Culturing of Retinal Pigment Epithelial Cells on an Ex Vivo Model of Aged Human Bruch’s Membrane
Summary
The goal of this protocol is to demonstrate the culturing of retinal pigment epithelial (RPE) cells on aged and/or diseased human Bruch's membrane. This method is suitable to study RPE cell behavior on a compromised extracellular matrix.
Abstract
Aside from vitamins and antioxidants recommended by the Age-Related Eye Disease Study, there is no effective therapy for "dry," or atrophic age-related macular degeneration (AMD) which represents 90% of the cases. Therapies are needed to slow or retard the development of geographic atrophy (GA), and understanding Bruch's membrane pathology is part of this process. Alterations in human Bruch's membrane precede the progression of AMD by contributing to the damage of retinal pigment epithelial (RPE) cells. Given the lack of sufficient animal models to study AMD, ex vivo models of aged human Bruch's membrane serve as a useful tool to study the behavior of RPE cells from immortalized and primary cell lines as well as RPE lines derived from induced pluripotent stem cells (iPSCs). Here, we present a detailed method that allows one to determine the effects of RPE cell behavior seeded on harvested human Bruch's membrane explants from human donors, including attachment, apoptosis and proliferation, ability to phagocytize photoreceptor outer segments, establishment of polarity, and gene expression. This assay provides an ex vivo model of aged Bruch's membrane to assess the functional characteristics of RPE cells when seeded on aged/compromised extracellular matrix.
Introduction
Age-related structural changes to human Bruch's membrane, which is caused by many factors, including nitrosative and oxidative stress, exerts multiple deleterious effects on the function of retinal pigment epithelial (RPE) cells and contributes to the pathology of age-related macular degeneration (AMD)1,2,3,4,5,6,7,8,9. When considering cell replacement therapy for advanced atrophic AMD or geographic atrophy, treatment will likely require the transplantation of cells onto a bed of RPE cell atrophy. Age-related changes within human Bruch's membrane can adversely affect the success of transplanted RPE cell grafts, given the damage to the extracellular matrix6,9,10,11,12,13,14,15,16,17,18,19,20,21,22. Investigating human Bruch's membrane biology and how structural changes within the matrix contribute to the progression to AMD is vital to understanding disease pathology. Thus, there is a critical need for the investigators in the age-related eye field to develop protocols that describe the ex vivo harvesting of aged and/or diseased human Bruch's membrane.
Historically, it has been difficult to model age-related disorders such as geographic atrophy and an aged human Bruch's membrane in animals23. This difficulty arises from multiple factors including the longevity of humans compared to rodents and other species frequently used for disease modeling, as well as the lack of a macula in most vertebrates24. The advantage of the method described herein is that cells can be tested directly on Bruch's membrane extracted from post mortem eyes of aged and/or diseased individuals. The overall goal of this article is to provide a detailed methodology for an ex vivo model of aged and/or diseased human Bruch's membrane, including the isolation of human Bruch's membrane explants from human donors and the seeding of RPE cells for downstream experiments. This model can serve as a relevant model to investigate the contribution of extracellular matrix damage on RPE cell function and pathology20,25,26,27,28.
Protocol
The following protocol is performed in adherence to the tenets of the Declaration of Helsinki and the Yale University Human Research Ethics Committee guidelines.
1. Sourcing Human Donor Eyes
- Obtain human donor globes from the National Disease Research Interchange (NDRI, Philadelphia, PA). Depending on the project, prepare no less than three pairs of donor eyes for each experimental group.
- Enucleate donor eyes within 10 h and ship within 48 h post mortem. Then transport the eyes to the laboratory in sterile Dulbecco's modified Eagle medium (DMEM) culture medium on ice. Previously reported studies have demonstrated that donor eyes harvested and transported in this fashion preserve the biological features and activities of Bruch's membrane25,26,29.
2. Harvesting of Human Bruch's Membrane Explants
- Place each eye on a 1.5 x 1.5 inch gauze soaked with carbon dioxide-independent DMEM media in a 100 mm culture dish, and use fine blade scissors to make an incision through the sclera 3 mm posterior to the limbus and circumferentially extending in either direction.
- Make a full-thickness circumferential incision (penetrating to vitreous) 1 mm posterior to the ora serrata. Remove and discard the anterior segment (cornea, lens, and iris), the vitreous and retina.
- Use fine surgical scissors to make four radial incisions in between the sclera and choroid, and then peel the sclera away from the periphery to the optic nerve, while taking care to avoid tearing the choroid/Bruch's membrane complex.
- Next, make a circumferential incision through the suprachoroidal space along the ora serrata and peel the RPE-Bruch’s membrane-choroid complex posteriorly from the sclera.
- Remove native RPE cells by bathing the explant with 0.02 M ammonium hydroxide in Dulbecco's phosphate-buffered saline (DPBS) in a 60 x 15 mm polystyrene Petri dish for 20 min at room temperature, followed by washing it three-times in DPBS (Figure 1).
- Float the Bruch's membrane complex explant in carbon dioxide-free media over an unlaminated, hydrophobic 65 µm-thick polytetrafluoroethylene membrane with 0.2 µm pores, with the basal lamina layer of each explant facing the membrane.
- Place a polytetrafluoroethylene membrane with Bruch's membrane explant on a fritted glass support in a glass microanalysis vacuum filter-holder system.
- Flatten the curled edges from the choroidal side with fine forceps coated with Teflon, taking care not to touch the Bruch's membrane basal laminin side.
- Heat 15 mL of agarose (4% in DPBS) and then cool it to 37 °C. Then pour 15 mL of the liquidized gel onto the Bruch's membrane-choroid complex from the choroidal-side while applying gentle suction to guarantee that the explant will remain flat. Keep the tissue at 4 °C for 2 – 3 min to solidify the agarose and then peel the polytetrafluoroethylene membrane away.
- Place the Bruch's membrane explant (1.5 inches in diameter, within solidified agarose gel) in a 60 x 15 mm polystyrene cell culture dish (with Bruch's membrane facing up) that has been lined with warm liquid agarose on the bottom of the dish to fix the Bruch's membrane explant on the bottom of the well with the explant basal laminin layer facing up.
- The agarose will solidify within 2 – 3 min at room temperature to stabilize the Bruch's membrane explants in the culture dish. Cover the Bruch's membrane explants in the culture dish with DPBS and store the dish at 4 °C for future use. Figure 2A demonstrates the isolated Bruch's membrane explants in a 60 x 15 mm culture dish.
- If the desired culture system is in a 96-well plate, place the Bruch's membrane explant (1.5 inches in diameter, with solidified agarose gel) on a flat Teflon sheet with the laminin-side up.
- Using a trephine, cut five or six, 6 mm-circular buttons from the Bruch's membrane complex explant and place each on 4% agarose at 37 °C in a non-treated polystyrene well of a 96-well plate. The agarose will solidify within 2-3 min at room temperature to fix the Bruch's membrane explants (buttons) on the bottom of each well.
NOTE: Figure 2B demonstrates trephined Bruch's membrane buttons in a 96-well plate. Typically, 9 - 11 buttons or explants can be harvested from each eye.
3. Culturing of Retinal Pigment Epithelial (RPE) Cells on Human Bruch's Membrane Explants
- Upon receipt of the donor eyes, clean the extraocular tissue with DPBS. Make a circumferential incision 5 mm below the iris-sclera touch point (pars plana) into the subretinal space. Discard the anterior segment, vitreous humor, and retina.
- Wash the eyecup, which contains the Bruch's membrane and RPE sheet, with cold DPBS. Dissociate the RPE cells with 5 mL of 0.25% trypsin for no longer than 10 min for each eye sample. Quench the trypsin reaction with 25 mL of DMEM complete medium with 15% fetal bovine serum (FBS) warmed at 37 °C in a 50 mL tube.
- Centrifuge the cells at 200 × g for 10 min at 24 °C, and resuspend the pellet in 5 mL of DMEM complete medium (with 15% FBS).
- Count the cells and seed 15,000 viable RPE cells onto the basal lamina of each various aged Bruch's membrane explants (buttons) in a 96-well plate in 200 µL of serum-free minimum essential media (MEM) containing antibiotics (100 IU/mL penicillin G, 100 mg/mL streptomycin, 5 mg/mL gentamicin, and 2.5 mg/mL amphotericin B). Assuming a cell diameter of 20 µm, plating at this density will allow RPE cells to cover approximately 15% of the plating area.
- Plate the cells onto the button, whereby the cell density can then be increased stepwise up to 100% confluence depending on the effect of each parameter on cell behavior.
- Allow the cells to attach to the explant for 24 h in a humidified atmosphere of 95% air/5% CO2 at 37 °C. Gently change medium with warm complete DMEM.
Representative Results
When this protocol is performed properly, one can determine the effects of aged and/or diseased human Bruch's membrane on RPE cell function by the establishment of polarity and the outer blood retinal barrier, polarized secretion of growth factors and cytokines, and phagocytosis of photoreceptor rod outer segments.
We have generated data that demonstrate a disease phenotype on aged human Bruch's membrane. For example, culturing RPE cells on aged human Bruch's membrane explants decreases RPE cell reattachment (Figure 3). RPE cells cultured on aged human Bruch's membrane decreases the capacity of these cells to phagocytize rod outer segments (ROS), a critical RPE function (Figure 4). Moreover, apoptotic cells can be identified and compared on these button explants using a terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) stain as described in previous work26. The effects of an aged and/or diseased human Bruch's membrane on RPE cell gene expression profile can be determined using microarray technology. We have demonstrated that RPE cell gene expression is altered when cultured on human Bruch's membrane from older individuals compared to explants from younger individuals (Figure 5)27. For statistical analysis, explants from at least 3 – 5 human donors are used per group.
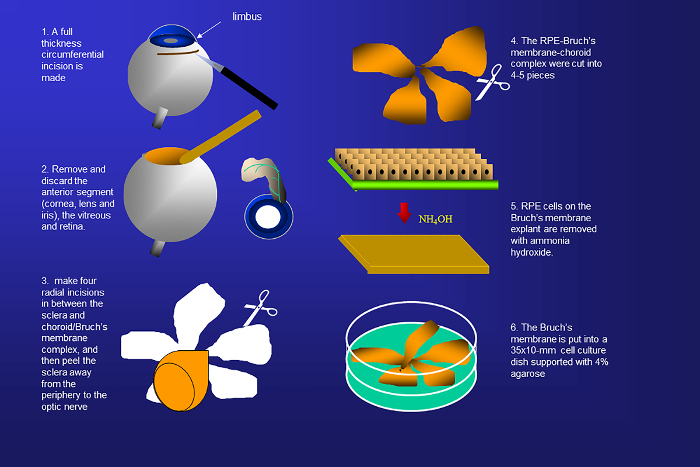
Figure 1. Schematic representation of the isolation of human Bruch's membrane from donor eyes. Human Bruch's membranes are harvested by removing the sclera, anterior segment, retina, vitreous, and native retinal pigment epithelial (RPE) cells. Please click here to view a larger version of this figure.
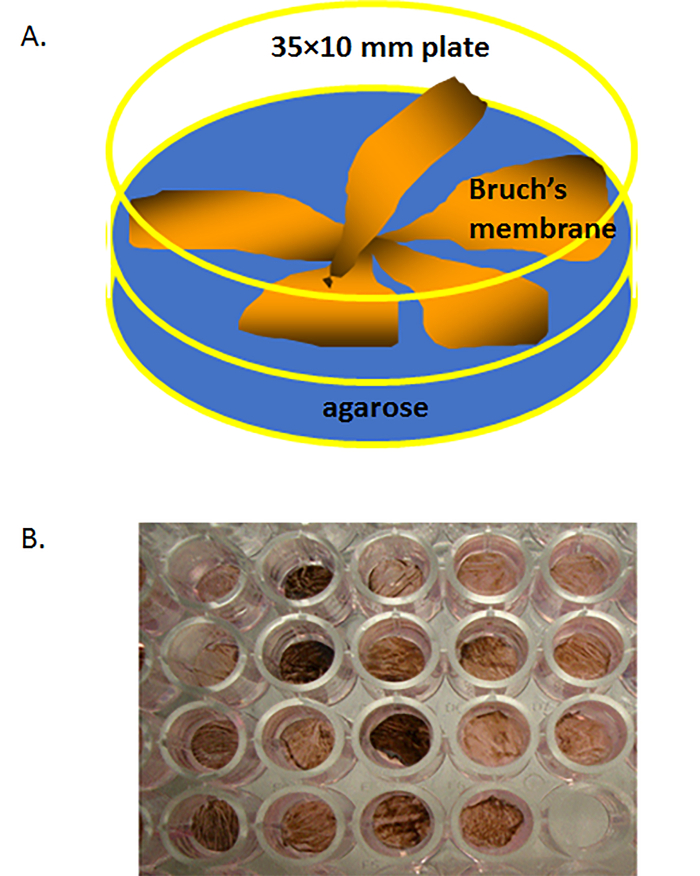
Figure 2. Isolated human Bruch's membrane (BM) in a culture dish. (A) Schematic of human Bruch's membrane explants (each 1.5 inches in diameter, with solidified agarose gel) placed in a 60 × 15 mm polystyrene cell culture dish (BM face-up) filled with warm liquid agarose on the bottom of dish. (B) A human Bruch's membrane explant that has been trephined into a few 6 mm circular buttons (explants), each ow which was then placed on 4% agarose at 37 °C in a non-treated polystyrene well of a 96-well plate. Please click here to view a larger version of this figure.
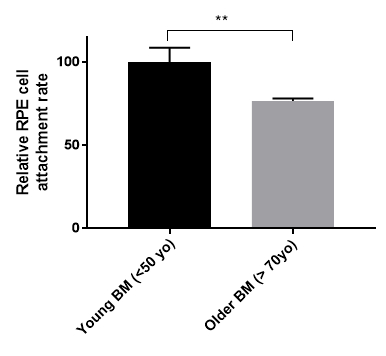
Figure 3. Retinal pigment epithelial (RPE) cell reattachment rate on young and older human Bruch's membrane (BM). Primary RPE cells were seeded onto human Bruch's membrane explants from young (<50-year-old, n = 3) or older (>70-year-old, n = 5) donor eyes for three weeks. Cell attachment rate was measured by MTT cell viability assay. Older human Bruch's membranes reduced the RPE cell attachment rate by 24% (young BM 100 +/-8.54 S.E. vs. older BM 76.65 +/-1.44 S.E.). **p <0.05, S.E. = standard error.
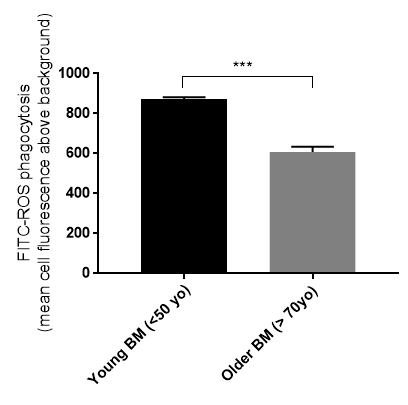
Figure 4. Retinal pigment epithelial (RPE) cell phagocytosis is affected by the age of the human Bruch's membrane (BM). Primary RPE cells cultured on older human Bruch's membrane explants had a reduced ability to phagocytize rod outer segments (ROS) (young BM 873 +/-9.3 S.E., n = 5 vs. older BM 608 +/-25.4 S.E., n = 5). ***p <0.01, S.E. = standard error.
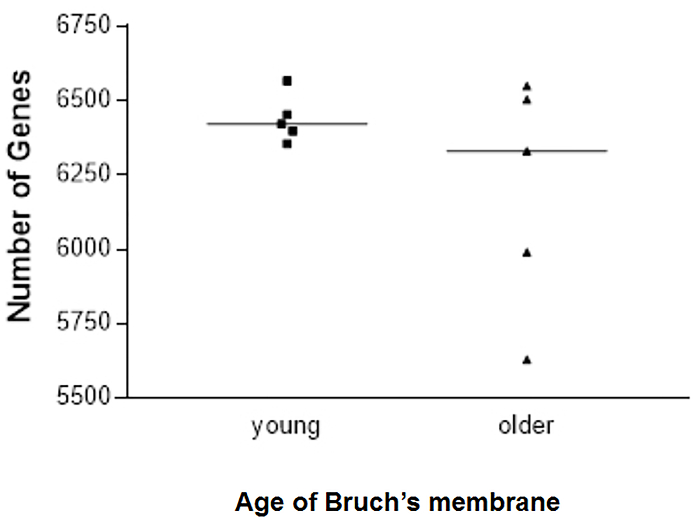
Figure 5. Number of retinal pigment epithelial (RPE) cell genes expressed in each sample cultured on young or older human Bruch's membrane explants. There was more scatter in the number of genes expressed in primary RPE cells seeded onto older versus younger human Bruch's membrane (6201 ± 388 vs. 6439 ± 80, respectively); five explants were tested for each age group. Presented with the full permission of all authors of Cai, H et al.27. Please click here to view a larger version of this figure.
Discussion
Aged human Bruch's membrane (extracellular matrix) contributes to the disease progression of AMD and thus there is a need to understand how this altered matrix contributes to RPE cell dysfunction. Given the lack of sufficient animal models to study age-related changes in AMD, ex vivo model systems that mimic the effects of disease can serve as a valuable tool to understand the pathophysiology. The methodology described in this manuscript can be used to consistently isolate human Bruch's membrane explants and culture RPE cells, including primary, immortalized, or stem cell-derived RPE cell lines.
As mentioned previously, human Bruch's membrane explants are sourced from the NDRI. A protocol is established with NDRI which specifies the criteria set for donor eyes to be acceptable. For example, donors must have no known retinal eye diseases, eyes/globes are retrieved within 10 h after death and globes arrive in the laboratory within 48 h after death (via overnight package delivery on ice). Specify the appropriate age range and number of eyes needed for statistical analysis of the study, e.g., five pairs of globes from donors between the ages of 20 – 49 years and five globe pairs from donors between the ages of 50 – 89 years.
The availability of eye globes varies, generally averaging ten pairs of globes available each month. The eye package arrives with basic, de-identified donor information: age, race, time of death, cause of death, and brief past medical history (disease listing or comorbidities).
Most importantly, harvesting human Bruch's membrane explants requires the removal of the anterior segment of the eye and vitreous, allowing for the isolation of the RPE-Bruch's membrane-choroid complex. Once the Bruch's membrane is isolated, RPE cells can be seeded onto the acellular explants at varying concentrations and cultured for downstream experiments. The preparation and procedural handling as described herein is critical, paying attention to the orientation of the isolated Bruch's membrane to make sure the laminal surface is facing-up while not touching the surface mechanically to avoid damaging the extracellular matrix surface structure.
It should also be noted that when using the Bruch's membrane explant systems, there are some variables that could affect the results of downstream experiments such as individual donor genomic background, age of the Bruch's membrane, or postmortem time of the Bruch's membrane, and the location of the Bruch's membrane, i.e., central or peripheral part of Bruch's membrane explants. As with any other human tissue study, one must recruit the appropriate donor eye sample number to have the necessary statistical power and match donor eye age if appropriate. Establishing strict criteria for accepting eyes from eye banks is a critical parameter. Only accept eyes that are enucleated less than 10 h postmortem and which the Bruch's membrane preparation can be harvested within 24 – 48 h after death. The optic nerve site may be used as a marker for orientation, and use the same locations for experimental and control study groups. By taking these measures, one can minimize experimental variability.
In summary, understanding of the RPE-Bruch's membrane-choroid complex and how it is affected by age and disease is critical to understanding its contribution to the pathophysiology of AMD. Model systems that employ ex vivo techniques are a valuable tool to investigate these effects using human tissue. Herein, we describe a methodology that employs human donor explants as a relevant ex vivo model of aged Bruch's membrane. This technique allows one to use aged and/or diseased human Bruch's membrane as a research tool to investigate how structural changes within the matrix can affect overlaying RPE cell behavior including attachment, proliferation, and gene expression25,27. Successful development of similar ex vivo model systems will further our understanding of AMD and facilitate the development of novel therapeutic options.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research is supported by Research to Prevent Blindness, New York, www.rpbusa.org, and the Greater New York Center for Retinal Degenerative Disease Foundation Fighting Blindness, www.blindness.org. The authors would like to thank Luanna Bartholomew, Ph.D., for her critical review of this manuscript.
Materials
| Human donor globes | National Disease Research Interchange, NDRI | ||
| Dulbecco's modified eagle medium (DMEM) | Thermo Fisher Scientific | 11995-065 | |
| Carbon-dioxide independent media | Thermo Fisher Scientific | 18045-088 | |
| 60-mm polystyrene petri dish | Corning Inc. | 351007 | |
| Dulbecco's phosphate buffered saline, PBS | Thermo Fisher Scientific | 14190144 | |
| Hydrophobic 65 µm-thick polytetrafluoroethylene membrane with 0.2 µm pores | Merck Millipore | JGWP04700 | |
| Fisherbrand glass microanalysis vacuum filter holder system | Thermo Fisher Scientific | 09-753-102 | |
| Agarose | Sigma-Aldrich | A2576-5G | |
| 35 × 10mm culture dish | VWR International | 25373-041 | |
| Trephine | Accutome | AM0570 60 | |
| 96 well plate | Corning Inc. | 3595 | |
| Minimum essential media (MEM) | Thermo Fisher Scientific | 11095-080 | |
| Penicillin G | Sigma-Aldrich | P3032-1MU | |
| Streptomycin | Sigma-Aldrich | S6501 | |
| Gentamicin | Sigma-Aldrich | G1914 | |
| Amphotericin B | Sigma-Aldrich | 1397-89-3 | |
| Ammonium hydroxide solution | Sigma-Aldrich | 1336-21-6 |
References
- Murdaugh, L. S., Wang, Z., Del Priore, L. V., Dillon, J., Gaillard, E. R. Age-related accumulation of 3-nitrotyrosine and nitro-A2E in human Bruch’s membrane. Exp. Eye Res. 90 (5), 564-571 (2010).
- Wiradjaja, F., DiTommaso, T., Smyth, I. Basement membranes in development and disease. Birth Defects Res C Embryo Today. 90 (1), 8-31 (2010).
- Nystrom, A., Bornert, O., Kuhl, T. Cell therapy for basement membrane-linked diseases. Matrix Biol. , (2016).
- Pauleikhoff, D., Harper, C. A., Marshall, J., Bird, A. C. Aging changes in Bruch’s membrane. A histochemical and morphologic study. Ophthalmology. 97 (2), 171-178 (1990).
- Marshall, G. E., Konstas, A. G., Reid, G. G., Edwards, J. G., Lee, W. R. Type IV collagen and laminin in Bruch’s membrane and basal linear deposit in the human macula. Brit. J. Ophthalmol. 76 (10), 607-614 (1992).
- Sarks, S. H., Arnold, J. J., Killingsworth, M. C., Sarks, J. P. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. Brit. J. Ophthalmol. 83 (3), 358-368 (1999).
- Spraul, C. W., Lang, G. E., Grossniklaus, H. E., Lang, G. K. Histologic and morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv. Ophthalmol. 44 (Suppl 1), S10-S32 (1999).
- Abdelsalam, A., Del Priore, L., Zarbin, M. A. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv. Ophthalmol. 44 (1), 1-29 (1999).
- Mullins, R. F., Aptsiauri, N., Hageman, G. S. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye. 15 (Pt 3), 390-395 (2001).
- Caldwell, R. B. Extracellular matrix alterations precede vascularization of the retinal pigment epithelium in dystrophic rats. Curr. Eye Res. 8 (9), 907-921 (1989).
- Pauleikhoff, D., Harper, C. A., Marshall, J., Bird, A. C. Aging changes in Bruch’s membrane. A histochemical and morphologic study. Ophthalmology. 97 (2), 171-178 (1990).
- Abdelsalam, A., Del Priore, L., Zarbin, M. A. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv. Ophthalmol. 44 (1), 1-29 (1999).
- Spraul, C. W., Lang, G. E., Grossniklaus, H. E., Lang, G. K. Histologic and morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 44, S10-S32 (1999).
- Del Priore, L. V., Tezel, T. H. Reattachment rate of human retinal pigment epithelium to layers of human Bruch’s membrane. Arch. Ophthalmol. 116 (3), 335-341 (1998).
- Ho, T. C., Del Priore, L. V. Reattachment of cultured human retinal pigment epithelium to extracellular matrix and human Bruch’s membrane. Invest. Ophth. Vis. Sci. 38 (6), 1110-1118 (1997).
- Tezel, T. H., Del Priore, L. V. Reattachment to a substrate prevents apoptosis of human retinal pigment epithelium. Graefes Arch. Clin. Exp. Ophthalmol. 235 (1), 41-47 (1997).
- Tezel, T. H., Del Priore, L. V. TGF beta secretion modulates the density-dependent growth of pig retinal pigment epithelium in vitro. Ophthalmic Res. 31 (3), 192-202 (1999).
- Gullapalli, V. K., Sugino, I. K., Van Patten, Y., Shah, S., Zarbin, M. A. Impaired RPE survival on aged submacular human Bruch’s membrane. Exp. Eye Res. 80 (2), 235-248 (2005).
- Wang, H., Yagi, F., Cheewatrakoolpong, N., Sugino, I. K., Zarbin, M. A. Short-term study of retinal pigment epithelium sheet transplants onto Bruch’s membrane. Exp. Eye Res. 78 (1), 53-65 (2004).
- Tezel, T. H., Del Priore, L. V. Repopulation of different layers of host human Bruch’s membrane by retinal pigment epithelial cell grafts. Invest. Ophth. Vis. Sci. 40 (3), 767-774 (1999).
- Tezel, T. H., Del Priore, L. V., Kaplan, H. J. Reengineering of aged Bruch’s membrane to enhance retinal pigment epithelium repopulation. Invest. Ophth. Vis. Sci. 45 (9), 3337-3348 (2004).
- Castellarin, A. A., Sugino, I. K., Vargas, J. A., Parolini, B., Lui, G. M., Zarbin, M. A. In vitro transplantation of fetal human retinal pigment epithelial cells onto human cadaver Bruch’s membrane. Exp. Eye Res. 66 (1), 49-67 (1998).
- Nguyen, H. V., Li, Y., Tsang, S. H. Patient-specific iPSC-derived RPE for modeling of retinal diseases. J. Clin. Med. 4 (4), 567-578 (2015).
- Fletcher, E. L., Jobling, A. I., Greferath, U., Mills, S. A., Waugh, M., Ho, T., De Longh, R. U., Phipps, J. A., Vessey, K. A. Studying age-related macular degeneration using animal models. Optometry Vision Sci. 91 (8), 878-886 (2014).
- Del Priore, L. V., Tezel, T. H. Reattachment rate of human retinal pigment epithelium to layers of human Bruch’s membrane. Arch. Ophthalmol. 116 (3), 335-341 (1998).
- Tezel, T. H., Kaplan, H. J., Del Priore, L. V. Fate of human retinal pigment epithelial cells seeded onto layers of human Bruch’s membrane. Invest. Ophth. Vis. Sci. 40 (2), 467-476 (1999).
- Cai, H., Del Priore, L. V. Bruch membrane aging alters the gene expression profile of human retinal pigment epithelium. Curr. Eye Res. 31 (2), 181-189 (2006).
- Moreira, E. F., Cai, H., Tezel, T. H., Fields, M. A., Del Priore, L. V. Reengineering human Bruch’s membrane increases rod outer segment phagocytosis by human retinal pigment epithelium. Transl. Vis. Sci. Technol. 4 (5), 10 (2015).
- Sun, K., et al. Bruch’s membrane aging decreases phagocytosis of outer segments by retinal pigment epithelium. Mol. Vis. 13, 2310-2319 (2007).

