肱动脉的临床研究超声评价血管内皮依赖性血流介导的血管舒张功能
Summary
内皮功能障碍与许多疾病有关,并预测在人类心血管不良事件。血流介导的舒张功能(FMD)是评价血管内皮功能的超声无创方法。方法的选择和操作者的经验可能会影响结果。在人类研究中的系统方法口蹄疫是在这里讨论。
Abstract
血管内皮细胞是细胞覆盖血管的内部,并提供其结构和功能的作用的单层。内皮细胞充当一个屏障,阻止白细胞粘附和聚集,以及控制渗透性血浆成分。在功能上,影响内皮血管张力。
内皮功能障碍是一种调节血管张力,thombroresistance,细胞增殖和有丝分裂的化学物质之间的不平衡。它是在动脉粥样硬化的第一步骤,并且有冠状动脉疾病,外周动脉疾病,心脏衰竭,高血压和高脂血症相关联。
血管内皮功能障碍的第一个示范涉及直接输注乙酰胆碱和定量冠状动脉造影。乙酰胆碱结合的毒蕈碱受体在血管内皮细胞表面,从而增加细胞内钙而增加镍TRIC氧化氮(NO)的产生。在受试者一个完整的内皮细胞,血管扩张,观察,同时患有血管内皮损伤经历了矛盾的血管收缩。
存在着一种非侵入性的体内测定方法内皮功能使用高分辨率的B模式超声外周动脉。外周动脉的内皮功能密切相关,冠状动脉功能。这种技术在测量肢体缺血的时间反应性充血肱动脉直径百分比变化。
这种技术被称为内皮依赖性,血流介导的血管舒张(FMD)具有在临床研究的设置值。然而,一些生理和技术问题可能影响结果和相应的准则,该技术的准确度已经公布。尽管指引,口蹄疫仍然严重依赖于操作者,并提出一个陡峭的学习曲线。本文介绍了一种标准化的方法,上臂测量口蹄疫的肱动脉,并提供建议,以降低运营商内部的变化。
Introduction
人类血管内皮细胞提供了在体内的结构和功能的作用。在组织切片中,内皮细胞出现小的,包括薄层细胞1-2微米厚坐在上面一层平滑肌细胞(媒体)和一个厚厚的一层结缔组织(外膜)的。作为一个整体,内皮细胞提供了一个广泛区域的血液和血管的平滑肌组织之间的信息交流。据估计,700 平方米和横截面面积的质量为1000-1500克体重70公斤的人,相当于在群众肝脏1。一个健康的内皮细胞可用于机械化学信号传导,保持血管的内环境稳定。内皮功能障碍是这些介质失衡,血管疾病的第一步,本前动脉粥样硬化的组织学证据。一种非侵入性的, 在活体内的方法用于定量人体的血管舒张作用动脉存在。该方法中,内皮依赖性,血流介导的血管舒张(FMD)是广泛用于临床试验。
内皮细胞充当血管的结构组分和生产的胞外基质组分,例如葡糖胺聚糖和纤连蛋白2。在血流和急性损伤动脉长期变化可能导致结构的改变。在功能上,血管内皮细胞参与血管张力,炎性过程,抗血栓形成和抗凝血的调节。内皮细胞的影响血管收缩,通过内皮素而血管舒张是由一氧化氮(NO),前列环素和内皮超极化因子(EDHF)3-6介导的。
内皮功能障碍是任何这些介质和动脉粥样硬化的第一步减值。这并不奇怪,因为疾病的一种机制,它与一些重要的临床相关条件,例如冠状动脉疾病,高血压和糖尿病7-11。重要的是,内皮功能障碍可以在个人未经诊断的心血管疾病进行观察,并预测未来的心血管事件7,12,13的。内皮功能障碍的一项措施,与Framingham评分相结合,可以提供单独或者14以上的措施更多的预后信息。
血管内皮功能障碍的措施可能涉及直接输注药物剂。 Intercoronary输注乙酰胆碱,例如,结合的定量血管造影术证明的受试者具有完整内皮的血管舒张。然而,随着血管内皮损伤的经验似是而非的血管收缩个人。15外周动脉,输液用计,应变容积描记药理剂流量的测量是可能的16。
代理Š直接影响内皮和引发一种化学信号,被称为内皮依赖性的血管扩张剂。乙酰胆碱,例如,作用于毒蕈碱性受体在内皮细胞上,从而增加细胞内钙浓度,活化一氧化氮合酶和血管舒张。影响血管舒张无内皮细胞的参与剂被称为内皮依赖性药物。硝酸甘油,例如,激活可溶性鸟苷酸环化酶和环鸟苷-3', – 5'-monophasphate(cGMP)的其中通过蛋白激酶介导的血管扩张,在血管壁调节细胞内钙浓度的17。
有一种非侵入性的, 在体内方法用于定量由Celermajer和同事引入血管内皮功能障碍称为“血流介导的内皮依赖性血管舒张”(FMD)18。简单地说,改变动脉血流开放的剪切力敏感的离子赞NELS在内皮。该信号通过第二信使级联tranduced并激活内皮型一氧化氮合酶(eNOS),生成NO。穿过细胞膜此物种扩散到邻近的平滑肌细胞(SMC)。内的SMC,信号转导,从而降低细胞内钙离子浓度,影响血管舒张19。动脉管腔的直径增大,从而增加血流量用的哈根 – Poiseullie方程一致。口蹄疫的效果,可以取消与NO合成酶抑制剂,如单甲基精氨酸(L-NMMA)20的施用。
Celermajer 等人的创新工作已经允许使用高分辨率B型超声后面缺血的反应性充血过程中,以评估在动脉直径的变化。在该技术中,人受试者休息仰卧和肱动脉的直径,测量在纵向平面上。 A型血,pressu再压脉袋是用来产生缺血肢体。以下的血压袖带动脉的直径,再次测量的释放。在剪应力的急剧变化是刺激NO介导的血管扩张。一个简单的公式说明了相对于基线直径( 式1)的直径的改变。这个方程,充血和基线直径的参数的全面讨论,可以在协议中找到和结果部分。
<!–Equation 1: Percent FMD
%FMD = 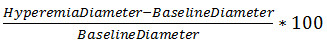
在多个研究中,百分之口蹄疫已发现预测患者的心血管事件既定的心血管疾病21-24。肱动脉FMD百分比和冠状动脉之间的相关性口蹄疫,建立了安德森等人 ,魔trating外围测量和更多的临床相关的缺血性变化之间的联系到心脏25。口蹄疫并不表明容器的最大血管舒张。为了评估此,口蹄疫可以跟随内皮依赖性,硝酸甘油介导的同一容器中的血管舒张。
有百分之影响口蹄疫的测量技术问题。自从引进该技术,一些研究显示,内学科,跨运营商的变化26度很高。它已经表明,生理因素如吸烟,抗高血压药物,一天中的时间,和空腹状态影响百分之口蹄疫。同样,技术的选择,如相对于测量和闭塞的持续时间的现场箍的位置已被证明影响测量27,28。指南已经出版,描述目前的共识,并允许技术与标准化实验室19,29。
尽管在技术不断发展的共识,血流介导的血管扩张仍严重依赖于操作者用长的学习曲线。 Corretti,例如,推荐一个经验丰富的调查员的监督下,超声医师完成100次独立运作之前。为了保持足够的专业知识水平,每年推荐的技师完成100次。对于研究人员用小样本的人口和有限的资源,学习曲线提供了一个进入门槛。本文将说明在上臂肱动脉血流介导的血管舒张的方法,并提供技术建议,以减少操作符内部的可变性。
Protocol
Representative Results
Discussion
内皮功能障碍是影响血管张力和在动脉粥样硬化的发展中的早期步骤中的化学介质的不平衡。测定动脉的反应性的方法,以评估这些化学途径的状态。评估反应的直接和间接方法的不同血管床的存在,从直接输注的内皮激动剂在冠状循环中的食指38的非侵入性,脉冲波形的分析。
肱动脉FMD是一个已建立的技术用于通过高频超声波间接地评价血管内皮功能。有优势,在?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
从血管集成生理学和实验治疗学(VIPERx)实验室,这项工作是由外科加州旧金山大学的系和北加州理工学院的研究与教育基金的支持。说明该项目是由国家研究资源中心支持奖号码KL2RR024130。内容完全是作者的责任,并不一定代表美国国家研究资源中心和美国国立卫生研究院的官方意见。
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| Philips HD 11XE ultrasound | Philips Healthcare | ||
| 5-12 MHz linear array transducer | Philips Healthcare | L12-5 | |
| Ultrasound gel | Parker Laboratories | ||
| Vascular Research Tools v.5.0 | Medical Imaging Applications, LLC | ||
| MIA Gating module | Medical Imaging Applications, LLC | ||
| Desktop PC | Dell, Inc | ||
| Windows XP | Microsoft, Inc | ||
| 5 cm tourniquet blood pressure cuff | Hokanson | SC 5 | |
| Hand-held aneroid manometer | Welch Allyn | DS66 |
References
- Gerlach, E., Nees, S., Becker, B. F. The vascular endothelium: a survey of some newly evolving biochemical and physiological features. Basic Res Cardiol. 80, 459-474 (1985).
- Sato, T., Arai, K., Ishiharajima, S., Asano, G. Role of glycosaminoglycan and fibronectin in endothelial cell growth. Experimental and molecular pathology. 47, 202-210 (1987).
- Yanagisawa, M., et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 332, 411-415 (1988).
- Ignarro, L. J., Buga, G. M., Wood, K. S., Byrns, R. E., Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proceedings of the National Academy of Sciences. 84, 9265-9269 (1987).
- Moncada, S., Higgs, E. A., Vane, J. R. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. The Lancet. 309, 18-21 (1977).
- Ozkor, M. A., et al. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation. 123, 2244-2253 (2011).
- Suwaidi, J. A., et al. Long-Term Follow-Up of Patients With Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation. 101, 948-954 (2000).
- Neunteufl, T., et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 129, 111-118 (1997).
- Taddei, S., et al. Hypertension Causes Premature Aging of Endothelial Function in Humans. Hypertension. 29, 736-743 (1997).
- Perticone, F., et al. Prognostic Significance of Endothelial Dysfunction in Hypertensive Patients. Circulation. 104, 191-196 (2001).
- Williams, S. B., Cusco, J. A., Roddy, M. -. A., Johnstone, M. T., Creager, M. A. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. Journal of the American College of Cardiology. 27, 567-574 (1996).
- Schindler, T. H., et al. Prognostic value of abnormal vasoreactivity of epicardial coronary arteries to sympathetic stimulation in patients with normal coronary angiograms. Arterioscler Thromb Vasc Biol. 23, 495-501 (2003).
- Halcox, J. P., et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 106, 653-658 (2002).
- Yeboah, J., et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study the multi-ethnic study of atherosclerosis. Circulation. 120, 502-509 (2009).
- Ludmer, P. L., et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. New England Journal of Medicine. 315, 1046-1051 (1986).
- Higashi, Y., et al. Effect of the angiotensin-converting enzyme inhibitor imidapril on reactive hyperemia in patients with essential hypertension: relationship between treatment periods and resistance artery endothelial function. Journal of the American College of Cardiology. 37, 863-870 (2001).
- Linke, A., Erbs, S., Hambrecht, R. Exercise and the coronary circulation—alterations and adaptations in coronary artery disease. Progress in cardiovascular diseases. 48, 270-284 (2006).
- Celermajer, D. S., et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. The Lancet. 340, 1111-1115 (1992).
- Thijssen, D. H. J., et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. American Journal of Physiology – Heart and Circulatory Physiology. 300, (2011).
- Doshi, S. N., et al. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clinical science. 101, 629-635 (2001).
- Brevetti, G., Silvestro, A., Schiano, V., Chiariello, M. Endothelial Dysfunction and Cardiovascular Risk Prediction in Peripheral Arterial Disease: Additive Value of Flow-Mediated Dilation to Ankle-Brachial Pressure Index. Circulation. 108, 2093-2098 (2003).
- Neunteufl, T., et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. The American Journal of Cardiology. 86, 207-210 (2000).
- Gokce, N., et al. Predictive value of noninvasivelydetermined endothelial dysfunction for long-term cardiovascular events inpatients with peripheral vascular disease. Journal of the American College of Cardiology. 41, 1769-1775 (2003).
- Gokce, N., et al. Risk Stratification for Postoperative Cardiovascular Events via Noninvasive Assessment of Endothelial Function: A Prospective Study. Circulation. 105, 1567-1572 (2002).
- Anderson, T. J., et al. Close relation of endothelial function in the human coronary and peripheral circulations. Journal of the American College of Cardiology. 26, 1235-1241 (1995).
- De Roos, N. M., Bots, M. L., Schouten, E. G., Katan, M. B. Within-subject variability of flow-mediated vasodilation of the brachial artery in healthy men and women: implications for experimental studies. Ultrasound in medicin., & biology. 29, 401-406 (2003).
- Berry, K. L., Skyrme-Jones, R. A., Meredith, I. T. Occlusion cuff position is an important determinant of the time course and magnitude of human brachial artery flow-mediated dilation. Clinical science. 99, 261-267 (2000).
- Betik, A. C., Luckham, V. B., Hughson, R. L. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. American journal of physiology. Heart and circulatory physiology. 286, 442-448 (2004).
- Corretti, M. C., et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial arteryA report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology. 39 (1001), 257-265 (2002).
- Grenon, S. M., et al. n-3 Polyunsaturated fatty acids supplementation in peripheral artery disease: the OMEGA-PAD trial. Vascular medicine. 18, 263-274 (2013).
- Moens, A. L., Goovaerts, I., Claeys, M. J., Vrints, C. J. Flow-mediated vasodilation. Chest. 127, 2254-2263 (2005).
- Gnasso, A., et al. Association between wall shear stress and flow-mediated vasodilation in healthy men. Atherosclerosis. 156, 171-176 (2001).
- Verma, S., et al. Cross-sectional evaluation of brachial artery flow-mediated vasodilation and C-reactive protein in healthy individuals. European Heart Journal. 25, 1754-1760 (2004).
- Donald, A. E., et al. Methodological Approaches to Optimize Reproducibility and Power in Clinical Studies of Flow-Mediated Dilation. Journal of the American College of Cardiology. 51, 1959-1964 (2008).
- Witte, D. R., et al. Is the Association Between Flow-Mediated Dilation and Cardiovascular Risk Limited to Low-Risk Populations. Journal of the American College of Cardiology. 45, 1987-1993 (2005).
- Benjamin, E. J., et al. Clinical Correlates and Heritability of Flow-Mediated Dilation in the Community: The Framingham Heart Study. Circulation. 109, 613-619 (2004).
- Nosova, E. V., et al. Short-term Physical Inactivity Impairs Vascular Function. Journal of Surgical Research. 10, (2014).
- Axtell, A. L., Gomari, F. A., Cooke, J. P. Assessing Endothelial Vasodilator Function with the Endo-PAT. Journal of Visualized Experiments. , (2000).
- Greenland, P., et al. ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic AdultsA Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Journal of the American College of Cardiology. 56, (2010).
- Inaba, Y., Chen, J. A., Bergmann, S. R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. The international journal of cardiovascular imaging. 26, 631-640 (2010).
- Black, M. A., Cable, N. T., Thijssen, D. H. J., Green, D. J. Importance of Measuring the Time Course of Flow-Mediated Dilatation in Humans. Hypertension. 51, 203-210 (2008).
- Chironi, G., Craiem, D., Miranda-Lacet, J., Levenson, J., Simon, A. Impact of shear stimulus, risk factor burden and early atherosclerosis on the time-course of brachial artery flow-mediated vasodilation. Journal of Hypertension. 26, 508-515 (2008).
- Bots, M. L., Westerink, J., Rabelink, T. J., Pd Koning, E. J. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. European Heart Journal. 26, 363-368 (2005).
- Hijmering, M. L., et al. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. Journal of the American College of Cardiology. 39, 683-688 (2002).

