固体脂质纳米粒(前哨淋巴结)细胞内定位的应用
Summary
In this study, a method for synthesizing ultra-small populations of biocompatible nanoparticles was described, as well as several in vitro methods by which to assess their cellular interactions.
Abstract
Nanoparticle-based delivery vehicles have shown great promise for intracellular targeting applications, providing a mechanism to specifically alter cellular signaling and gene expression. In a previous investigation, the synthesis of ultra-small solid lipid nanoparticles (SLNs) for topical drug delivery and biomarker detection applications was demonstrated. SLNs are a well-studied example of a nanoparticle delivery system that has emerged as a promising drug delivery vehicle. In this study, SLNs were loaded with a fluorescent dye and used as a model to investigate particle-cell interactions. The phase inversion temperature (PIT) method was used for the synthesis of ultra-small populations of biocompatible nanoparticles. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylphenyltetrazolium bromide (MTT) assay was utilized in order to establish appropriate dosing levels prior to the nanoparticle-cell interaction studies. Furthermore, primary human dermal fibroblasts and mouse dendritic cells were exposed to dye-loaded SLN over time and the interactions with respect to toxicity and particle uptake were characterized using fluorescence microscopy and flow cytometry. This study demonstrated that ultra-small SLNs, as a nanoparticle delivery system, are suitable for intracellular targeting of different cell types.
Introduction
纳米颗粒为基础的运载工具显示细胞内定位的应用具有很大的承诺,提供了一个机制,专门改变细胞信号传导和基因表达。这些车辆可装载药物,蛋白质,并设计成影响的细胞反应,并实现在靶组织中的期望的效果的核酸。许多类型的纳米载体的已探索用于治疗和诊断益处包括脂质,聚合物,硅,和磁性材料。这些系统是有吸引力的,因为它们的潜力用于局部药物递送,增加的治疗浓度在靶组织,并降低全身毒性。
固体脂质纳米颗粒(前哨淋巴结)是已成为一种有希望的药物递送载体,近年来的纳米颗粒递送系统的充分研究的实例。前哨淋巴结可以容易地配制成用于多种应用中,包括生物传感1,化妆品2,和叔herapeutic交付3-7。其效用源于这样一个事实,他们是完全由可再吸收的,无毒的脂质,从而增强了生物相容性。在合成过程中,亲脂性药物可以掺入SLN车辆,从而提高药物的溶解度和适宜用于肠胃外施用。 SLN汽车还有助于稳定封装的治疗,减少其降解和清除,并最大限度地发挥治疗作用。这些车辆特别适合于长效,控制释放制剂由于在体温3,4,8,9其稳定性。重要的是,药物在脂质纳米粒封装改变药物分子的内在药动学曲线。这提供了一个潜在的优点,允许药物具有窄治疗指数的控制释放。的前哨淋巴结-掺入治疗剂的释放速率可以基于所述脂质的降解率或药物扩散速度在调谐脂类基质。
前哨淋巴结通常改造以积聚在特定靶组织。例如,它们的大小(通常大于10nm)potentiates保留在循环中,其中肿瘤组织的泄漏脉管促进沉积。此外,颗粒施用途径已经显示出改变生物分布与潜在的目标特定生理结构如淋巴结10,11。一旦沉积在靶组织,以获得适当的细胞相互作用和纳米颗粒的最终的内化是具有挑战性,因为细胞膜为选择性地控制离子和分子的流入和流出所述单元 12的能力。为了便于细胞吸收,这是可以修改的纳米载体与特定的配体,包括肽,小分子,和单克隆抗体13,14。一些机制,包括被动渗透和纳米粒子的主动运输穿过细胞膜先前已经描述3,12,15。在一般情况下,它已经证明,细胞的纳米粒子相互作用由纳米颗粒的物理化学性质,包括大小,形状,表面电荷和表面化学的影响,除了特定小区参数诸如细胞类型或细胞周期时相12。
先前的调查显示亚10纳米前哨淋巴结的合成使用相转化温度(PIT)17的方法,局部16和生物标志物检测的应用1。这是一个温和的合成方法,其中2的组合物保持不变,同时使温度逐渐变化。加热的溶液连续搅拌下,在冷却至RT的结果中的纳米乳剂。这个过程的结果在前哨淋巴结的合成与较小粒度的1比以前报道使用各种方法对脂质楠的合成oparticles 17-22。所得大小比例,小于20纳米,提供的优点为胞内靶向应用,由于增加的表面积和用于增强细胞相互作用的潜力。
示意图前哨淋巴结,旨在提供一种荧光染料或治疗, 显示在图1的前哨淋巴结组成的脂质内部(例如,线性烷)使亲脂性化合物(例如,染料或治疗剂)的掺入和表面活性剂外的( 如线性离子表面活性剂),四面环水。在这项研究中,前哨淋巴结装载用荧光染料和用作模型来研究粒子 – 细胞相互作用。原代人真皮成纤维细胞和小鼠的树突细胞暴露于染料加载SLN随时间以表征相对于毒性和颗粒摄取的相互作用。甲3-(4,5-二甲基吡啶-2-基)-2,5- diphenylphenyltetrazolium溴化物(MTT)法是utili捷思,以确定合适的剂量水平。荧光显微镜,流式细胞术用来检查颗粒吸收体外两种方法。
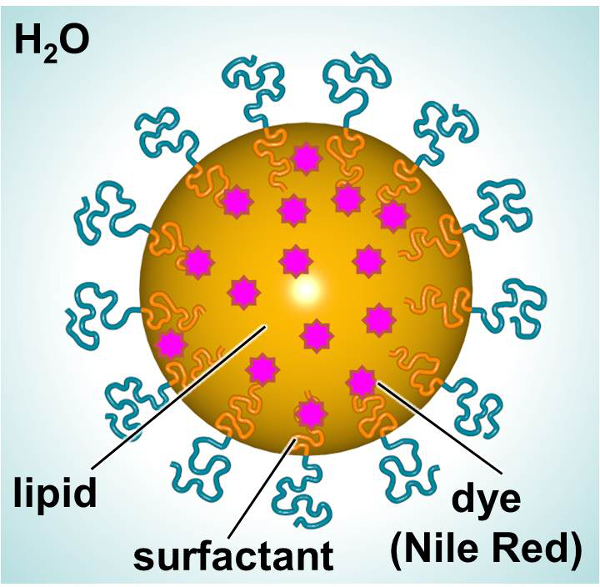
图1.原理SLN的展示的主要成分。 请点击此处查看该图的放大版本。
Protocol
Representative Results
Discussion
在这项研究中,前哨淋巴结的合成和它们的适用性的细胞内靶向应用进行了探索。这些生物相容性纳米粒子希望作为运载工具多种应用,包括药物输送,基因沉默,和疫苗技术25-30。合成超小型前哨淋巴结用一种简便方法,以及它们与初级皮肤细胞和初级免疫细胞相互作用进行了探讨。前哨淋巴结被设计为包括荧光染料(NIR),其中担任一个模型治疗货物的封装。
PIT…
Disclosures
The authors have nothing to disclose.
Acknowledgements
Research reported in this publication was supported by The Johns Hopkins Applied Physics Laboratory’s Research and Exploratory Development Department, Office of Technology Transfer, and Stuart S. Janney Fellowship Program, in addition to the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R21HL127355.
Materials
| Nile Red (NiR) | Sigma | 19123 | BioReagent, suitable for fluorescence, ≥98.0% |
| Heneicosane | Aldrich | 286052 | 98% |
| Brij O10 | Sigma | P6136 | Brij 97, C18-1E10, Polyoxyethylene (10) oleyl ether |
| Water | Sigma | W3500 | Sterile-filtered, BioReagent, suitable for cell culture |
| Syringe Filter 0.2 µm Supor Membrane Low Protein Binding | Life Sciences | PN4612 | Non-Pyrogenic |
| Nanotrac Ultra | Microtrac | serial number U1985IS | Instrument |
| Differential Scanning Calorimeter | Mettlet-Toledo | —- | Instument |
| Primary human fibroblasts | Life Technologies | C-004-5C | Neonatal (HDFn) |
| Medium 106 | Life Technologies | M-106-500 | A sterile, liquid medium for the culture of human dermal fibroblasts. |
| Low Serum Growth Supplement Kit (LSGS Kit) | Life Technologies | S-003-K | All the components of complete LSGS |
| MTT Cell Proliferation Assay Kit | Trevigen | 4890-025-K | Sensitive kit for the measurement of cell proliferation based upon the reduction of the tetrazolium salt, 3,[4,5-dimethylthiazol-2- yl]-2,5-diphenyl-tetrazolium bromide (MTT) |
| Safire2 microplate reader | Tecan | —- | Instrument |
| Phosphate buffered saline | Sigma | P5493 | For molecular biology |
| Recombinant murine GM-CSF | R&D Systems | 415 | >97%, by SDS-PAGE under reducing conditions and visualized by silver stain. |
| Recombinant murine IL-4 | R&D Systems | 404 | >97%, by SDS-PAGE under reducing conditions and visualized by silver stain. |
References
- Calderon-Colon, X., et al. Synthesis of sub-10 nm solid lipid nanoparticles for topical and biomarker detection applications. J Nanopart Res. 16 (2252), 1-10 (2014).
- Patwekar, S., et al. Review on nanoparticles used in cosmetics and dermal products. World Journal of Pharmacy and Pharmaceutical Sciences. 3 (8), 1407-1421 (2014).
- Martins, S., et al. Solid lipid nanoparticles as intracellular drug transporters: An investigation of the uptake mechanism and pathway. International Journal of Pharmaceutics. 430, 216-227 (2012).
- Yadav, N., Khatak, S., Sara, U. V. S. Solid Lipid Nanoparticles – A Review. International Journal of Applied Pharmaceuticals. 5 (2), 8-18 (2013).
- Weber, S., Zimmer, A., Solid Pardeike, J. Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 86 (1), 7-22 (2014).
- Mahajan, A., Kaur, S., Grewal, N. K., Kaur, S. Solid Lipd Nanoparticles (SLNs) – As Novel Lipd based Nanocarriers for Drugs. International Journal of Advanced Research. 2 (1), 433-441 (2014).
- Buse, J., El-Aneed, A. Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: current research and advances. Nanomedicine (Lond). 5, 1237-1260 (2010).
- Malam, Y., Loizidou, M., Seifalian, A. M. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 30 (11), 592-599 (2009).
- Lim, S. B., Banerjee, A., Onyuksel, H. Improvement of drug safety by the use of lipid-based nanocarriers. Journal of controlled release : official journal of the Controlled Release Society. 163, 34-45 (2012).
- Ali Khan, A., Mudassir, J., Mohtar, N., Darwis, Y. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. International journal of nanomedicine. 8, 2733-2744 (2013).
- Oussoren, C., Storm, G. Liposomes to target the lymphatics by subcutaneous administration. Advanced drug delivery reviews. 50, 143-156 (2001).
- Shang, L., Nienhaus, K., Nienhaus, G. U. Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnology. 12, 5 (2014).
- Joshi, M. D., Muller, R. H. Lipid nanoparticles for parenteral delivery of actives. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 71, 161-172 (2009).
- Torchilin, V. P. Micellar nanocarriers: pharmaceutical perspectives. Pharmaceutical research. 24, 1-16 (2007).
- Ashley, C. E., et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nature materials. 10, 389-397 (2011).
- Patchan, M., et al. Nanotech; Nanotechnology 2013: Bio Sensors Instruments, Medical, Environment and Energy; Chapter 3: Materials for Dru., and Gene Delivery. Nanobandage for controlled release of topical therapeutics. 3, 255-258 (2013).
- Forgiarini, A., Esquena, J., Gonzalez, C., C, S., Koutsoukos, P. Formation and stability of nano-emulsions in mixed nonionic surfactant systems. Trends in colloid and interface science XV. Progress in Colloid and Polymer Science. 118, 184-189 (2001).
- Nantarat, T., Chansakaow, S., Leelapornpisid, P. Optimization, characterization and stability of essential oils blend loaded nanoemulsions by PIC technique for anti-tyrosinase activity. International Journal of Pharmacy and Pharmaceutical Sciences. 7, 308-312 (2015).
- Sevcikova, P., Vltavska, P., Kasparkova, V., Formation Krejci, J. Characterization and Stability of Nanoemulsions Prepared by Phase Inversion. , 132-137 (2011).
- Forgiarini, A., Esquena, J., Gonzalez, C., Solans, C., Buckin, V. Studies of the relation between phase behavior and emulsification methods with nanoemulsion formation. Trends in colloid and interface science XIV. Progress in Colloid and Polymer Science. 115, 36-39 (2000).
- Forgiarini, A., Esquena, J., Gonzalez, C., Solans, C. Formation of nano-emulsions by low-energy emulsification methods at constant temperature. Langmuir. 17 (7), 2076-2083 (2001).
- Cabone, C., Tomasello, B., Ruozi, B., Renis, M., Puglisi, G. Preparation and optimization of PIT solid lipid nanoparticles via statistical factorial design. Eur J Med Chem. 49, 110-117 (2012).
- Raimondi, G., et al. Mammalian Target of Rapamycin Inhibition and Alloantigen-Specific Regulatory T Cells Synergize To Promote Long-Term Graft Survival in Immunocompetent Recipients. J Immunol. 184, 624-636 (2010).
- Jhunjhunwala, S., Raimondi, G., Thomson, A. W., Little, S. R. Delivery of rapamycin to dendritic cells using degradable microparticles. J Control Release. 133 (13), 191-197 (2009).
- Kapse-Mistry, S., Govender, T., Srivastava, R., Yergeri, M. Nanodrug delivery in reversing multidrug resistance in cancer cells.. Front Pharmacol. 5 (159), 1-22 (2014).
- Musacchio, T., Torchilin, V. P. Recent developments in lipid-based pharmaceutical nanocarriers. Front Biosci (Landmark Ed). 1 (16), 1388-1412 (2011).
- Cerpnjak, K., Zvonar, A., Gašperlin, M., Vrečer, F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs). Acta Pharm. 63 (4), 27-445 (2013).
- Rodrìguez-Gascòn, A., Pozo-Rodrìguez, A., Solinìs, M. A. Development of nucleic acid vaccines: use of self-amplifying RNA in lipid nanoparticles. Int J Nanomedicine. 9, 1833-1843 (2014).
- Almeida, A. J., Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 59, 478-490 (2007).
- Pardeshi, C., et al. Solid lipid based nanocarriers: an overview. Acta Pharm. 62, 433-472 (2012).
- Attama, A. A., Momoh, M. A., Builders, P. F., Sezer, A. D. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage Form Design and Development. Recent Advances in Novel Drug Carrier Systems. , 107-140 (2012).

