Quantitative and Qualitative Examination of Particle-particle Interactions Using Colloidal Probe Nanoscopy
Summary
Colloidal probe nanoscopy can be used within a variety of fields to gain insight into the physical stability and coagulation kinetics of colloidal systems and aid in drug discovery and formulation sciences using biological systems. The method described within provides a quantitative and qualitative means to study such systems.
Abstract
Colloidal Probe Nanoscopy (CPN), the study of the nano-scale interactive forces between a specifically prepared colloidal probe and any chosen substrate using the Atomic Force Microscope (AFM), can provide key insights into physical interactions present within colloidal systems. Colloidal systems are widely existent in several applications including, pharmaceuticals, foods, paints, paper, soil and minerals, detergents, printing and much more.1-3 Furthermore, colloids can exist in many states such as emulsions, foams and suspensions. Using colloidal probe nanoscopy one can obtain key information on the adhesive properties, binding energies and even gain insight into the physical stability and coagulation kinetics of the colloids present within. Additionally, colloidal probe nanoscopy can be used with biological cells to aid in drug discovery and formulation development. In this paper we describe a method for conducting colloidal probe nanoscopy, discuss key factors that are important to consider during the measurement, and show that both quantitative and qualitative data that can be obtained from such measurements.
Introduction
Atomic force microscopy (AFM) is a technique that enables qualitative and quantitative imaging and probing of a material surface.4-6 Traditionally, AFM is used for the evaluation of surface topography, morphology and structure of multi-phasic materials. AFM has the capability to quantitatively evaluate nano-scale interactions, such as charge, attraction, repulsion and adhesion forces between a specific probe and substrate in both air and liquid mediums.7,8 The AFM originally developed by Binning, Quate and Gerber9 uses a probe of known/determined sensitivity and spring constant to approach and/or scan a sample. Due to the physical interactions between the probe and the sample, the cantilever is deflected during contact or proximity and depending on the mode of operation, this deflection can be translated to acquire the topography of the sample or measure forces present between the probe and sample. Modifications to the AFM technique, such as colloidal probe nanoscopy,10 have allowed scientist to directly evaluate the nano-force interactions between two materials present in a colloidal system of interest.
In colloidal probe nanoscopy, a spherical particle of choice is attached to the apex of a cantilever, replacing the traditional conical and pyramidal tips. A spherical particle is ideal to allow comparison with theoretical models such as the Johnson, Kendal, Roberts (JKR)11 and Derjaguin, Landau, Vervwey, Overbeek (DLVO)12-14 theories and to minimize the influence of surface roughness on the measurement.15 These theories are used to define the contact mechanics and inter-particle forces expected within a colloidal system. The DLVO theory combines the attractive van der Waal forces and repulsive electrostatic forces (due to electrical double layers) to quantitatively explain the aggregation behavior of aqueous colloidal systems, while the JKR theory incorporates the effect of contact pressure and adhesion to model elastic contact between two components. Once an appropriate probe is produced, it is used to approach any other material/particle to evaluate the forces between the two components. Using a standard manufactured tip one will be able to measure interactive forces between that tip and a material of choice, but the benefit of using a custom made colloidal probe allows the measurement of forces present between materials present within the studied system. Measurable interactions include: adhesive, attractive, repulsive, charge, and even electrostatic forces present between the particles.16 Additionally, the colloidal probe technique can be used to explore tangential forces present between particles and material elasticity.17,18
The ability to conduct measurements in various media is one of the major advantages of colloidal probe nanoscopy. Ambient conditions, liquid media, or humidity-controlled conditions can all be used to mimic environmental conditions of the system studied. The ability to conduct measurements in a liquid environment enables the study of colloidal systems in an environment that it naturally occurs; thus, being able to quantitatively acquire data that is directly translatable to the system in its natural state. For example, particle interactions present within metered dose inhalers (MDI) can be studied using a model liquid propellant with similar properties to the propellant used in MDIs. The same interactions measured in air would not be representative of the system existent in the inhaler. Furthermore, the liquid medium can be modified to evaluate the effect of moisture ingress, a secondary surfactant, or temperature on the particle interactions in an MDI. The ability to control temperature can be used to mimic certain steps in the manufacturing of colloidal systems to evaluate how temperature either in the manufacturing of or storage of colloidal systems may have an impact on particle interactions.
Measurements that can be obtained using colloidal probes include; Topography scanning, individual force-distance curves, force-distance adhesion maps, and dwell force-distance measurements. Key parameters that are measured using the colloidal probe nanoscopy method presented in this paper include the snap-in, max load, and separation energy values. Snap-in is a measurement of the attractive forces, max load the value of the maximum adhesion force, and the separation energy conveys the energy required to withdraw the particle from contact. These values can be measured through instantaneous or dwell force measurements. Two different types of dwell measurements include deflection and indentation. The length and type of dwell measurement can be specifically chosen to mimic specific interactions that are present within a system of interest. An example is using deflection dwell – which holds the samples in contact at a desired deflection value – to evaluate the adhesive bonds that develop in aggregates formed in dispersions. The adhesive bonds formed can be measured as a function of time and can provide insight into the forces required to redisperse the aggregates after prolonged storage. The plethora of data that can be obtained using this method is a testament to the versatility of the method.
Protocol
1. Preparing the Colloidal Probe and AFM Substrate
- To prepare colloidal probes, use a method developed previously by the authors.19
- In brief, use a 45° angle holder to affix a tipless cantilever at the specific angle of 45° (Figure 1A).
- Prepare an epoxy slide by smearing a thin layer of epoxy onto a microscope slide. Use a clean spatula or a slow stream of nitrogen to ensure that the layer of epoxy added to the microscope slide is of minimal height.
- Affix the epoxy slide to a 40X optical zoom microscope lens using a custom designed holder (Figure 1B). Then use the cantilever to approach the epoxy slide and acquire a small amount of epoxy on the cantilever.
- Repeat these steps to also attach a single particle of interest at the apex of the cantilever (Figure 1C).
- Prepare the AFM substrate by affixing colloidal particles onto an AFM coverslip using a thermoplastic mounting adhesive.
- Heat a 35 mm round cover slip to 120 °C, and apply a small amount of the adhesive to the coverslip. The high temperature is necessary to melt the thermoplastic adhesive for application.
- Then cool, the coverslip to 40 °C before dusting the colloidal particles onto the glue. NOTE: At 40 °C the glue is sufficiently set that the particles will not become embedded into the glue, but the glue is sticky enough to ensure that the particles will adhere to the substrate.
- Further cool the coverslip to RT and use a gentle stream of nitrogen to blow off any excess unattached particles.
- Wash the substrate several times with the liquid medium that will be used for colloidal probe measurements to ensure that all unattached particles are removed from the substrate. NOTE: This is important to reduce the effects of free flowing particles during the measurement, which can interact with the cantilever and introduce errors in the results.
2. Mounting the Colloidal Probe, Aligning Laser, and Equilibrating System
- Mount the coverslip with the colloidal particles into the bottom half of a liquid cell, making sure that the O-ring is seated properly to prevent any leaking.
- Place a hydrophobic transparent sheet onto the microscope stage to guard against any liquid that may leak during the experiment, especially if only using the bottom half of a liquid cell for the measurement, and place the liquid cell onto the microscope stage. NOTE: For simplicity one can use only the bottom half of a liquid cell, given that the system can be equilibrated adequately; tip – the evaporation changes the condition of the measurement and impacts the results/reading.
- Attach the colloidal probe to the AFM scanning head and assemble onto the AFM. With the AFM instrument software on, use the knobs on the scanning head to bring the cantilever tip into focus. NOTE: All procedural steps and measurements were completed using an MFP-3D-Bio AFM with Asylum Research software.
- To maximize intensity, align the laser onto the tip of the cantilever using the appropriate adjustment knobs on the scanning head.
- Allow the system to equilibrate for 5-10 min or until the deflection value stabilizes. Use the deflection adjustment knob to bring the deflection to zero or slightly negative.
- After the system has equilibrated in air, use the AFM software (Thermal Panel in the Master Panel window) to thermally calculate the InvOLS (sensitivity) and spring constant of the colloidal probe. NOTE: This sensitivity will be temporarily used until the true sensitivity is measured at the completion of the measurement (see step 4).
- Select either “Cal Spring Constant” or “Cal InvOLS” and then click on “Capture Thermal Data”.
- Once a prominent peak is apparent, stop capturing data, and click to zoom over the main peak.
- Click on “Initialize Fit” followed by “Fit Thermal Data,” to obtain the automatically calculated spring constant or InvOLS values.
- Slowly add 2 ml of the liquid medium to the liquid cell using a syringe and ensure that no bubbles are present around the cantilever. Re-align the laser, since the refractive index of the medium has now changed, and once again equilibrate the system allowing the deflection value to stabilize before adjusting the deflection back to zero. NOTE: If a large temperature difference exists between the environment and liquid, equilibration will take longer.
3. Imaging and Data Acquisition
- Set the initial scan size to 20 µm, scan rate to 1 Hz, scan angle to 90°, set point to 0.2 V and obtain a scan of the sample. Adjust the gain as needed to obtain overlapped trace and retrace curves.
- Once a particle of interest is found, immediately zoom onto that particle to limit extended probe interactions with the substrate prior to obtaining force volume measurements.
- Once zoomed in, acquire a sufficient image of a single particle or portion of a single particle. Then switch to the Force Panel in the software. Bring the red position bar to the highest position, set the force distance to 5 µm, scan rate to 0.1 Hz, trigger channel to none and conduct a single force measurement. Make sure the probe does not contact the substrate.
- From the single measurement graph obtained, calculate the virtual deflection line by right clicking on the graph window, and selecting the “Calculate Virtual Def Line” option. This will automatically calculate the virtual deflection and update the value as needed within the software.
- Change the trigger channel to deflection and set the trigger point to 20 nm. Set the force distance to 1 µm and adjust the scan velocity as desired depending on the measured forces of interest.
- Manually adjust the value for the deflection Inverse Optical Lever Sensitivity (InvOLS) in the Review Force Panel after conducting 2-3 consecutive preliminary single force measurements.
- Conduct a single force measurement, then click on the “Review” button on the Force Panel which opens up a Master Force Panel.
- Highlight the most recently completed force measurement. Under the “Axis” heading ensure that only “DeflV” is checked. Change the “X-Axis” input field to “Sep” using the dropdown menu and click on “make graph.”
- Click on the “Parm” tab on the Master Force Panel and adjust the value of “InvOLS” until the contact region of the graph is completely vertical. Then populate this value in the “Defl InvOLS” field located under the Cal sub-tab in the Force tab located on the main Master Panel window.
- Repeat this 2-3 times to ensure that the InvOLS value does not change significantly.
- Now that all parameters have been set up, ensure that the liquid medium level is still sufficient and that the deflection is still stable. NOTE: At this time, single force curves or force maps can be obtained. If dwell force measurements are desired, the dwell options can be accessed in the Force Panel.
4. Post-tuning of Sensitivity for Analysis
- After the completion of measurement acquisition, measure the true sensitivity of the colloidal probe. To do this, conduct a force measurement using a relatively large deflection/force with the colloidal probe in the same liquid medium against an “infinitely” hard surface such as mica. NOTE: Sensitivity was obtained after completion of the experiments because the large deflection/force may damage colloidal probes prepared with porous or fragile colloids.
- The slope of the contact region is used by the software to automatically calculate the sensitivity (Figure 2). Use this true value of sensitivity during data analysis of all the curves obtained using that particular colloidal probe.
Representative Results
Liquid colloidal systems are used for several pharmaceutical drug delivery systems. For inhalation drug delivery, a common colloidal system is the suspension pressurized metered dose inhaler (pMDI). Particle interactions present within the pMDI play a vital role in formulation physical stability, storage, and drug delivery uniformity. In this manuscript, inter-particle forces between porous lipid-based particles (~2 µm optical mean particle diameter) in a model propellant (2H,3H-perfluoropentane) were evaluated at RT to convey the functionality of and possible errors associated with the presented procedure.
Figure 3 shows two representative colloidal probes prepared using the lipid-based inhalable particles that can be used for colloidal probe nanoscopy. It is important that a single colloidal particle is affixed to the apex of the cantilever such that it is the most prominent feature and will be the first point of contact during the measurement. This ensures that the interactions measured are solely due to the colloid particle. Attaching multiple particles or particle agglomerates can produce erroneous results (Figure 4) due to multiple cantilever deflections caused by both particles simultaneously sensing the same single particle present on the substrate. Using properly prepared colloidal probes, topographical images of a particle substrate such as those shown in Figure 5 can be achieved in a liquid medium.
Topography scans using a colloidal probe will be less defined than those obtained using a sharpened conical tip; however, in CPN, the main purpose of a topographical scan is to locate a particle on the substrate that can be used to evaluate the inter-particle interactions. Figure 6 conveys several force curves one may encounter when conducting colloidal probe measurements in a liquid medium. Liquid measurements contain more sources of error during measurement and one should be aware of all sources to appropriately minimize their effect on the accuracy of the measurement (Figure 6A).
The rapid and sharp peaks evident in the force curve in Figure 6B are indicative of a sudden disturbance to the system during the measurement. This can be attributed to AFM instrument movement or a sudden noise in the background (ex. door slamming, sneezing) that results in a short period of instantaneous and rapid destabilization. In Figure 6C the fluctuation of the baseline in the approach and retraction of the cantilever suggest a problem with the liquid medium. This may occur if the liquid cell is not adequately filled allowing the evaporation of the medium to have a large impact on the stability of the system and measurement. An alternative source of this instability can be from improper equilibration of the cantilever in the liquid medium prior to analysis. The cantilever is sensitive to changes in temperature and actions such as ‘topping off’ the liquid cell require adequate re-equilibration time. Figure 6D depicts a baseline shift during the approach and retract cycle. This clean shift is non-existent during instantaneous force measurements, but is more apparent in dwell force measurements. This drift is an effect of cantilever thermal drift, which can occur for several reasons including: slow evaporation of the liquid medium leading to a change in the temperature of the medium, using a medium that is still equilibrating to the surrounding temperature, or conducting the measurement in an environment that is not ideally controlled. Minimal steady shifts in the temperature of the liquid medium during the measurement produce such drifts. This type of measurement drift is difficult to control for high evaporating liquids, unless a closed liquid cell is used during measurement; however, most AFM analysis software can correct such drifts.
After all controllable sources of error have been mitigated and the system is appropriately equilibrated, adhesion mapping can be employed to obtain a large statistical body of data across a determined size of the sample. Force mapping can be used independently or in conjunction with topographical scanning to evaluate the effect of topography on particle adhesive forces (Figure 7). Force mapping will provide two major graphs of interest: a topographical map of the sample based on the height at which the cantilever contacts the substrate (Figure 7A) and an adhesion map conveying the max pull force of each individual force curve (Figure 7B). The graph in Figure 7B can also be used to obtain a numerical average and standard deviation of adhesion and snap-in forces, as well as separation energies across the entire sample. These raw data graphs can be viewed as three-dimensional representations of the topography or spread of adhesion measurements across the sample (Figure 7C/D) and overlaying them will produce a three-dimensional illustration of the distribution of the adhesion forces as a function of the topography (Figure 7E). This type of data provides critical understanding of the forces present between colloids and how the surface of the colloids further effect interactions.
Additionally dwell force measurements can be used to evaluate the effect of contact mechanics and length of contact on the adhesive forces. Solid lipid particles were used to convey the effect of the dwell on the measured adhesive forces (Figure 8). Figure 8 indicates that adhesive forces increase as a function of time using indentation dwell, while they plateau using deflection dwell. This trend becomes more obvious at longer dwell times (180 sec).
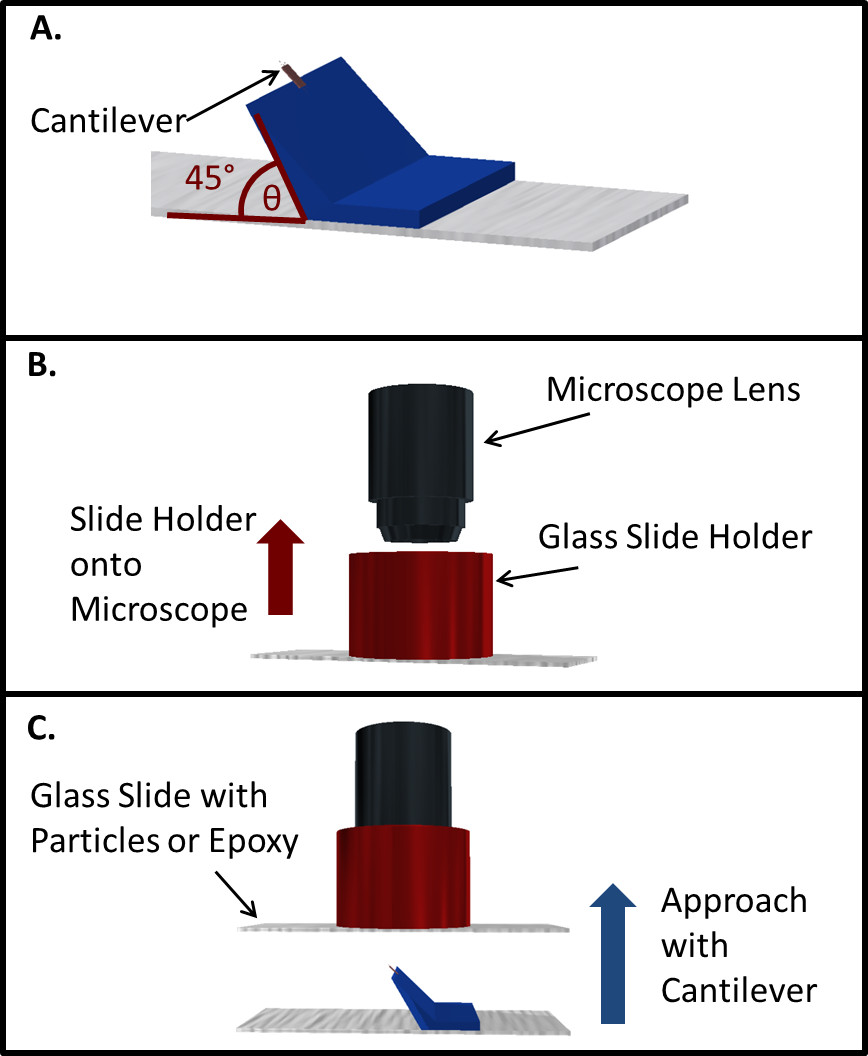
Figure 1. Depiction of the method used to produce colloidal probes for colloidal probe nanoscopy. (A) AFM cantilever, attached to a custom designed 45° cantilever holder; (B) Epoxy/Particle slide is affixed to a secondary holder that is slid onto the microscope lens; (C) The cantilever is slowly raised to acquire epoxy and a particle.
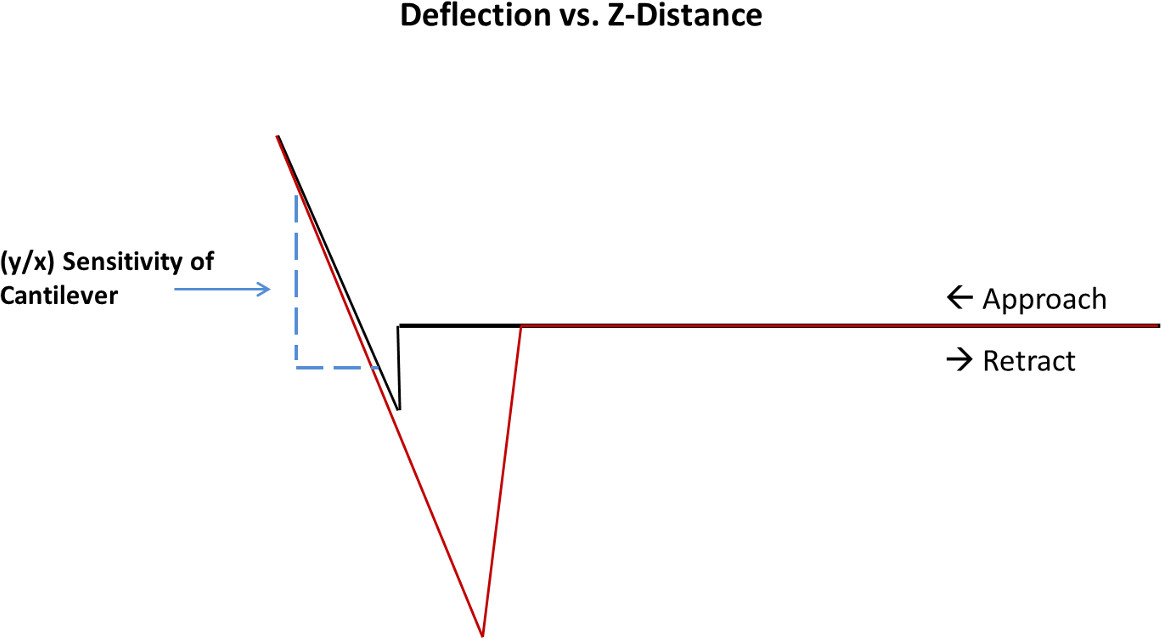
Figure 2. The sensitivity of the cantilever is the slope of the contact region of a deflection vs. z-distance curve.
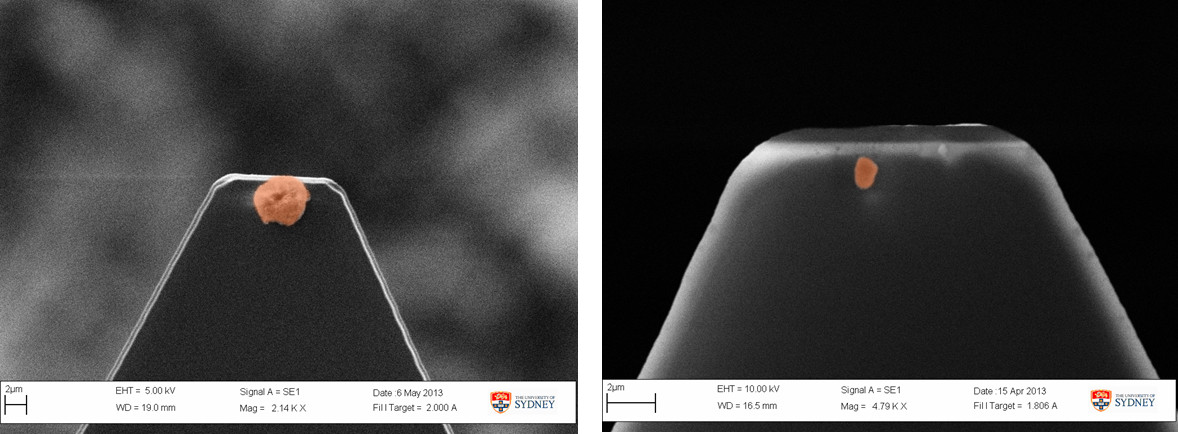
Figure 3. Properly prepared Colloidal Probes that can be used to conduct the colloidal probe measurements.
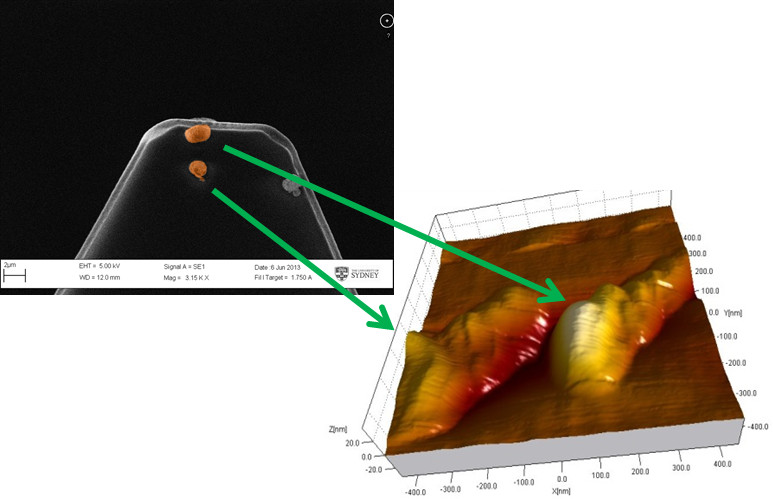
Figure 4. The use of a colloidal probe that has multiple particles affixed may result in the erroneous duplication of a single particle present on the substrate during the topographical scanning of the substrate.
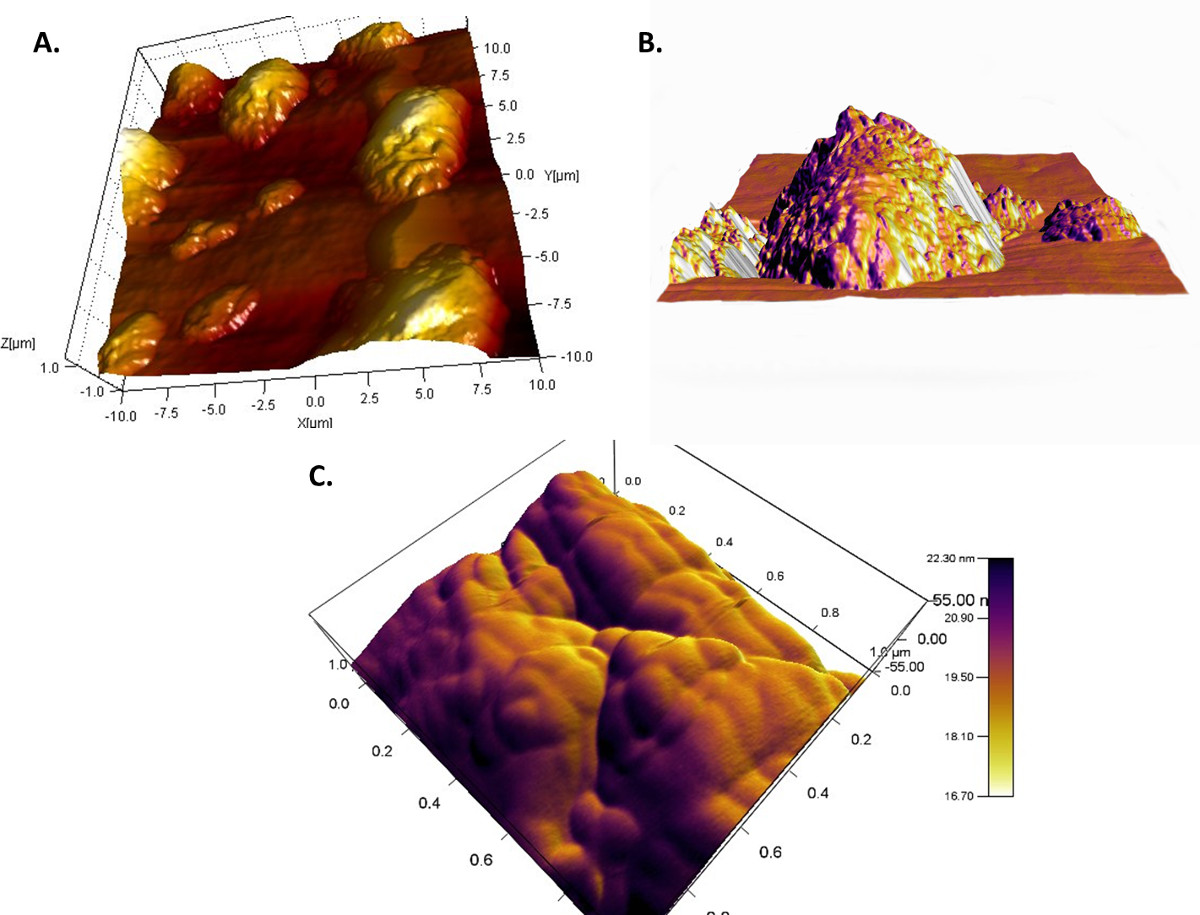
Figure 5. Topography scans obtained using a properly prepared colloidal probe. (A) A large scan revealing multiple particles of interest; (B) a more focused scan revealing one major particle of interest; (C) a scan focused on the surface of a single particle.
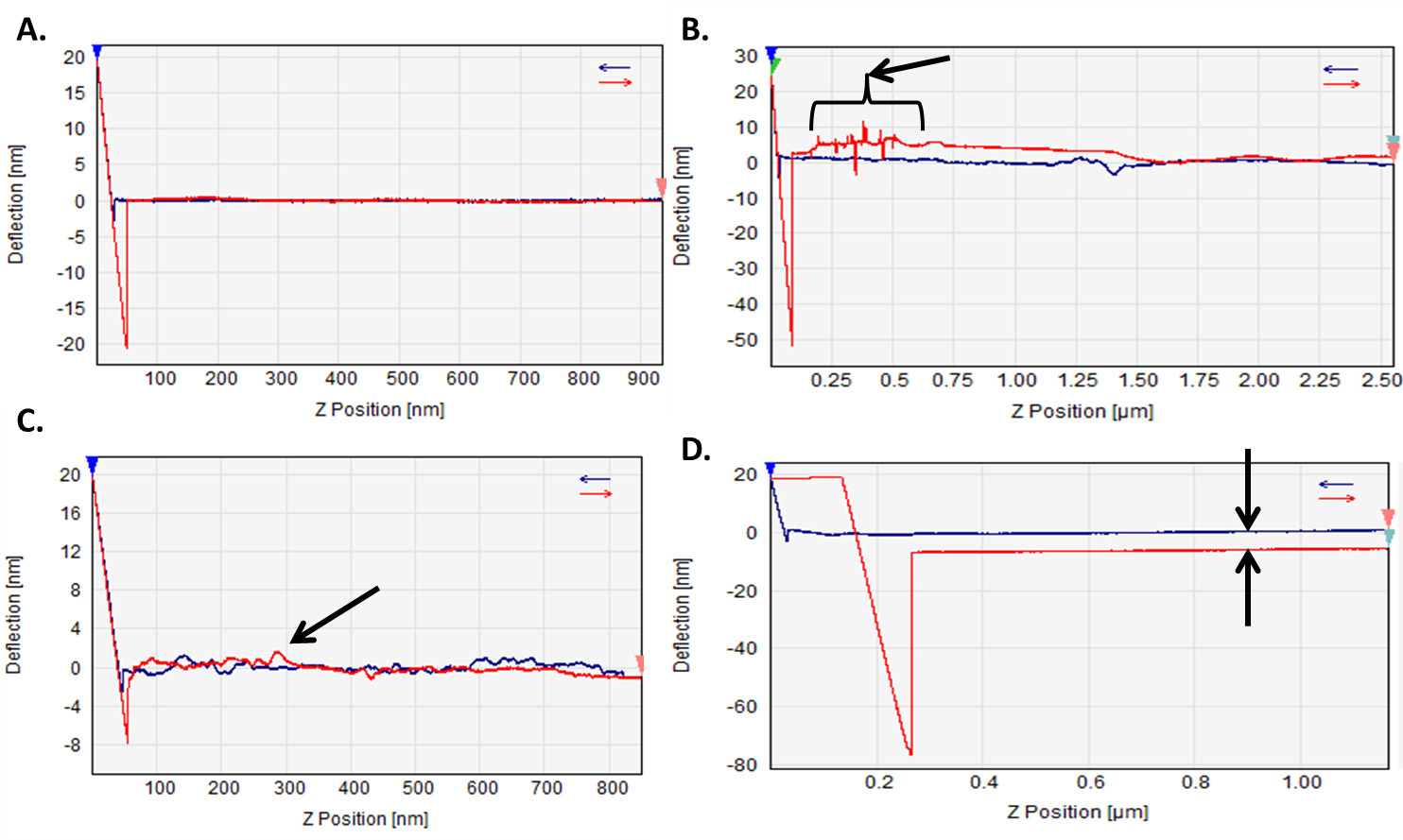
Figure 6. Force curves obtained with various limitations that one must be aware of. (A) Example of a good force curve; (B) force curve showing a disturbance either by movement of the AFM or by noise present during the measurement; (C) fluctuation due to un-equilibrated cantilever can lead to unstable approach/retract; (D) thermal drift present during a measurement, existent because of slow evaporation leading to cooling of the medium or unstable environmental control.
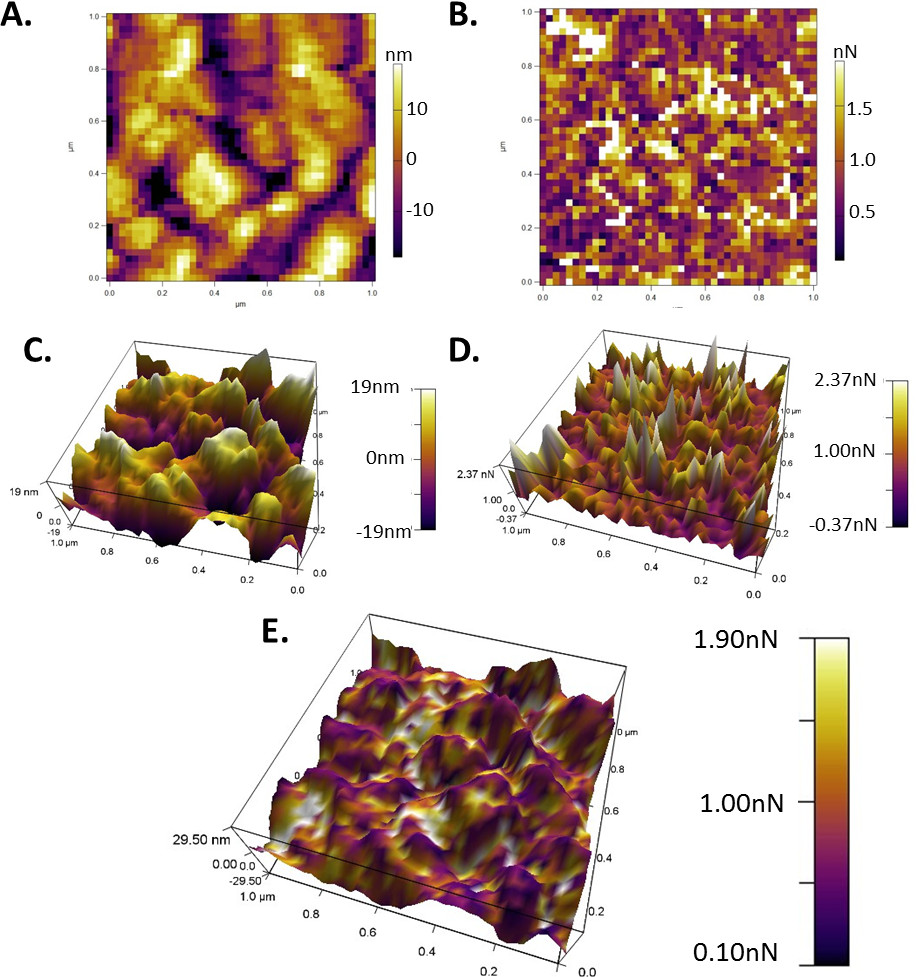
Figure 7. Adhesion force mapping that can be obtained using colloidal probe nanoscopy. (A) Topographical distribution of the sample surface; (B) distribution of max adhesion force across the sample. (C/D) 3-dimensional representations of the graphs shown in a and b respectively; (E) overlay of the topography and adhesion forces producing a single three-dimensional illustration of the adhesion forces as a function of topography.
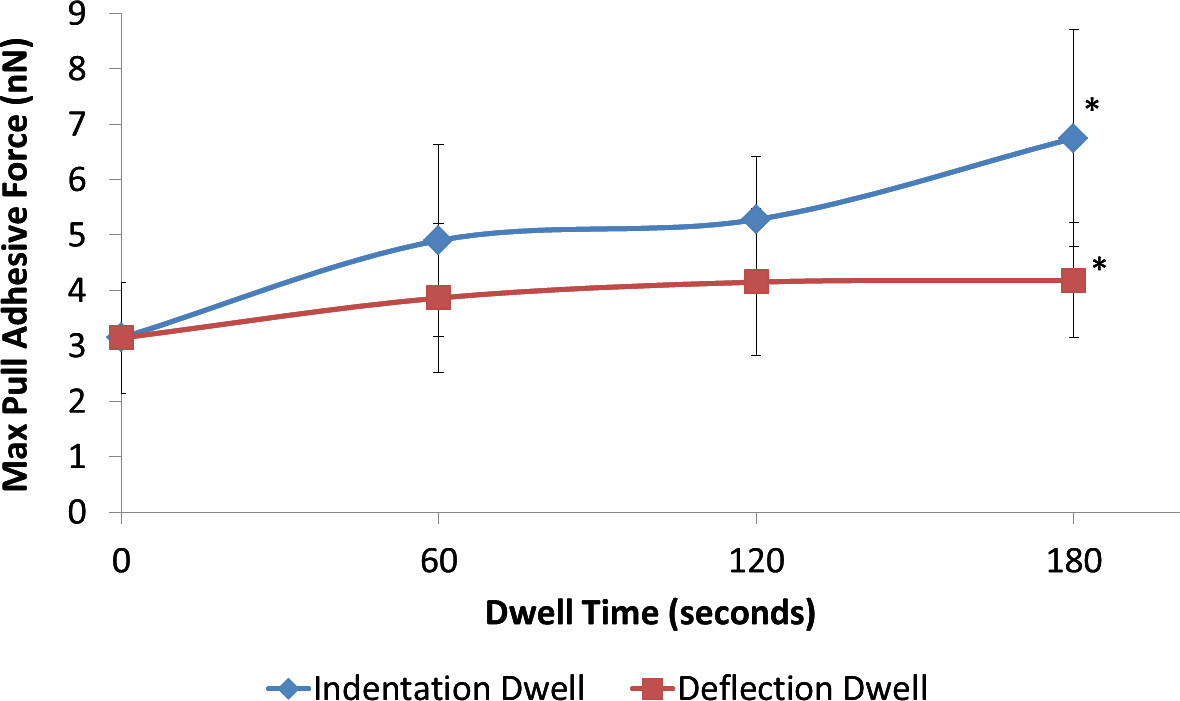
Figure 8. Adhesion forces measured as a function of dwell time using two varying dwell measurements (n=30), (◊) indentation (□) deflection; * indicates a significant difference between values at the specific time point using a two-tailed T-test with 95% confidence (P < 0.05).
Discussion
Several sources of system instability present during liquid colloidal probe nanoscopy can easily be mitigated through proper equilibration procedures. Instabilities as discussed previously result in erroneous results and force curves that are more difficult to analyze objectively. If all sources of instability have been tended and graphs similar to that shown in Figure 4 are still present, another measurement parameter may be the reason. Other measurement parameters that are important to consider during colloidal probe nanoscopy include the speed at which the cantilever is engaged and retracted from the sample and the trigger point of the force measurement. Additionally, it should be noted that the location of the colloidal probe's center may be different from a traditional AFM tip. Hence, it is advised to position the laser spot directly above the center of the probing particle to maximize measurement accuracy.
It is important to choose a speed that is sufficient to the force one is interested in measuring and one that is suitable to use in the liquid medium. If only interested in the adhesion forces present between the particles, the speed of approach is not critical. However, for attractive and repulsive force measurements between the particles, choosing an approach and retract speed that is slow enough is important. An approach speed should be chosen to allow the interactions and not the speed to dominate the deflection of the cantilever. A fast approach will overshadow and not allow time for the attractive interactions to form, while a very slow approach in a liquid medium will produce unstable baselines similar to Figure 6C. The instability caused by a slow approach is because the liquid buoyant force on the cantilever is similar to the force used in the cantilever approach.
Another measurement parameter that should be considered prior to data acquisition is the final trigger force. Too large a trigger force can result in large deformations during the measurement and may even crush the probe or sample depending on the material properties. Alternatively, too small of a force will produce inaccurate results, as the liquid layer between the probe and sample may not be sufficiently pushed out from between the particles, thus the measured interaction is not particle-particle. Method optimization is important to properly screen and test various measurement parameters to ensure that the data obtained is representable and accurate.
Force maps shown in Figure 7 can provide large easily obtained data sets. The resolution of the topographical map and subsequently overlaid three-dimensional representations are directly related to the number of measurements conducted. However, even though a larger number of data points will produce higher resolution images, scan times can be greatly increased. Keeping liquid measurement systems stable throughout force mapping can be challenging depending on the liquid medium and environmental controls. Liquid evaporation, which is one of the biggest concerns, can be limited by regularly ‘topping off’ of the system with additional liquid. However, it is imperative that the scan is paused and sufficient time is given for re-equilibration of the system prior to resuming the measurement. A suitable scan time should be chosen to ensure that the system can be kept stable to ensure the accuracy of the measurements.
The ability to conduct instantaneous force curves, dwell force curves, and large data sets of force maps conveys the versatility of colloidal probe nanoscopy in evaluating interactions present in colloidal systems in environments that mimic naturally occurring. Experimental data obtained using the method detailed here can provide key insights into colloidal stability, electrostatic interactions, and coagulation kinetics. This information can be used to screen and or improve upon present colloidal systems throughout various industries. Additionally this method can be used with biological cell lines to evaluate the effect of certain drugs or materials (prepared on a colloidal probe) on cell interactions and functions. This can provide great insight in small molecule, drug discovery and formulation design. Furthermore, with recent advances in the ability to produce submicron and even nano-colloidal probes, one can use the method presented here to study even nano-colloidal systems.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge (1) financial supports from Department of Nanobiomedical Science & BK21 PLUS NBM Global Research Center for Regenerative Medicine in Dankook University, and from the Priority Research Centers Program (No. 2009-0093829) funded by NRF, Republic of Korea, (2) the facilities, and the scientific and technical assistance, of the Australian Centre for Microscopy and Microanalysis at the University of Sydney. HKC is grateful to the Australian Research Council for the financial supports through a Discovery Project grant (DP0985367& DP120102778). WCh is grateful to the Australian Research Council for the financial supports through a linkage Project grant (LP120200489, LP110200316).
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| Double-Bubble Epoxy | Hardman | 4004 | |
| Veeco Tipless Probes | Veeco | NP-O10 | |
| Porous Particles | Pearl Therapeutics | N/A | |
| Atomic Force Microscope (MFP) | Asylum | MFP-3D | |
| SPIP Scanning Probe Image Processor Software | NanoScience Instruments | N/A | |
| 35 mm Coverslips | Asylum | 504.003 | |
| Tempfix | Ted Pella. Inc. | 16030 |
References
- Sindel, U., Zimmermann, I. Measurement of interaction forces between individual powder particles using an atomic force microscope. Powder Technology. 117, 247-254 (2001).
- Ducker, W. A., Senden, T. J., Pashley, R. M. Direct measurement of colloidal forces using an atomic force microscope. Nature. 353, 239-241 (1991).
- Israelachvili, J. N., Adams, G. E. Measurement of forces between two mica surfaces in aqueous electrolyte solutions in the range 0–100 nm. Journal of the Chemical Society, Faraday Transactions. 1, 975-1001 (1978).
- Upadhyay, D., et al. Magnetised thermo responsive lipid vehicles for targeted and controlled lung drug delivery. Pharmaceutical Research. 29, 2456-2467 (2012).
- Chrzanowski, W., et al. Biointerface: protein enhanced stem cells binding to implant surface. Journal of Materials Science: Materials in Medicine. 23, 2203-2215 (2012).
- Chrzanowski, W., et al. Nanomechanical evaluation of nickel–titanium surface properties after alkali and electrochemical treatments. Journal of The Royal Society Interface. 5, 1009-1022 (2008).
- Tran, C. T., Kondyurin, A., Chrzanowski, W., Bilek, M. M., McKenzie, D. R. Influence of pH on yeast immobilization on polystyrene surfaces modified by energetic ion bombardment. Colloids and Surfaces B: Biointerfaces. 104, 145-152 (2013).
- Page, K., et al. Study of the adhesion of Staphylococcus aureus to coated glass substrates. Journal of materials science. 46, 6355-6363 (2011).
- Binnig, G., Quate, C. F., Gerber, C. Atomic force microscope. Physical Review Letters. 56, 930-933 (1103).
- Butt, H. -. J. Measuring electrostatic, van der Waals, and hydration forces in electrolyte solutions with an atomic force microscope. Biophysical Journal. 60, 1438-1444 (1991).
- Johnson, K., Kendall, K., Roberts, A. Surface energy and the contact of elastic solids. Proceedings of the Royal Society of London. A. Mathematical and Physical Sciences. 324, 301-313 (1971).
- Deraguin, B., Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solution of electrolytes. Acta Physicochim: USSR. 14, 633-662 (1941).
- Derjaguin, B., Muller, V., Toporov, Y. P. Effect of contact deformations on the adhesion of particles. Journal of Colloid and Interface Science. 53, 314-326 (1975).
- Verwey, E. J. W., Overbeek, J. T. G. Theory of the stability of lyophobic colloids. DoverPublications.com, doi:10.1021/j150453a001. , (1999).
- Kappl, M., Butt, H. J. The colloidal probe technique and its application to adhesion force measurements. Particle & Particle Systems Characterization. 19, 129-143 (2002).
- Tran, C. T., Kondyurin, A., Chrzanowski, W., Bilek, M. M., McKenzie, D. R. Influence of pH on yeast immobilization on polystyrene surfaces modified by energetic ion bombardment. Colloids and Surfaces B: Biointerfaces. , (2012).
- Sa, D. J., de Juan Pardo, E. M., de Las Rivas Astiz, R., Sen, S., Kumar, S. High-throughput indentational elasticity measurements of hydrogel extracellular matrix substrates. Applied Physics Letters. 95, 063701-063701 (2009).
- Zauscher, S., Klingenberg, D. J. Friction between cellulose surfaces measured with colloidal probe microscopy. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 178, 213-229 (2001).
- Sa, D., Chan, H. -. K., Chrzanowski, W. Attachment of Micro- and Nano-particles on Tipless Cantilevers for Colloidal Probe Microscopy. International Journal of Colloid and Interface. , (2014).

