images acquisition and analysis. Use a Typhoon 9400 scanner to obtain the fluorescence gels images (12 images for one experiment). Scan Cy2, Cy3 and Cy5 images for each gel at 488/520 nm, 532/580 nm and 630/670 nm excitation/emission wavelengths, respectively. Use informatic software to analyze the images. Data are representative of the changes of spot volume associated with the distribution of the signal across the four gels among the two samples studied.
- Coomassie staining for big gels. Fix the preparative gels in 50% (v/v) EtOH and 3% (v/v) orthophosphoric acid solution for at least 24 hr. Then after, wash twice with ultra pure water for 20 min. Perform coloration in 34% (v/v) MeOH, 17% (w/v) ammonium sulfate and 2% orthophosphoric acid for 1 hr before adding 0.1% (w/v) Coomassie Blue G-250. Keep the gels in coloration for at least 48 hr and decolorized in ultra pure water.
- Spots for MS identification. Once fluorescence images are analyzed, the software provides a list of spots whose expression is statistically different. Manually excise these spots from the preparative gels. This procedure requires practice and some manual dexterity in order to avoid keratin contamination. Then samples can be kept in water at -20 °C until sending to MS. Spot identification is perfectly compatible with Tandem mass spectrometry (MS/MS) and relevance of protein identities is judged according to the probability based Mowse score calculated with a p-value of 0.05 (p < 0.05)18.
3. Qualitative Mini-2DE Assay
- Resuspend maximum 100 µg of protein sample in 2D buffer until a final volume of 200 µl for a 11 cm IPG strip. In case of overexpression system or purified protein, the starting amount can be lowered to 20 µg.
- Add 1.1% (v/v) Destreak Reagent, 0.55% (v/v) IPG buffer and traces of Bromophenol Blue to a final volume of 200 µl. Then, vortex, make a fast spin and load into an IPG strip for passive rehydration (to bring the strip to its original thickness) overnight covered with mineral oil. Note: the proportion of IPG buffer is the same for all the interval of pH desired (pH 3-11, 4-7, 7-11, etc), but its composition varies in function of the pH range selected.
- Isoelectrofocalisation. Perform IEF at 20 °C. The duration of the program depends on the pH interval selected. Noteworthy, parameters such as electroendosmosis may disturb the IEF profile and in these cases, optimization in electrofocalisation time is required19. Note: it is not necessarily a longer IEF that provides better results but shortening the time of IEF can also improve the resolution. After IEF, IPG strips can be stored at -20 °C for months.
- IPG strips equilibration. Equilibrate the strips in a buffer containing 25 mM Tris-HCl pH 6.8, 20 mM DTT, 10% glycerol, 5% SDS, 0.05% Bromophenol Blue. Make three equilibration bathes for 15 min with changing the buffer solution between each bath.
- SDS-PAGE. Layer the IPG strip onto a precast gel (IPG+1 well, 11 cm IPG strip) with a percentage of polyacrylamide chosen depending on the MW of the protein of interest. Then, blot the gel onto a nitrocellulose/PVDF membrane at 100 mV during 33 min.
- Incubate overnight with the required antibody and visualize by peroxidase activity. Perform the alignments of immunoblots using unrelated polypeptides revealed by the secondary antibody. Reach a semi-quantitative analysis by densitometry of the volume and intensity for the traces/ spots with ImageJ software.
Representative Results
Proteomics on brain tissue remains challenging since no ideal buffer exists to recover 100% of proteins, especially membrane-associated or cytoskeleton proteins. The first set of experiments were focused on the search for a suitable lysis buffer compatible with the two approaches and which enables to recover a large panel of protein. Thus, three lysis buffers were assayed to determine the most appropriate one. Firstly, it was used the common biochemical and molecular biology buffer Tris-HCl20 at 10 mM with 1% (w/v) SDS (Figure 1A). Furthermore, Tris-HCl has a robust application as an electrophoresis buffer for polyacrylamide and agarose gels. The other two lysis buffers were UTS (Figure 1B) and the 2DE one. Tris-SDS yield a poor resolution and the number of spots were considerably lowered (Figure 1B) in comparison with the profile observed with UTS, where a high spot separation, no distorsion and a far better recovering of proteins were observed (Figure 1B). This point is supported by the fact that UTS extraction yields almost no residual pellet, whereas Tris-HCl owes a thick one after bench centrifugation. The pattern showed in Figure 1B clearly gives evidence of the higher protein solubility in UTS regarding other buffers such as Tris-HCl. Notheworthy that even 2DE buffer was also tested, its use was ruled out since despite pellet was neither observed, lipids were not dissolved and they remained in the upper layer difficulting the manipulation. In agreement with these data, it is recommended the use of UTS buffer for several reasons 1) its neutral chaotrope that denatures proteins by disrupting nonconvalent and ionic bonds between the amino acid residues and avoid the activity of proteases which degrade cellular proteins21 2) addition of thiourea to the urea solution enhances drastically the solubility of hydrophobic membrane proteins and proteins prone to aggregate22,23 and 3) the adjunction of the anionic detergent SDS breaks lipids bind proteins via hydrophobic interactions, as well as increases protease and phosphatase activity inhibition24,25. Furthermore it was decided to add a precipitation step to eliminate impureties that may disrupt the IEF. In this way, as precipitation method determines the solubility of the proteins26 and methanol has already been described as the most suitable solvent to remove lipids which are abundant in brain tissue, it brought us to choose the chloroform/methanol precipitation27. The utilization of the zwitterionic detergent CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-Propane sulfonate) for resuspending the proteins pellet in the 2D buffer is due to its suitability for facilitating the protein entrance in the first dimension step28.
The profile of the human and mice brain proteome by 2D-DIGE is shown in Figures 2A and 2B respectively. In the Figure 2A merged fluorescent images coming from human control and AD cortex samples can be observed, in Figure 2B idem for mice samples from hippocampus of a model of AD-like Tau pathology29. Cy2, Cy3 and Cy5 are respectively illustrated for the human samples in Figures 2C-2E. Both merged patterns (Figure 2A and 2B) present a high spot resolution reflecting a high-quality IEF and second dimension separation in agreement with the profile observed in Figure 1B. This spot definition quality together with the fact that cross-linking is performed in order to eliminate a possible preference of the proteins to a particular cyanine, make these fluorescence images (Figures 2C-2E) very easy to align throughout the software analysis. Moreover, the preparative gels stained with blue Coomassie evidence an enriched protein map with abundant spots all over the pH range utilized, allowing one to excise the spots after software analysis and for their posterior identification by MS/MS.
The qualitative mini 2DE analysis by immunoblotting for a protein provides high value information for its identification and modifications, not only for post-translational ones but also for oligomers and truncations (Figures 3A and 3B). The feasibility to perform this technique in mini 2DE provides fast and precise data about the protein of interest. Regarding tissue from a patient affected by Lewy Body Dementia (LBD), the characterization of alpha-synuclein enables to visualize a well defined 4-7 pH profile where the native protein is at a pI of 4.67 and a MW of 18 kDa, nonetheless with mini-2DE it can be further seen the presence of dimers (Figure 3A, asterisk) at the same pI than the native forms but also the existence of ubiquitinated forms that are more basic and with a higher MW when more ubiquitinated they are (Figure 3A, arrows). Another protein intimately linked to AD is the amyloid-beta peptide, generated by the complex catabolism of the amyloid precursor protein (APP). In the Figure 3B it is compared the plot of a Sporadic AD case with a familial one. In the case of the familiar AD it can be observed how amyloid-beta 1-42 is decreased respect to the sporadic AD, and moreover, the isovariants amyloid-beta x-42 (4 kDa) are also down-regulated all over the gap, meanwhile the oligomers found at 8 kDa reflect that this fact is not due to a lower quantity of the protein, since spots intensity is more relevant in familiar AD (Figure 3B).
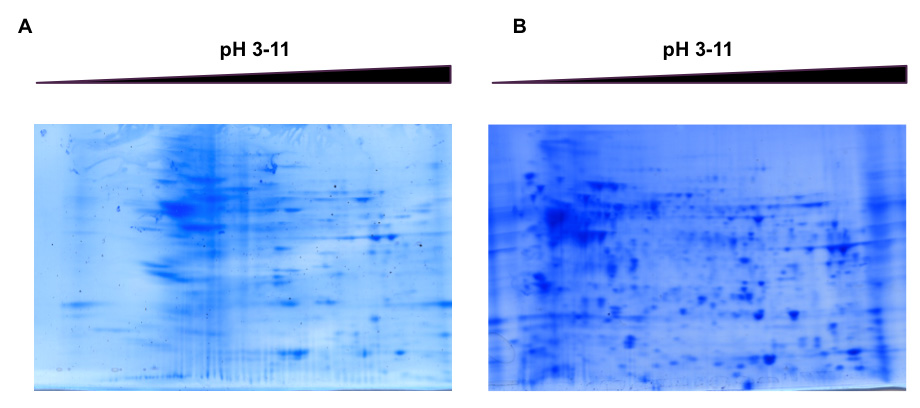
Figure 1: 2DE profile after 10 mM Tris 1% SDS w/v buffer extraction (A) and the pattern obtained after UTS utilization (B). In the panel B it can be seen how the number of spots, intensity and resolution is much higher than the one of panel (A). The use of UTS allows an almost total protein extraction that it is reflected in the 2DE Coomassie staining. Click here to view larger image.
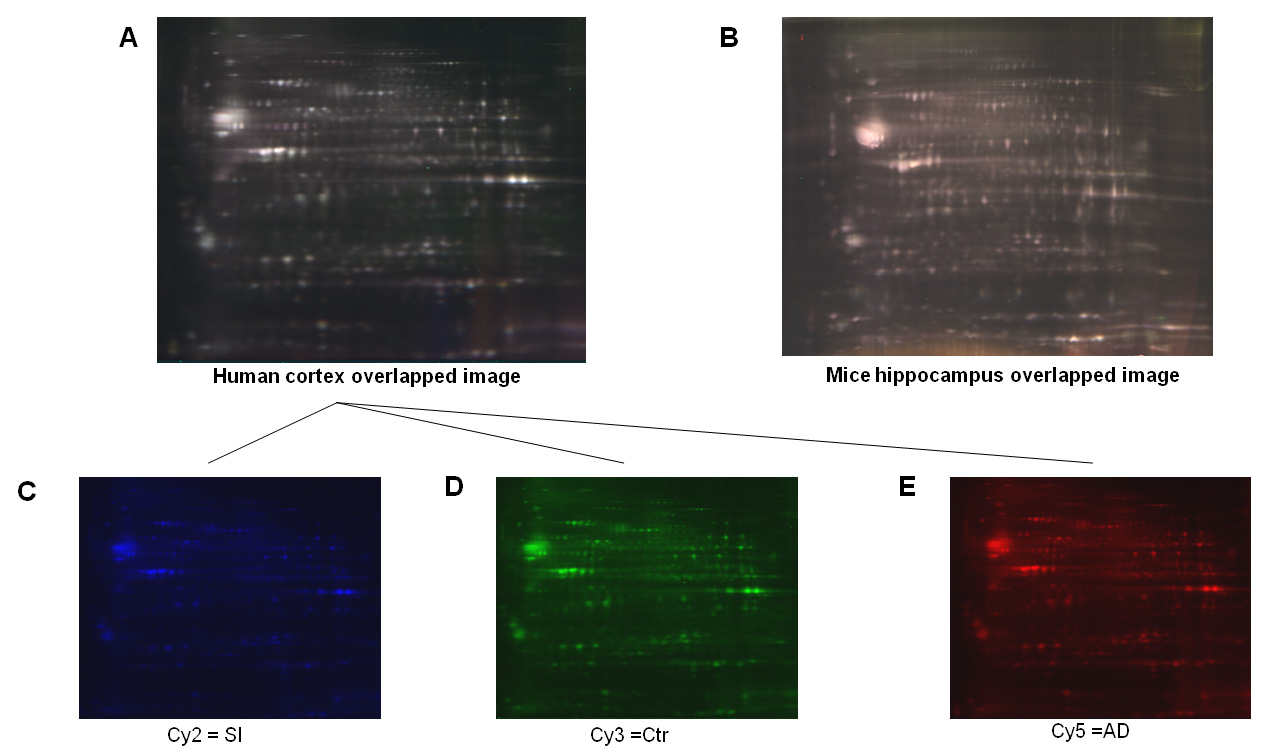
Figure 2: 2D-DIGE profiles for human (A) and mice (B) are illustrated. Images (A) and (B) represent the merged images for the three cyanines in a 3-11 pH range of 18 cm strip. In the pictures (C), (D) and (E) cyanines are exposed independently (Cy2 in blue, Cy3 in green and Cy5 in red). Click here to view larger image.
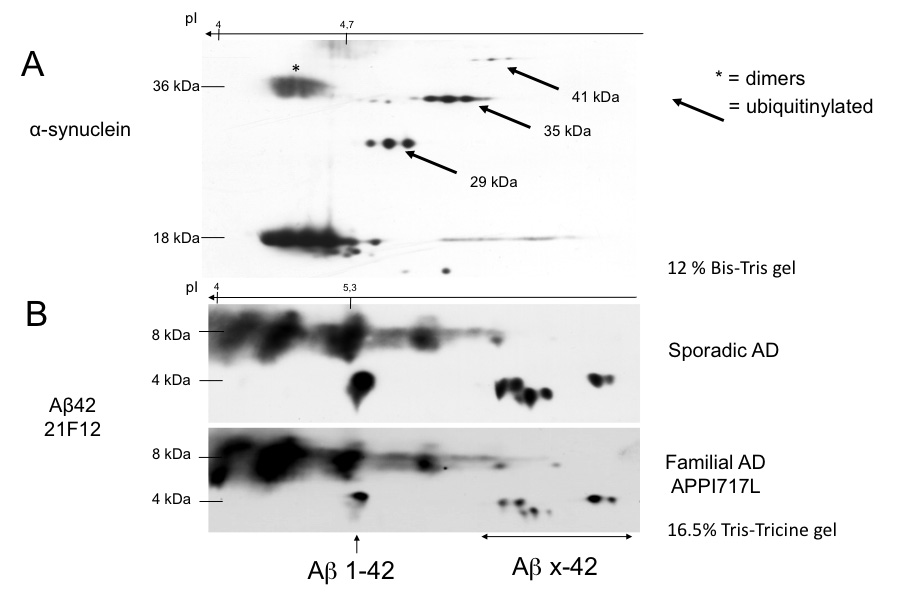
Figure 3: In the upper panel it can be observed the mini-2DE profile of the protein alpha synuclein in an LBD patient. The native pI (4.4) and MW (18 kDa) change in function of the dimerization (asterisk) where the MW is the double, and ubiquitination that shifts the pI until a more basic one (arrows) (A). The down picture reveals the profile of an amyloid-beta peptide antibody in a sporadic and genetic case of AD. 2DE pattern show how oligomerization and degradation take place in a different manner according to the etiology of the disease. This immunoblot clearly points out the differences in the APP catabolism between the two conditions. On one hand there is an increase in the catabolic products in the in the sporadic AD regarding the familial one (arrows), while the oligomers seem to be less marked (B). These experiments have been performed in 11 cm strips in a 4-7 pH gap in a 12% Bis-Tris gel and in a 16.5% Tris-Tricine one respectively (A, B). Click here to view larger image.
Discussion
Discovering pathological expression changes of proteins, search for biomarkers and the modulation of potential pathways for pharmacological targets are among the aims of the neuroproteomics approaches30. Amid the emerging tools, the 2DE field adds a promising expectative. Nevertheless, a consensus should be reached in order to minimize variability and increase reproducibility in the experiments. In line of this idea a standardized protocol to perform 2D-DIGE (Figure 2) and the subsequent 2DE immunoblotting (Figure 3) for human and mice brain tissue fully compatible with mono-dimensional immunoblotting is herein described in detailed.
One of the biggest dilemmas in proteomics is the solubilization buffer choice. In order to increase reproducibility among the different approaches, UTS was chosen since it is completely compatible with the IEF. Furthermore, UTS extraction buffer yields no pellet or a really slight one after centrifugation at 12,000 x g (do not confuse with the SDS precipitation at 4 °C). This is a crucial point taking into account that many neurodegenerative disorders present proteins aggregation, including AD. The fact that there is no pellet simplifies the performance and data interpretation, since no independent studies should be done for soluble and non-soluble fraction.
Whatever 2D-DIGE or mini-2D approaches are going to be performed, there are some limitations that should be indicated. Firstly the chemical properties of the UTS buffer, this solution must not be overheated, or carbamylation occurs on primary amines and can therefore interfere with both cyanine dye coupling and 2D profile, worsening spots resolution by impairing IEF15. Secondly, the available pH ranges, nowadays the widest pH interval is among 3 to 11, this factor joined to the probability that not all proteins entry onto the IPG strip may explain the stacked proteins bands in the both sides of the gels after staining. This limitation might expound why not the whole proteome can be studied by 2D. Another important limitation is the controversy use of the precipitation. On one hand, it is highly recommendable to do the precipitation step with chloroform/methanol that enables to efficiently reduce the lipid content27, salts, nucleic acids and charged detergents that might interfere with the IEF parameters and impact the resolution and/or reproducibility of 2D. Moreover, on the other hand it cannot be ruled out that some proteins strongly attached to the membrane may be lost. Nonetheless, in the case that other extraction buffers are used instead of UTS, either with or without the addition of proteases or phosphatases inhibitors, the use of precipitation step is highly recommended to any pitfalls during IEF. Lastly, it is noteworthy that 2D-DIGE images analysis presents the inconvenience that overlapped fluorescence spots may lead to a loss of information, since to software analysis does not consider this like a significant variation.
The novelty of this combined method lies in their reproducibility, versatility and proteins characterization. Only one extraction buffer allows one to perform two complementary techniques. 2D-DIGE provides quantitative information about proteins expression changes and their subsequent identification by MS/MS. However, it is possible that isoforms, isovariants, zymogens, post-translational modifications and catabolic products could not be identified since the overlapped fluorescence with other proteins. To overcome this limitation it is proposed in parallel the use of mini-2DE. In fact, to our knowledge one of the most important pitfall that can be committed is to take the mono-dimensional immunoblotting as a complete validation of the 2D-DIGE results, or even to consider that any proteomics modification is not necessary to validate. Furthermore, technical advantages of mini 2DE are 1) the feasibility of using small SDS-PAGE systems, 2) the relatively low material necessary, 3) the broad ranges of pH available, 4) the immunoblot sensibility and 5) the easy estimation of the changes. The fussiest aspects of mini 2DE technique is the quantification, despite mini-2DE cannot be considered a quantitative approach, after spots or plots alignment it is possible to establish a ratio between the acidic and basic part or vice versa, furnishing in this way a reliable estimation of the changes observed9.
Several data in the literature accurate the use mini-2DE to accomplish protein characterization, such as for overexpressed proteins with and without post-translational modification31, oligomerization and degradation pathways described for dementia with Lewy bodies32,33 and in the Figure 3A for an AD patient. Another example of the information provided by mini 2DE is the truncation of beta amyloid species performed in non-demented and AD cases as a potential target for vaccination approach34. The presence of oligomers and its differentially degradation is also shown in Figure 3B depending on a Sporadic or familiar AD patient. In addition, mini 2DE approach opens a wide and practical window of possibilities since different treatments may be performed. For instance in the field of AD, phosphatase inhibitors can be added and uncover their impact in Tau phosphorylation. Also the pharmacological drugs effect on proteolysis pathways, given that pI and MW identification of the truncated forms may provide the cleavage site according to the antibody's epitope34. Interestingly, recent data have elucidated using mini-2DE the association of lifestyle and drug treatments with cognitive impairments in mouse models, as well as the biochemical profile of a new Tau mutation35-37.
The development of a method enabling the 2D-DIGE of brain tissue obtain from human or mouse origin as well as the validation using mini-2DE behind, may shed light into uncovering new pharmacological targets and biomarkers for the study of neurological diseases. The fact that these disorders have their origin in the brain, oblige to study this organ as an ideal information source. However, human samples are limited, so the use of animal models becomes indispensable to achieve this goal. Herein the importance of using approaches in parallel for both types of samples and validate the results. It is desirable that in the near future reliable candidates will be found and tested in corporal fluids such as plasma and cerebrospinal fluid in order to establish an early diagnosis, evaluation of the disease progression and eventually the evaluation of the plausible treatments available.
Critical steps within the protocol must be taken into account for its suitable application. In the case of 2D-DIGE, samples test quality is a crucial point. This test permits to know proteins profile and validate the real amount of proteins extract existing in the 2D buffer after precipitation. Stained profiles give us information concerning the quality and homogeneity between samples. Only samples with similar patterns must be used for 2D-DIGE studies, otherwise the 2D-DIGE may lead to poor staining and poor resolution of protein profile. Differences in these samples patterns are more common in human than in mouse brain tissue, since human brain samples for research deal with the many inconvenient38,39. In addition, the identification of protein degradation due to post-mortem interval has been already reported by 2D-DIGE40,41. Verification of the pH before cyanine dye labeling, this step must impair all posterior procedure. Cyanine dye labeling and SDS-PAGE electrophoresis should be done in darkness as much as possible in order not to impact fluorescence labeling. Finally, polyacrylamide gels must be as homogeneous as possible among them, in order to eliminate inter-variability during electrophoretic migration and to facilitate patterns overlap and posterior analysis42,43.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by Inserm, University of Lille 2, MEDIALZ, LabEx (excellence laboratory, program invest for the future) and DISTALZ (Development of Innovative Strategies for a Transdisciplinary approach to Alzheimer’s disease). F-J.F-G is currently a recipient fellowship of ANR (French National Research Agency/ NeuroSplice de Tau Projet ANR- 2010-BLAN-1114 01), but this work was also under the support of a grant from the JCCM (Spain).
Materials
| CyDye DIGE FLUOR MIN KIT 5nmol 1 * 5 Nmo | GE Healtcare | 25-8010-65 | |
| Immobiline DryStrip | GE Healtcare | Reference in function of the pH interval/size | |
| IPG Buffer, pH X-X | GE Healtcare | Reference in function of the pH interval desired | |
| AMBERLITE IRN-150L MIXED BED RESIN 1 | GE Healtcare | 551797N | |
| DRYSTRIP COVER FLUID IMMOBILINE 1 * 1 l | GE Healtcare | 17-1335-01 | |
| KIT BOX IPG 1 * 1 KIT | GE Healtcare | 28-9334-92 | Plastic box to make the passive rehydration |
| MILLEX GS Filter | Millipore | SLGS033SB | |
| Criterion XT Precast Gels 13.3 x 8.7 cm (W x L) | Bio-Rad | Reference in function of the MW to separate | |
| IPGphor III Isoelectric Focusing Unit | GE Healtcare | 11-0033-64 | |
| Ettan DALTsix Large Electrophoresis System | GE Healtcare | 80-6485-08 | |
| OneTouch 2D Gel SpotPicker 1.5 mm | Gel Company | P2D1.5 |
References
- Collins, P. Y., et al. Grand challenges in global mental health. Nature. 475, 27-30 (2011).
- De Deyn, P. P., Van Dam, D., Sergeant, N., Buée, L. . Animal Models of Dementia Vol. 48 Neuromethods 449-468. , 449-468 (2011).
- O’Farrell, P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 250, 4007-4021 (1975).
- Riederer, B. M. Non-covalent and covalent protein labeling in two-dimensional gel electrophoresis. J Proteomics. 71, 231-244 (2008).
- Marouga, R., David, S., Hawkins, E. The development of the DIGE system: 2D fluorescence difference gel analysis technology. Anal Bioanal Chem. 382, 669-678 (2005).
- Westermeier, R., Scheibe, B. Difference gel electrophoresis based on lys/cys tagging. Methods Mol Biol. 424, 73-85 (2008).
- Gong, L., et al. Drosophila ventral furrow morphogenesis: a proteomic analysis. Development. 131, 643-656 (2004).
- Gharbi, S., et al. Evaluation of two-dimensional differential gel electrophoresis for proteomic expression analysis of a model breast cancer cell system. Mol Cell Proteomics. 1, 91-98 (2002).
- Ando, K., et al. Tau pathology modulates Pin1 post-translational modifications and may be relevant as biomarker. Neurobiol Aging. 34, 757-769 (2013).
- Bretteville, A., et al. Two-dimensional electrophoresis of tau mutants reveals specific phosphorylation pattern likely linked to early tau conformational changes. PLoS One. 4, 4843 (2009).
- Sergeant, N., et al. Two-dimensional characterization of paired helical filament-tau from Alzheimer’s disease: demonstration of an additional 74-kDa component and age-related biochemical modifications. J Neurochem. 69, 834-844 (1997).
- Sergeant, N., et al. Different distribution of phosphorylated tau protein isoforms in Alzheimer’s and Pick’s diseases. FEBS Lett. 412, 578-582 (1997).
- Rabilloud, T., Luche, S., Santoni, V., Chevallet, M. Detergents and chaotropes for protein solubilization before two-dimensional electrophoresis. Methods Mol Biol. 355, 111-119 (2007).
- Wrobel, K., Caruso, J. A. Pretreatment procedures for characterization of arsenic and selenium species in complex samples utilizing coupled techniques with mass spectrometric detection. Anal Bioanal Chem. 381, 317-331 (2005).
- McCarthy, J., et al. Carbamylation of proteins in 2-D electrophoresis–myth or reality. J Proteome Res. 2, 239-242 (2003).
- Chafey, P., et al. Proteomic analysis of beta-catenin activation in mouse liver by DIGE analysis identifies glucose metabolism as a new target of the Wnt pathway. Proteomics. 9, 3889-3900 (2009).
- Kahn, J. E., et al. Comparative proteomic analysis of blood eosinophils reveals redox signaling modifications in patients with FIP1L1-PDGFRA-associated chronic eosinophilic leukemia. J Proteome Res. 10, 1468-1480 (2011).
- Pottiez, G., et al. A large-scale electrophoresis- and chromatography-based determination of gene expression profiles in bovine brain capillary endothelial cells after the re-induction of blood-brain barrier properties. Proteome Sci. 8, 57 (2010).
- Stochaj, W. R., Berkelman, T., Laird, N. Preparative 2D Gel Electrophoresis with Immobilized pH Gradients: IPG Strip Equilibration. CSH Protoc. , (2006).
- Azimzadeh, O., et al. Formalin-fixed paraffin-embedded (FFPE) proteome analysis using gel-free and gel-based proteomics. J Proteome Res. 9, 4710-4720 (2010).
- Singh, S., et al. Identification of the p16-Arc subunit of the Arp 2/3 complex as a substrate of MAPK-activated protein kinase 2 by proteomic analysis. J Biol Chem. 278, 36410-36417 (2003).
- Rabilloud, T. Use of thiourea to increase the solubility of membrane proteins in two-dimensional electrophoresis. Electrophoresis. 19, 758-760 (1998).
- Molloy, M. P., et al. Extraction of membrane proteins by differential solubilization for separation using two-dimensional gel electrophoresis. Electrophoresis. 19, 837-844 (1998).
- Ericsson, C., Peredo, I., Nister, M. Optimized protein extraction from cryopreserved brain tissue samples. Acta Oncol. 46, 10-20 (2007).
- Shaw, M. M., Riederer, B. M. Sample preparation for two-dimensional gel electrophoresis. Proteomics. 3, 1408-1417 (2003).
- Ye, X., et al. Optimization of protein solubilization for the analysis of the CD14 human monocyte membrane proteome using LC-MS/MS. J Proteomics. 73, 112-122 (2009).
- Centlow, M., Hansson, S. R., Welinder, C. Differential proteome analysis of the preeclamptic placenta using optimized protein extraction. J Biomed Biotechnol. 2010, 458-748 (2010).
- Perdew, G. H., Schaup, H. W., Selivonchick, D. P. The use of a zwitterionic detergent in two-dimensional gel electrophoresis of trout liver microsomes. Anal Biochem. 135, 453-455 (1983).
- Schindowski, K., et al. Alzheimer’s disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am J Pathol. 169, 599-616 (2006).
- Veenstra, T. D., Marcus, K. Multidimensional advancement of neuroproteomics. Expert Rev Proteomics. 5, 149-151 (2008).
- Hernandez-Hernandez, O., et al. Myotonic dystrophy CTG expansion affects synaptic vesicle proteins, neurotransmission and mouse. 136, 957-970 (2013).
- Deramecourt, V., et al. Biochemical staging of synucleinopathy and amyloid deposition in dementia with Lewy bodies. J Neuropathol Exp Neurol. 65, 278-288 (2006).
- Tofaris, G. K., Razzaq, A., Ghetti, B., Lilley, K. S., Spillantini, M. G. Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem. 278, 44405-44411 (2003).
- Sergeant, N., et al. Truncated beta-amyloid peptide species in pre-clinical Alzheimer’s disease as new targets for the vaccination approach. J Neurochem. 85, 1581-1591 (2003).
- Le Freche, H., et al. Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology. 116, 779-787 (2012).
- Leboucher, A., et al. Detrimental effects of diet-induced obesity on tau pathology are independent of insulin resistance in tau transgenic mice. Diabetes. 62, 1681-1688 (2013).
- Deramecourt, V., et al. Clinical, neuropathological, and biochemical characterization of the novel tau mutation P332S. J Alzheimers Dis. 31, 741-749 (2012).
- Birdsill, A. C., Walker, D. G., Lue, L., Sue, L. I., Beach, T. G. Postmortem interval effect on RNA and gene expression in human brain tissue. Cell Tissue Bank. 12, 311-318 (2011).
- Bell, J. E., et al. Management of a twenty-first century brain bank: experience in the BrainNet Europe consortium. Acta Neuropathol. 115, 497-507 (2008).
- Crecelius, A., et al. Assessing quantitative post-mortem changes in the gray matter of the human frontal cortex proteome by 2-D DIGE. Proteomics. 8, 1276-1291 (2008).
- Swatton, J. E., Prabakaran, S., Karp, N. A., Lilley, K. S., Bahn, S. Protein profiling of human postmortem brain using 2-dimensional fluorescence difference gel electrophoresis (2-D DIGE). Mol Psychiatry. 9, 128-143 (2004).
- Viswanathan, S., Unlu, M., Minden, J. S. Two-dimensional difference gel electrophoresis. Nat Protoc. 1, 1351-1358 (2006).
- Tannu, N. S., Hemby, S. E. Two-dimensional fluorescence difference gel electrophoresis for comparative proteomics profiling. Nat Protoc. 1, 1732-1742 (2006).

