Patch Clamp Recordings on Intact Dorsal Root Ganglia from Adult Rats
Summary
This manuscript describes how to prepare intact dorsal root ganglia for patch clamp recordings. This preparation maintains the microenvironment for neurons and satellite glial cells, thus avoiding the phenotypic and functional changes seen using dissociated DRG neurons.
Abstract
Patch clamp studies from dorsal root ganglia (DRGs) neurons have increased our understanding of the peripheral nervous system. Currently, the majority of recordings are conducted on dissociated DRG neurons, which is a standard preparation for most laboratories. Neuronal properties, however, can be altered by axonal injury resulting from enzyme digestion used in acquiring dissociated neurons. Further, dissociated neuron preparations cannot fully represent the microenvironment of the DRG since loss of contact with satellite glial cells that surround the primary sensory neurons is an unavoidable consequence of this method. To overcome the limitations in using conventional dissociated DRG neurons for patch clamp recordings, in this report we describe a method to prepare intact DRGs and conduct patch clamp recordings on individual primary sensory neurons ex vivo. This approach permits the fast and straightforward preparation of intact DRGs, mimicking in vivo conditions by keeping DRG neurons associated with their surrounding satellite glial cells and basement membrane. Furthermore, the method avoids axonal injury from manipulation and enzyme digestion such as when dissociating DRGs. This ex vivo preparation can additionally be used to study the interaction between primary sensory neurons and satellite glial cells.
Introduction
Sensation is essential to an organism's survival and wellbeing. The transmission of stimuli is dependent on the sensory pathways starting at peripheral endings of axons from primary sensory neurons. Primary sensory neurons, with the exception of the mesencephalic nucleus of the trigeminal nerve, are located in the trigeminal ganglia and dorsal root ganglia (DRGs). They serve as gatekeepers of the sensory information 1. At the perikarial membrane, just as at the central and peripheral terminals, DRG neurons express receptors and ion channels, such as glutamate receptors, TNF alpha receptors, transient receptor potential cation channel subfamily V member 1 (TRPV1), sodium channels, etc. 2-7. Patch clamp recordings of the perikarial membrane allow understanding functional changes of many of these receptors and channels throughout the neuron.
The patch clamp recording technique is a powerful tool for studying the activities of channels or receptors and a great number of studies have been conducted by applying this technique on DRG neurons 8-10. In most studies the DRG is removed by cutting the dorsal rootlets and spinal nerve close to the ganglion. After mincing, the ganglion is then placed in digestive enzymes that result in dissociation of the DRG neurons, which may then be recorded immediately or cultured for several days prior to recording. Unfortunately, dissociation of DRG neurons involves a necessary axotomy close to the perikarya. Once dissociated and axotomized, DRG neurons undergo phenotypic changes as well as changes in membrane excitability 11,12. The loss of contact between the perikarya of individual neurons and the satellite glial cells that normally surround them is likely to contribute to these changes 13. The crosstalk between neurons and satellite glial cells is both essential in physiological conditions and in adaptation to pathological conditions such as those leading to intractable pain 14,15. It would be challenging to study the interaction between neurons and satellite glial cells using a dissociated DRG preparation.
Intact DRGs, on the other hand, provide closer to in vivo conditions. In the past several years, our laboratory, as well as some other groups, has been using intact DRGs from adult rats to investigate changes of primary sensory neurons in different conditions associated with chronic pain 3-5,11,15-17. Although the techniques used in these studies are somewhat established, a step-by-step description has not yet been published. In the present manuscript, we describe a convenient and fast way to prepare intact DRGs and their use for patch clamp recordings.
Protocol
Ethics Statement: All procedures for the maintenance and use of the experimental animals conformed to the regulations of UCSF Committees on Animal Research and were carried out in accordance with the guidelines of the NIH regulations on animal use and care (Publication 85 – 23, Revised 1996). The UCSF Institutional Animal Care and Use Committee approved the protocols used in this study.
1. Preparation of Instruments, Solutions and Dishes
- Prepare Artificial Cerebrospinal Fluid (aCSF).
- Prepare 500 ml of 10x low cation solution, and 500 ml of 10x bicarbonate solution (see Table 1 and 2 for solution preparation). Store at 4 oC and use within one month.
- Prepare 600 ml of fresh aCSF by mixing 60 ml of 10x low cation solution with 60 ml of bicarbonate solution, and then add 480 ml of deionized water to reach a final volume of 600 ml. Bubble the aCSF with carbogen (95% O2 and 5% CO2) for at least 10 min before use.
- Pull Patch Pipette from Capillary Glass.
- Place thin walled capillary glass into a pipette puller to prepare recording pipettes.
Note: In our lab, the following setting parameters of the puller are used to achieve the ideal pipette shape: heat (510), velocity (32). The ideal pipette shape for intact DRG recording should have a gradual slender taper with a low cone angle, and this can best be achieved by trial and error. - Ensure that the resistance of the pipette is 3-5 MΩ when filled with internal solutions (see Table 3 for solution preparation). Please refer to Axon Guide for the detailed method of measuring pipette resistance 18.
- Place thin walled capillary glass into a pipette puller to prepare recording pipettes.
- Add 1 mg of collagenase to 400 µl of aCSF, to give a final concentration of 13 units/ml. Then fill each glass pipette with about 20 µl of collagenase solution. Store the filled pipettes in a -20 oC freezer and use them within three weeks.
- Prepare 200 ml of cold aCSF (about 4 oC). To cool the aCSF quickly place it into a beaker, seal with plastic wrap and place in a -20 oC freezer for approximately 20 min until it starts to freeze. Place the beaker in an ice bath and bubble with carbogen for 10 min. Bubble the remaining 400 ml of aCSF with carbogen at RT.
- While the aCSF is cooling, cut the end of a plastic disposable transfer pipette to enlarge the opening to a diameter of 5 mm (this will be used to transfer the DRG). Collect the following surgical instruments: scalpel (#15), Mayo straight and curved scissors, fine 2 mm tip rongeur, Adson (toothed) forceps, iris scissors, spring scissors and two fine forceps. Pour the oxygenated cold aCSF into 3 glass petri dishes (Outer diameter: 10 cm).
2. DRG Dissection
- Anesthetize a rat (200-220 g) with sodium pentobarbital (100 mg/kg, i.p.) and confirm that the level of anesthesia is adequate by testing pedal reflex and eye blink reflex.
- After verifying that the rat is anesthetized, shave the lumbar area and incise the skin and subcutaneous tissue along the midline. Detach the paraspinal muscles from the spinous processes at the L1-S2 level using a fine periosteal elevator.
- Use curved scissors to cut the spinous processes from L1 to S2. Use straight Mayo scissors to make transverse cuts in the exposed vertebral column at approximately L1 and S1 and remove this segment of the vertebral column en bloc and quickly submerge it into cold aCSF (prepared in step 1.5) for 2-3 min. Following the removal of lumbar DRGs, euthanasia is carried out by bilateral thoracotomy while the rat is still under deep pentobarbital anesthesia.
- Flush off the blood from the removed vertebral column and attached tissue and transfer to a petri dish with cold aCSF. Use a fine rongeur to remove the laminae. Cut the dura mater along the midline with microscissors to expose the spinal cord. Gently lift the spinal cord, and cut the nerve roots at their entry point in the spinal cord using iris scissors, then remove the spinal cord.
- Transfer the remaining vertebra with DRGs to the second petri dish filled with cold aCSF. Identify the L4 and L5 DRGs based on their relative position to transverse processes and sciatic nerve.
Note: Maintaining the sciatic nerve intact will help to identify L4 and L5 DRG (the sciatic nerve originates from the L4-6 spinal nerves). - Use Dumont forceps and the Noyes scissors to free the DRGs from the surrounding connective tissues. Keep the nerve root and spinal nerve attached to the DRGs.
- Use the transfer pipette to move the DRGs into the third petri dish for further dissection.
- Under the dissection microscope, carefully remove as much as possible of the epineurium surrounding the DRG.
- Use the Noyes spring scissors to cut an opening where the dorsal and ventral roots join the DRG and separate the ventral root from the DRG. Use Dumont #5 forceps with blunt tips to hold the epineurium, and use another fine forceps to roll the DRG from the epineurium.
- Continue to remove as much as possible of epineurium attached to DRGs using the fine forceps.
Note: A well-dissected DRG is important to obtain a good digestion in the next step.
3. DRG Digestion
- Transfer the DRG to the recording chamber. Make sure the side of DRG where the nerve roots originate faces down.
Note: In this way, most neurons are accessible. - Use an anchor to stabilize the DRG. Perfuse DRG with oxygenated aCSF at a rate of 0.5 ml/min through plastic tubes connected with a peristaltic pump, which is placed on a table next to the recording rig.
- Wait for 30 min to allow the cells to recover. Assess the quality of the DRG under infrared differential interface contrast (IR-DIC) optics at 40X objective magnification through a CCD camera.
Note: Good DRGs usually contains many round, well contrasted, neurons surrounded by satellite glial cells on its surface. - Digest a small area of the surface of the DRG.
Note: The glass patch pipettes are placed in pipette holders, which are small couplings that allow tubing to be attached to the glass pipette with an airtight seal.- Individually connect two 1 ml syringes to the pipette holders through two pieces of small diameter rubber tubing. Put one glass pipette filled with collagenase into one of the pipette holders and an empty glass pipette into the other one.
- Use the micromanipulator to position the pipettes just above the DRG. Then, under microscope magnification, collide the tips of two pipettes against each other gently to enlarge the openings of pipette tips (Ideally a diameter of 5-10 µm).
- Move the pipette filled with enzyme close to the surface of DRG and briefly apply positive pressure to the pipette containing collagenase through the tube connecting the holder and the syringe by displacing the plunger about 0.5 ml.
Note: Under pressure, the flow of the enzyme from the pipette will slightly enlarge the space between the DRG neurons, which can serve as sign for enzyme application. - After 10 to 15 min, when debris of the remaining epineurium is observed, apply gentle negative pressure to the empty pipette to suck away the debris.
Note: In this way, the neurons and surrounding satellite glial cells will be clearly exposed and ready for patch recording. - Discard the two pipettes into the sharps container.
4. Patch Clamp Recordings
- Place the electrode solution (see Table 3) on ice to prevent degradation. Fill the glass patch pipette with filtered intracellular solution (syringe filter, pore size: 0.2 µm) and place the pipette in the headstage pipette holder. Apply a gentle positive pressure to the pipette through a 5 ml syringe by displacing the plunger about 1 ml before lowering the pipette into bath solution.
Note: This prevents the pipette from becoming blocked. - Choose "V-clamp" mode by turning the mode knob on the amplifier, then open "Membrane test" interface in the software. Move the pipette close to the target neuron under microscope inspection.
Note: In the intact DRG preparation, a thin layer of satellite glial cells encapsulates each neuron. - Use positive pressure from the pipette to traverse the satellite glial cell layer until a sudden enlargement of space between the neuron and surrounding layer of satellite glial cells is observed. Keep moving the pipette towards the neuron until a "dimple" is observed on the neuron.
Note: Compared with recordings from disassociated neurons, the positive pressure needs to be a slightly larger, which will help to penetrate the satellite glial cell layer and increase the chances for successful patching. - Reduce the positive pressure. Next, achieve a giga ohm seal with gentle suction.
Note: With this method, the success rate of recording is at least 70%. - Obtain whole-cell recording configuration as described previously19.
- Briefly, penetrate the neuron cell membrane via a short but strong suction. Alternatively, use the "zap" function on the amplifier while suction is applied.
- Once a whole cell mode is established, compensate whole-cell capacitance and series resistance (Rs) by turning the capacitance and resistance compensation knobs on the amplifier.
Note: Rs is normally 5-20 MΩ. - Abandon the cell if Rs is initially greater than 30 MΩ; or Rs changes by more than 20% during the recording. Also measure the resting membrane potential; abandon a cell if the resting membrane potential is more than -50 mV.
- Close the "Membrane test" window. Choose "I-clamp normal" mode by turning the mode knob on the amplifier.
- Click "open protocol" in the software, select and load the protocol for measuring rheobase. Click "record" to start recording. Measure input resistance and rheobase to examine the neuronal excitability by injecting a graded series of depolarizing currents in steps of 100 pA.
- Click "open protocol" again and select the protocol for measuring membrane threshold. A 500 ms depolarizing ramp current (2,000 pA/s) will be injected to the neuron.
- Use data acquisition and analysis software (e.g. Clampfit) to analyze the recorded traces according to manufacturer's instructions. Use the software to calculate the input resistance (Rin) on the basis of the steady-state I-V relationship during the hyperpolarizing currents delivered. Move cursor to measure the value for rheobase and membrane threshold.
Note: The amplitude of current required to induce the AP is defined as the rheobase, and the lowest voltage for inducing AP is defined as membrane threshold.
- Record the Ligand Induced Currents.
- Fill a pipette with the specific agonists.
Note: In the current report, we used 100 µM glutamate and 100 µM AITC to induce the currents mediated by glutamate receptors and TRPA1 receptors respectively. - Check the pipette to make sure there are no air bubbles inside. Place the pipette in the pipette holder. Connect the pipette holder with a tube connected to a drug dispensing system.
- Use the manipulator to move the pipette within 50 µm of the neuron. Set the drug dispensing system pressure to 1 psi and the duration to 1 sec. Switch the recording mode to voltage clamp, and clamp at -70 mV. Briefly apply the pressure via the drug dispensing system to record a drug-induced current.
- Fill a pipette with the specific agonists.
Representative Results
Figure 1 shows the process of preparing intact DRG for patch recording. Figure 1A shows the exposure and location of the ganglia after laminectomy. Figure1B shows L3, L4 and L5 DRGs with the nerve roots attached after removing the spinal cord. Then L4 and 5 DRGs are carefully dissected and freed from the vertebrae. Next, the epineurium, a transparent membrane surrounding the DRG, is removed (yellow arrow, Figure 1D). The best location to separate the epineurium is through the site where the dorsal and ventral roots join at the DRG, as indicated by the black arrow in Figure 1D. After peeling off the epineurium, the ventral root is removed and discarded, leaving the dorsal rootlets, spinal nerve and DRG, as shown in Figure 1E. Digestion with collagenase removes the residual epineurium, exposing the neurons and the satellite cells that surround them (Figure 1F).
After completing the DRG preparation, the excitability of small diameter DRG neuron is examined by measuring the rheobase and membrane threshold. As shown in Figure 2A, the rheobase is 260 pA. Figure 2B shows the membrane threshold measured with this method. With the increasing current, the membrane is continuously depolarized until an AP is evoked. In this example the membrane threshold (the potential at which the AP is evoked) is -11.9 mV. Therefore, by maintaining the relationship between the neurons and satellite glial cells, our preparation is closer to the in-vivo situation. Based on our results, the rheobase recorded from small DRG neurons is around 300 pA, and the input resistance is 451.3 ± 27.3 MΩ. Both are higher compared with those measured from dissociated neurons (around 150 pA and 635 MΩ), suggesting that dissociation increased the excitability of DRG neurons 11.
With this preparation, we also measured the inward currents induced by glutamate and allyl isothiocyanate (AITC), which are agonists for glutamate receptors and transient receptor potential cation channel member A1 (TRPA1) respectively. The amplitude of inward currents induced by these ligands will reflect the number of the receptors distributed in the neurons. Figure 3A shows an inward current in a small diameter DRG neuron induced by a 1 sec puff application of glutamate (1 mM). Glutamate induced an inward current (198 pA), which can be largely blocked by co-application of the NMDA receptor antagonist APV and the AMPA/kainate receptor antagonist CNQX (data not shown), confirming the current is mediated by glutamate receptors on the DRG neurons. This data established that there are functional glutamate receptors on DRG neurons. The neuron illustrated in Figure 3B showed inward currents (260 pA) induced by 1 sec puff application of allyl isothiocyanate (AITC, 100 µM), a selective TRPA1 agonist. The inward current was blocked by the selective TRPA1 antagonist 10 µM HC 030031 (data not shown), confirming the currents was mediated by TRPA1 receptors and establishes the presence of TRPA1 receptors on DRG neurons.
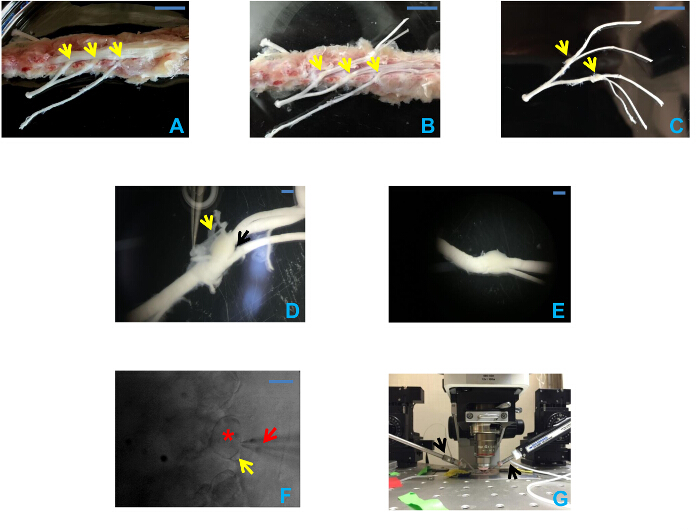
Figure 1: Preparation of intact DRGs. (A) Exposure of the spinal cord after laminectomy and the segmentation of DRGs. Arrows from right to left indicate L3-5 DRGs respectively. Scale bar = 1 cm. (B) Exposure of L3, L4, and L5 DRGs and the attached nerve roots after removal of spinal cord. Arrows indicate L3, L4, and L5 DRGs from right to left. Scale bar = 1 cm. (C) Intact DRGs with nerve roots attached after being dissected from the spinal cord. Arrows indicate L4 and L5 DRGs from right to left. Scale bar = 1 cm. (D) Intact DRG with epineurium attached. In this picture, the epineurium is intact, and the yellow arrow shows the semi-transparent epineurium held by a Dumont forceps. The black arrow indicates the junction formed by dorsal root and ventral root, which serves as a good starting point to peel off the epineurium. Scale bar = 1 mm. (E) The same DRG with the dorsal root attached after the epineurium and ventral root have been removed. Scale bar = 1 mm. (F) Infrared microscope images of DRG following digestion with collagenase. Small- and medium-sized neurons are visible. A satellite glial cell (yellow arrow) is visible surrounding a neuron (asterisk). The red arrow points to the pipette. Scale bar = 10 µm. (G) Recording equipment configuration. The black arrows indicate the pipette holders. The left one holds the drug filled pipette, and recording pipette is on the right. Please click here to view a larger version of this figure.
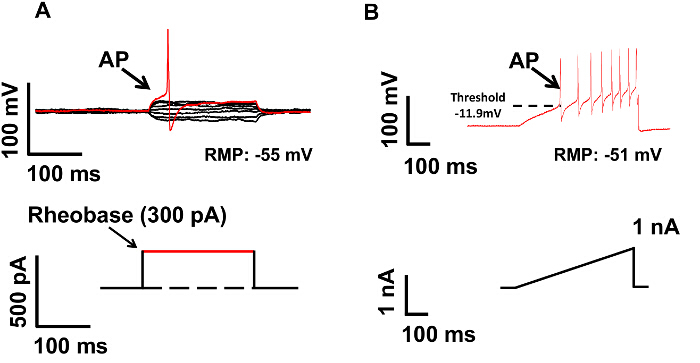
Figure 2: Measurement of Neuronal Excitability in Current Clamp Mode. (A) Rheobase was measured by injecting a series of currents into the DRG neuron (upper panel). The lowest current intensity, which can induce an action potential, is defined as rheobase, as indicated by the arrow in lower panel. The rheobase for this neuron is 300 pA. (B). The membrane threshold was measured by injecting a ramp current. The potential, when an action potential is evoked, is defined as membrane threshold, as labeled by the dashed line in the upper panel. The membrane threshold for this neuron is -11.9 mV. Please click here to view a larger version of this figure.
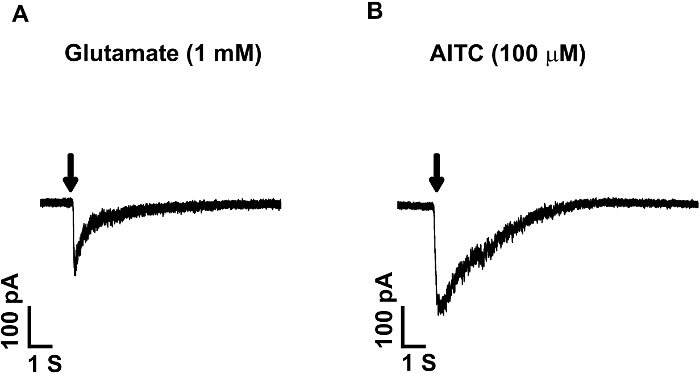
Figure 3: Ligand Induced Currents in Small DRG Neurons. Currents induced by puff application of glutamate (1 mM) or allyl isothiocyanate (TRPA1 agonist, AITC, 100 µM) for 1 sec. Both agonists induced inward currents, suggesting the presence of the glutamate receptors and TRPA1 receptors on small DRG neurons. Glutamate application induced an inward current of 198 pA (A), and AITC induced an inward current of 260 pA (B). Neurons are clamped at -70 mV. Please click here to view a larger version of this figure.
| 2 liters of 10x low-cationic stock | |||
| Final Concentration (mM) | Component | MW | Weight (g) |
| 3 | KCl | 74.6 | |
| 11 | Glucose | 180.2 | |
| 123 | NaCl | 58.4 | |
| 1.25 | NaH2PO4*H2O | 138 | |
| 1 | MgCl2*6H2O | 203.3 | |
| 2 | CaCl2*2H2O | 147 | |
Table 1
| 1 liter of 10x bicarbonate stock | |||
| Final Concentration (mM) | Component | MW | Weight (g) |
| 26 | NaHCO3 | 84.01 | 21.84 |
Table 2
| 100 ml of intracellular solution | |||
| Concentration (mM) | Component | MW | g |
| 130 | KGluconate | 234.24 | |
| 10 | KCl | 74.55 | |
| 10 | HEPES | 238.3 | |
| 10 | EGTA | ||
| 2 | MgCl2*6H2O | 203.3 | |
| Notes: Adjust pH to 7.4 with KOH, and osmolarity to 260 – 280 mOsm with sucrose and distilled water. Add 2 mM MgATP, 0.5 mM Na2GTP and filter before immediate use. | |||
Table 3
Discussion
We report a method to prepare whole DRGs for patch clamp studies. There are several key elements for preparing an ideal specimen. Firstly, it is important to dissect the DRGs with dorsal roots attached. After that, the epineurium need to be carefully removed while avoiding damage to the neurons. Finally, to expose the neurons and their surrounding satellite glial cells, it is necessary to digest the remaining connective tissue. Intact DRGs from adult rats prepared with the method described here will maintain good viability for 6 to 8 hr and can be stably recorded during that period. They can be used for many different studies of DRGs including patch clamp recordings on neurons or satellite glia cells, or evoked responses of DRG neurons.
During the dissection procedure, it is important to quickly dissect the DRG and keep it at a low temperature (4 oC) to slow the neuronal metabolism and thus maintains its viability. During the dissection of DRGs, one should avoid stretching the dorsal or spinal nerves entering the DRGs. Another key step in this preparation is the removal of the epineurium surrounding the DRG. Since the epineurium is difficult to penetrate, any epineurium remaining on the DRG will prevent the patch pipettes from reaching the neurons. Application of collagenase is an important step for successful preparations. Collagenase will digest the remaining epineurium and clean the surface of cells at the same time, both of which are critical for successful patching. To avoid over-digestion of the tissue, three factors needs to be considered. First, collagenase comes in several different forms; see the Table of Materials for our recommendation, the one currently used is potent but gentle, thus avoiding over-digestion. Second, the enzyme should be delivered at a light pressure to avoid the dispersing of the enzyme beyond the recording area. We have found the pressure produced from applying 0.5 ml of air out of a 1ml syringe of is sufficient. Third, the best concentration range for the collagenase is from 10-13 units/ml. Higher concentrations might lead to over-digestion and faster degradation of the tissue, while lower concentrations involves an unnecessarily long digestion time. In our experience, a concentration of 13 units/ml and a digestion time around 15 min produces the best results. After applying the enzyme the remaining epineurium surrounding the DRG should become loose, and can be cleared away with gentle suction. Collagenase is sensitive to repeat freezing-thawing cycles, therefore, once diluted the enzyme solution should be divided into aliquots and used within 3 weeks.
Zhang and colleagues were the first to describe a method for patch clamp recording from intact DRGs. The rats they used, however, were very young (10-15 days postnatal) 20 which has the advantage that the DRGs are easier to prepare given that they have a thinner epineurium and they have an increased viability. DRGs from neonatal rats, however, have the disadvantage that the expression of proteins and ion channels like TRPV1 and NaV1.8 differ from those of adult rats 21,22. Also, behavioral and pharmacological studies are usually conducted in adult rats. Thus the method of recording detailed here allows making a correlation between electrophysiology and behavior in adult animals. The use of intact dorsal root ganglia as a preparation to conduct patch clamp recordings ex vivo has increased recently 15-17,23,24, and some studies have used in vivo recordings 25. Most of ex vivo recordings expose the DRG neurons by incubating the whole DRG into enzyme cocktail for around 30-60 min. While this method produces more neurons, it has a risk of over-digestion of the neurons at the superficial layers. Our method minimizes the possibility of over-digestion by using visual inspection to monitor the extent of the digestion, a method also used by Ma et al for in vivo recordings25.
Compared with dissociated DRG neurons, one of the advantages of the intact DRG preparation comes from the preservation of both neurons and satellite glial cells (Figure 1). This is not the case in conventional preparations that uses dissociated DRG neurons. Satellite glial cells forms a barrier surrounding individual DRG neurons , and their presence is essential for maintaining the physiological environment of these neurons 13. As noted in the Results the electrophysiological properties of neurons prepared by this means differ from those obtained by studies using dissociated cells.
One of the most interesting future directions for this preparation is to investigate the crosstalk between DRG neurons and satellite glial cells. After nerve injury, for example, satellite glial cells have been shown to undergo plastic changes, which are closely related to the pain behavior that ensues 13-15,26. The preservation of satellite glial cells in the intact DRGs, will enable us to determine whether transmitter receptors such as the glutamate receptor, or ATP receptors are present on satellite glial cells and how they respond to change in neuronal activity. By patching satellite glial cells and stimulating the neuron simultaneously, the satellite glial cells can act as a biological sensor and show whether there is transmitter release from the nearby neurons. Furthermore, intact DRG preparation keeps both afferent and efferent nerve roots. This greatly expands the experimental scope by adding the possibility of stimulating the axons entering or exiting the neurons with suction electrode, which would be a better mimic of in vivo situation.
There are some limitations to the current preparation. The existence of the barrier formed by satellite glial cells, makes it more difficult to patch neurons and one should be aware that it may limit the concentration of drugs reaching the neurons. Therefore, to gain access to the neuron in our preparation it is important to maintain the pipette under positive pressure to aid penetrating satellite glial cells layer. Another limitation is that the dorsal roots and spinal nerves have to be cut in order to dissect out the DRG. Therefore, it is impossible to leave the axon totally intact. However, the presence of the remaining axon should be considered, as the peripheral or central axons may not be clamped to the same voltage as the soma, which could cause potential recording error or interference when voltage clamp is conducted. Also in order to get good exposure of the neurons, gentle digestion with collagenase is necessary and although this can be controlled with experience, it is impossible to completely protect neurons from the exposure to the digesting enzyme. Compared with conventional dissociated DRG neurons, however, whole DRGs are faster to prepare (around 30 min), can be used in many applications and closely mimics in vivo conditions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the Painless Research Foundation for support of the work. This work was also supported by the NIH grants R01 NS080921-01 and R21 NS079897-01A1.
Materials
| Pentobarbital sodium | vortech Pharmaceuticals | ||
| syringe | BD | 309659 | 1 ml, 5 ml. |
| scalpel | BD | size: 15 | |
| Mayo straight scissor | Fine Science Tools | 14010-15 | |
| Mayo curved scissor | Fine Science Tools | 14011-15 | |
| Rongeur | Fine Science Tools | 16021-14 | |
| Adson toothed forceps | Fine Science Tools | 11027-12 | |
| Iris Scissor | Fine Science Tools | 14084-08 | |
| Noyes spring scissor | Fine Science Tools | 15124-12 | |
| Bone scissors | Fine Science Tools | 16044-10 | Special for cutting the bones. |
| Forceps: Dumont, Dumoxel Biologie #5 | Fine Science Tools | 11252-30 | These have the fine tips that do not need sharpening when first purchased. |
| periosteal elevator | Sklar | 97-0530 | |
| Dissection microscope | WILD | ||
| Transfer pipette | Fisher brand | 13-711-5AM | |
| Petri dish (10 cm) | Pyrex | Glass petri dish can avoid damaging the tips of fine forceps | |
| Collagenase (Liberase TM) | Roche | 05-401-119-001 | dissolve at the concentration of 13 u/ml, aliquot into glass pipette. Avoid repeated freeze and thaw. |
| filter | Thermo scientific | 7232520 | Filter the internal solutions for patch clamp recording to avoid clog. |
| Glass pipette | Sutter | BF150-110-7.5 | |
| Anchor | Havard apparatus | 64-0250 | stabilize the DRG to avoid drift. |
| Peristaltic pump | WPI | ||
| Pipette puller | Sutter | P97 | |
| Amplifier | Molecular devices | Axopatch 200B | |
| Digitizer | Molecular devices | 1440D | |
| Microscope | NIKON | FN600 | |
| Micro-manipulator | Sutter | MPC200 | |
| microinjection dispense system | General Valve | Picrospitzer II | fast drug application system |
| Carbogen (95% O2, 5% CO2) | Local Medical Gas supplier |
References
- Basbaum, A. I., Bautista, D. M., Scherrer, G., Julius, D. Cellular and molecular mechanisms of pain. Cell. 139, 267-284 (2009).
- Caterina, M. J., et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 389, 816-824 (1997).
- Gong, K., Bhargava, A., Jasmin, L. GluN2B N-methyl-D-aspartate receptor and excitatory amino acid transporter 3 are upregulated in primary sensory neurons after 7 days of morphine administration in rats: implication for opiate-induced hyperalgesia. Pain. 157, 147-158 (2016).
- Gong, K., Kung, L. H., Magni, G., Bhargava, A., Jasmin, L. Increased response to glutamate in small diameter dorsal root ganglion neurons after sciatic nerve injury. PloS one. 9, 95491 (2014).
- Gong, K., Zou, X., Fuchs, P. N., Lin, Q. Minocycline inhibits neurogenic inflammation by blocking the effects of tumor necrosis factor-alpha. Clin Exp Pharmacol Physiol. , (2015).
- Ohtori, S., Takahashi, K., Moriya, H., Myers, R. R. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine. 29, 1082-1088 (2004).
- Waxman, S. G., Cummins, T. R., Dib-Hajj, S., Fjell, J., Black, J. A. Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain. Muscle nerve. 22, 1177-1187 (1999).
- Zhang, J. M., Song, X. J., LaMotte, R. H. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol. 82, 3359-3366 (1999).
- Dib-Hajj, S. D., et al. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain. 83, 591-600 (1999).
- Cummins, T. R., et al. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci. 19, RC43 (1999).
- Zheng, J. H., Walters, E. T., Song, X. J. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP. J Neurophysiol. 97, 15-25 (2007).
- Schoenen, J., Delree, P., Leprince, P., Moonen, G. Neurotransmitter phenotype plasticity in cultured dissociated adult rat dorsal root ganglia: an immunocytochemical study. J Neurosci Res. 22, 473-487 (1989).
- Hanani, M. Satellite glial cells: more than just ‘rings around the neuron’. Neuron Glia Biol. 6, 1-2 (2010).
- Takeda, M., Nasu, M., Kanazawa, T., Shimazu, Y. Activation of GABA(B) receptors potentiates inward rectifying potassium currents in satellite glial cells from rat trigeminal ganglia: in vivo patch-clamp analysis. Neuroscience. 288, 51-58 (2015).
- Zhang, H., et al. Altered functional properties of satellite glial cells in compressed spinal ganglia. Glia. 57, 1588-1599 (2009).
- Fan, N., Donnelly, D. F., LaMotte, R. H. Chronic compression of mouse dorsal root ganglion alters voltage-gated sodium and potassium currents in medium-sized dorsal root ganglion neurons. J Neurophysiol. 106, 3067-3072 (2011).
- Fan, N., Sikand, P., Donnelly, D. F., Ma, C., Lamotte, R. H. Increased Na+ and K+ currents in small mouse dorsal root ganglion neurons after ganglion compression. J Neurophysiol. 106, 211-218 (2011).
- Sherman-Gold, R. . The Axon Guide. , (2008).
- Cummins, T. R., Rush, A. M., Estacion, M., Dib-Hajj, S. D., Waxman, S. G. Voltage-clamp and current-clamp recordings from mammalian DRG neurons. Nat Protoc. 4, 1103-1112 (2009).
- Zhang, J. M., Donnelly, D. F., LaMotte, R. H. Patch clamp recording from the intact dorsal root ganglion. J Neurosci Methods. 79, 97-103 (1998).
- Benn, S. C., Costigan, M., Tate, S., Fitzgerald, M., Woolf, C. J. Developmental expression of the TTX-resistant voltage-gated sodium channels Nav1.8 (SNS) and Nav1.9 (SNS2) in primary sensory neurons. J Neurosci. 21, 6077-6085 (2001).
- Funakoshi, K., et al. Differential development of TRPV1-expressing sensory nerves in peripheral organs. Cell Tissue Res. 323, 27-41 (2006).
- Hayar, A., Gu, C., Al-Chaer, E. D. An improved method for patch clamp recording and calcium imaging of neurons in the intact dorsal root ganglion in rats. J Neurosci Methods. 173, 74-82 (2008).
- Yagi, J., Sumino, R. Inhibition of a hyperpolarization-activated current by clonidine in rat dorsal root ganglion neurons. J Neurophysiol. 80, 1094-1104 (1998).
- Ma, C., Donnelly, D. F., LaMotte, R. H. In vivo visualization and functional characterization of primary somatic neurons. J Neurosci Methods. 191, 60-65 (2010).
- Vit, J. P., Jasmin, L., Bhargava, A., Ohara, P. T. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol. 2, 247-257 (2006).

