Use of Electromagnetic Navigational Transthoracic Needle Aspiration (E-TTNA) for Sampling of Lung Nodules
Summary
We describe the novel use of electromagnetic navigational guided transthoracic needle aspiration for the pathologic assessment of human lung nodules.
Abstract
Lung nodule evaluation represents a clinical challenge especially in patients with intermediate risk for malignancy. Multiple technologies are presently available to sample nodules for pathological diagnosis. Those technologies can be divided into bronchoscopic and non-bronchoscopic interventions. Electromagnetic navigational bronchoscopy is being extensively used for the endobronchial approach to peripheral lung nodules but has been hindered by anatomic challenges resulting in a 70% diagnostic yield. Electromagnetic navigational guided transthoracic needle lung biopsy is novel non-bronchoscopic method that uses a percutaneous electromagnetic tip tracked needle to obtain core biopsy specimens. Electromagnetic navigational transthoracic needle aspiration complements bronchoscopic techniques potentially allowing the provider to maximize the diagnostic yield during one single procedure. This article describes a novel integrated diagnostic approach to pulmonary lung nodules. We propose the use of endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) for mediastinal staging; radial EBUS, navigational bronchoscopy and E-TTNA during one single procedure to maximize diagnostic yield and minimize the number of invasive procedures needed to obtain a diagnosis. This manuscript describes in detail how the navigation transthoracic procedure is performed. Additional clinical studies are needed to determine the clinical utility of this novel technology.
Introduction
A solitary pulmonary nodule (SPN) is a frequent clinical scenario that is increasing due to the number of radiographic chest studies and the implementation of lung cancer screening programs. A lung nodule by definition is less than 3 cm in diameter and one that typically lies beyond the bronchoscopically visualized segmental bronchi. A lesion larger than 3 cm is considered a lung mass. The etiologies of the majority of lung nodules are benign conditions (infections, inflammatory, vascular) and in a smaller percentage are caused by lung cancer and other malignancies. Evaluation of a SPN starts with assessment of risk factors, radiographic characteristics, size, growth, location, surgical risk, etc. Risk stratification helps to select the most appropriate individual management, which ranges from radiologic surveillance to primary surgical resection. Diagnostic modalities include bronchoscopic techniques: ( bronchoalveolar lavage (BAL), cytology brush, transbronchial biopsy (TBBx), transbronchial needle aspiration (TBNA), radial probe endobronchial ultrasound (R-EBUS), electromagnetic navigation (EMN), ultrathin bronchoscopy, virtual bronchoscopy navigation) and non-bronchoscopic: image guided transthoracic needle biopsy and surgical resection.
The bronchoscopic yield using simple conventional techniques for lesions less <3 cm ranges from 14 – 50%. 1,2 Lung cancer remains the leading cause of cancer deaths. It represents 14% of all invasive cancers diagnosed each year and 28% of all cancer deaths in the United States.3 Prognosis and treatment management are determined by the stage. The staging of non-small cell lung cancer (NSCLC) is defined by the TNM system. Determination of the N or nodal status requires strict assessment of the mediastinal and hilar lymph nodes.
Endobronchial ultrasound (EBUS) is a minimally invasive bronchoscopic technique that uses ultrasound to identify structures contiguous to the airways facilitating transbronchial needle aspiration (TBNA). For lung cancer staging a recent meta-analysis reported a median sensitivity of 89% with values ranging from 46% to 97% and a median negative predictive value PV of 91% for EBUS-TBNA. 4 Radial EBUS is another bronchoscopic tool that can be used to localize parenchymal lung lesions. It is a 1.4 mm diameter instrument with a 20-MHz ultrasound probe at the tip that generates 360 degree images with a penetration of 5 cm. Recently reported data of this technique showed a sensitivity of identifying neoplasia of 73% (95% CI, 0.70 – 0.76) for nodules of any size and 71% (95% CI, 0.66 – 0.75) for lesions smaller than 2.5 cm 5 .
Electromagnetic Navigational Bronchoscopy (ENB) is a technology that creates a magnetic field around the patient allowing determination of the spatial location of a sensor device within the magnetic field. This information is superimposed on previously obtained computed tomography (CT) images, which permit a display of the sensor location in relationship to the anatomy. A virtual bronchoscopy reconstruction of the patient's airways facilitates navigation to the target lesion. Once in the target, samples are taken directly using one of the two available technologies; trackable bronchoscopic instruments or through an extended working channel. Overall diagnostic accuracy was 73.9% (95% CI 68.0% - 79.2%) and negative predictive value 52.1% (95% CI 43.5% – 60.6%) and when using at least one of the other technologies (virtual bronchoscopy, ENB, Radial-EBUS, guide sheath, ultrathin bronchoscope) the diagnostic yield was 70.0% with a 95% CI of 67.1% to 72.9% 67 One of the commercially available systems has the added feature to complement bronchoscopic navigation with the ability to convert from a bronchoscopic approach to a transthoracic needle approach in cases where the lesion is unable to be accessed bronchoscopically. This system uses an electromagnetic navigation guided 19 G tracking needle that allows for the sampling of core lung biopsies using a 20 G automatic biopsy device.
Transthoracic needle aspiration (TTNA) is a non bronchoscopic diagnostic approach to lung nodules and masses. TTNA of a peripheral lung lesion can be performed under ultrasonography, fluoroscopy, CT scan or electromagnetic guidance. In an updated meta-analysis by Rivera et al the pooled sensitivity of TTNA for the diagnosis of peripheral bronchogenic carcinoma was 0.90 (95% CI, 0.88 to 0.91).8 In a study by Wiener RS that included 15,865 patients with a reported risk of pneumothorax after TTNA of 15% (95% CI, 14% – 16%) with 7% (95% CI, 6% – 7.2%) requiring management with a chest tube. 9 Clinical trials investigating the use of E-TTNA are currently underway.
CASE PRESENTATION
An 83 year old female former smoker with oxygen dependent chronic obstructive pulmonary disease was incidentally found with a PET fluorodeoxyglucose avid 1.6 x 1.3 cm spiculated right upper lobe (RUL) nodule (Figure 1). Although her probability for malignancy was high, the patient refused surgical interventions and opted for a diagnostic procedure before considering other therapies. The typical scenario that may prompt consideration for an evaluation for biopsy would be a case of intermediate probability for malignancy or with a high probability for malignancy with a contraindication for surgical resection.
Protocol
A protocol was approved by the local Institutional Review Board (IRB). Participants provide signed informed consent prior to completing the protocol.
1. Pre-procedure
- Ensure that the candidates for the electromagnetic navigational transthoracic needle aspiration have a peripheral pulmonary nodule. Determine the pulmonary nodule use of imagining studies.
NOTE: The most common one and needed for this protocol is by evaluating previously obtained chest CT. - On the day of the procedure attach the electronic reference point pads to the contralateral hemithorax of the target lesion to minimize pad overlap with the TTNA biopsy entrance point. Obtain a baseline inspiratory and expiratory non-contrast chest tomography with 0.5 mm intervals and 0.67 – 0.75 mm thickness.
- Transfer the digital imaging and communication in medicine (DICOM) data from the CT scanner to the planning software. If DICOM is unavailable, a compact disk (CD) copy of the CT can be directly loaded onto the system for planning. Digitally select the target lesion and reconstruct a three-dimensional virtual airway map.
- Transport the patient by stretcher from the CT scanner to the bronchoscopy suite with electronic reference points in place.
- Evaluate the patient for anesthesia, establish I.V. access and attach standard anesthetic monitoring.
class=”jove_title”2. Procedure (Bronchoscopic Phase)
- Transfer the patient to the bronchoscopy suite. Administer deep sedation anesthesia using a propofol infusion (100 – 200 mcg/kg/min). An airway is established with laryngeal mask airway (LMA) or endotracheal tracheal tube (ETT).
NOTE: Anesthesia can only be administered by a certified provider. - Perform a white light bronchoscopy (WLB) inspection in the standard fashion examining the tracheobronchial tree to the segmental level.
- Perform an EBUS-TBNA following the International Association for the Study of Lung Cancer (IASLC) lymph node map for lung cancer staging. If available, fine needle aspiration (FNA) specimens are cytological analyzed on-site. If not, the sample is place in formalin for later evaluation.
- Use the WLB bronchoscope with the EMN bronchoscopy program phase following the previously computer recognized or manually planed endobronchial pathway to the target lesion with an instrument of choice at the proceduralists discretion (Cytology brush, jaw biopsy forces, FNA Needle).
- Advance the R-EBUS through the working channel and attempt to confirm the location of the lesion.
- Once in the target, sample the lesion with the operator selected trackable instrument used to navigate. (Cytology brush, jaw biopsy forces, FNA Needle).
- If the target is unable to be verified by radial EBUS or the airway anatomy prohibits endobronchial navigation remove the bronchoscope and prepare the patient for a transthoracic approach. If the lesion cannot be diagnosed bronchoscopically prepare the patient for a transthoracic approach.
3. Procedure (Electromagnetic Transthoracic Needle Aspiration)
- Allocate all supplies needed for the TTNA. (see Material Table)
- Position the patient supine in a similar fashion used for chest CT scan imaging acquisition.
- Using a test electromagnetic navigational needle select the entrance point to the chest cavity and mark it on the skin surface. Entrance point should be superior to the surface of the nearest rib and avoid osseous structures and any vascular structure.
- Prepare the skin with the 2% chlorhexidine solution, drape the field using sterile technique and anesthetize locally (subcutaneous infiltration of about 1 to 2 cc of 1% lidocaine).
- Place the sterile electromagnetic navigational needle on top of the entrance point and select the angle to the target based on observation of the transverse and coronal views seen on the electromagnetic system screens. (Crosshair marks in at least two different planes.)
- Stabilize the needle and firmly advanced through the chest wall into the target lesion.
- Remove the guidance needle stylet from the needle and take care to avoid needle movement. At this point extreme care should be use to prevent inadvertent needle displacement. Cover the needle hub with your finger. Insert the 20 G FNA needle through the 19 G needle. Sample the lesion and provide the specimen for ROSE.
- Program the automatic needle biopsy to the desired distance or throw based on the size of the lesion, and then insert it through the 19 G needle. Stabilize both needle and biopsy gun, trigger the needle biopsy gun mechanism to perform the biopsy and remove from the inside of the electromagnetic navigational guide needle making sure it remains in appropriate position.
- Gently advance the guidance probe back into the lumen of the percutaneous needle and keep it stable.
- While the needle is continuously stabilized, the assistant uses the scalpel blade to gently remove the specimen from the inner cannula of the core biopsy needle and place it on the 1/2 x 1/2 inch previously cut non adherent pad. Immerse the pad in a formalin solution.
- Reconfirm appropriate placement of the needle within the lesion and then repeat the operation 4 to 5 times making sure the core needle is rotated clockwise or counterclockwise to sample different areas.
- Once satisfied with the specimens remove the needle, apply pressure and place a small bandage over the puncture site.
4. Post Procedure
- If available, perform bedside ultrasonography to assess for the presence of lung sliding (absence of pneumothorax) or use any other imaging modality (fluoroscopy,etc) .
- Transfer the patient to the recovery area and observe under standard ambulatory surgical unit protocols until fully awake.
- Obtain a chest radiograph to rule out complications.
- Discharge patient home after education is provided regarding the potential post procedure complications including bleeding and pneumothorax.
Representative Results
All tissue samples obtained by EBUS-TBNA and E-TTNA are processed by the central pathology laboratory for further analysis. Pathological evaluation of the samples includes cytological and histological assessment. Rapid on-site evaluation (ROSE) uses a modified Romanowsky stain that allows adequacy evaluation and a cytomorphological diagnostic assessment of the pathological specimens. A preliminary intra procedure assessment using ROSE of the EBUS-TBNA biopsies taken from the mediastinal lymph node stations 4L,4R and 7 was unrevealing of obvious neoplasm. The navigational bronchoscopic phase, supported with radial EBUS, failed to localize the right upper lobe lesion due to anatomical limitations. The procedure was rapidly converted to E-TTNA. E-TTNA was successfully performed and the obtained tissue was sufficient for further pathological and genetic analysis. The final pathology of the E-TTNA core sample biopsies was diagnostic and consistent with adenocarcinoma. No immediate complications were noted.
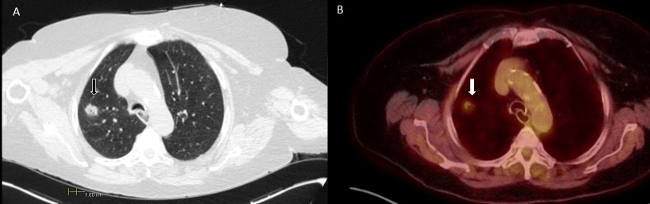
Figure 1. Chest Computed Tomography (Panel A) and Positron Emission Tomography (PET) (Panel B). Right upper lobe nodule with a PET negative mediastinum. Scale in cm.
Discussion
Electromagnetic guidance facilitates the percutaneous transthoracic biopsy technique. It is critical during the procedure to verify adequate equipment calibration. During needle insertion maintaining a steady angle and keeping visual alignment using at least two different planes is essential to effectively reach the target lesion.
Needle insertion depth can be modified between biopsies to maximize the cover area. Preferably, the procedure could be assisted by a second operator, especially at the time of biopsy gun operation, but a single operator procedure is feasible.
Familiarization with the instruments and technique takes approximately 2 to 3 needle insertions. If available training on phantom models or cadaveric models under direct supervision of an experienced operator is advisable. Electromagnetic navigational transthoracic needle aspiration (E-TTNA) is a novel technology that supplements the current armamentarium for the diagnosis of peripheral pulmonary nodules. Potential limitations of the procedure are inherent to the electromagnetic navigational technology, like CT to body divergence and related to the dynamic nature of the respiratory system. We recommend anticipation for the management of complications like pneumothorax and bleeding should be taken during any percutaneous procedure.
In this article we describe how the E-TTNA is performed by interventional pulmonologist and the incorporation of the technique to the current available bronchoscopic methods for the evaluation of lung nodules.
E-TTNA potentially can reduce the need for other image guided percutaneous transthoracic procedures providing full integration of bronchoscopic and non-bronchoscopic techniques in one single procedure potentially increasing diagnostic yield, improving safety and decreasing the overall number of procedures. Further studies are needed to determine clinical utility of this modern biopsy technique.
Disclosures
The authors have nothing to disclose.
Materials
| 20 G x 15 cm Programmable automatic biopsy needle | SuperCore Argon Medical devices | 701120150 | |
| Non Adherent Pads | Telfa | ||
| Sterile scissor | |||
| 2% chlorhexidine gluconate pad | |||
| Surgical Blade #11 | |||
| Sterile surgical drape | |||
| 1% lidocaine | |||
| Sterile gowns | |||
| Sterile gloves | |||
| Mask | |||
| Scrub hair cap | |||
| Electronic reference points | vPAD2 (Veran Medical Technologies) | INS-0049 | |
| Planning software | SPiNDrive 2.0 (Veran Medical Technologies) | ||
| Eelectromagnetic navigation platform | SPiNView (Veran Medical Technologies) | ||
| 19 G x 105 mm Electromagnetic navigational needle | SPiNPerc Veran Medical Technologies | INS-0029 | |
| Standard diagnostic Fiberoptic Bronchoscope | |||
| EBUS Bronchoscope | |||
| Radial EBUS probe UM-S20-17S | Olympus | ||
| Formaldehyde-based fixative solution. | |||
| Ethanol based fixative |
References
- Govert, J. A., Dodd, L. G., Kussin, P. S., Samuelson, W. M. A prospective comparison of fiberoptic transbronchial needle aspiration and bronchial biopsy for bronchoscopically visible lung carcinoma. Cancer. 87, 129-134 (1999).
- Gasparini, S. Bronchoscopic biopsy techniques in the diagnosis and staging of lung cancer. Monaldi Arch Chest Dis. 52, 392-398 (1997).
- . United States cancer statistics: 1999-2010 incidence and mortality web-based report. US Department of Health and Human Services Centers for Disease Control and Prevention and National Cancer Institute. , (2014).
- Silvestri, G. A., et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 143, e211S-e250S (2013).
- Steinfort, D. P., Khor, Y. H., Manser, R. L., Irving, L. B. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. The European respiratory journal. 37, 902-910 (2011).
- Gex, G., et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration; international review of thoracic diseases. 87, 165-176 (2014).
- Wang Memoli, J. S., Nietert, P. J., Silvestri, G. A. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 142, 385-393 (2012).
- Lee, K. A., Raval, A. A., Amir, L. Cost-effectiveness of endobronchial percutaneous biopsy compared with transthoracic biopsy for diagnosis of peripheral lung lesions. Lung Cancer Management. 3 (2), 135-148 (2014).
- Rivera, M. P., Mehta, A. C. American College of Chest, P. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 132, 131S-148S (2007).
- Wiener, R. S., Schwartz, L. M., Woloshin, S., Welch, H. G. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Annals of internal medicine. 155, 137-144 (2011).

