组分变熵稳定氧化物的体积和薄膜合成
Summary
高质量的散装和薄膜 (Mg0.25 (1 x)Cox镍0.25 (1-x)铜0.25 (1-x)锌0.25 (1-x)) O 和 (毫克0.25 (1 x)Co0.25 (1 x)镍0.25 (1 x)铜x锌0.25 (1-x)) O 熵稳定氧化物的提出。
Abstract
这里, 我们提出了合成散装和薄膜多组分 (镁0.25 (1 x)Cox镍0.25 (1-x)铜0.25 (1-x)锌0.25 (1-x)) O (Co 变体) 和 (毫克0.25 (1 x)Co0.25 (1-x)镍0.25 (1 x)铜x锌0.25 (1-x)) O (cu 变体) 熵稳定氧化物。相纯和化学均质 (毫克0.25 (1 x)Cox镍0.25 (1-x)铜0.25 (1 x)锌0.25 (1 x)) O (x = 0.20, 0.27、0.33) 和 (毫克0.25 (1 x)Co0.25 (1 x)镍0.25 (1 x)Cux锌0.25 (1 x)) O (x = 0.11, 0.27) 陶瓷颗粒被合成并用于沉积超高质量, 相纯, 单晶薄膜的靶化学计量。本文介绍了用脉冲激光沉积 (001) 取向氧化镁基片沉积光滑、化学均匀、熵稳定氧化物薄膜的详细方法。用 X 射线衍射法确定了大块薄膜材料的相和结晶度。通过 x 射线光电子能谱和能量色散 x 射线光谱法证实了成分和化学均匀性。用扫描探针显微镜测量薄膜的表面形貌。合成高质量, 单晶, 熵稳定的氧化物薄膜, 使研究的界面, 大小, 应变和无序影响的性质, 这一新类高无序氧化物材料。
Introduction
自2004年发现高熵金属合金以来, 高熵材料引起了极大的兴趣, 原因是硬度增加了1,2,3, 韧性4,5和耐腐蚀性3,6。最近发现了高熵氧化物7、8和硼化物9, 为物质爱好者开辟了一个大操场。特别是氧化物可以显示有用和动态功能属性, 如铁电性10、磁电11、12、热电 13和超导14。.熵稳定氧化物 (ESOs) 最近被证明具有有趣的, 组分依赖功能属性15,16, 尽管有重大的障碍, 使这种新的材料类特别令人兴奋。
熵稳定材料是化学上均匀的, 多组分 (通常有五个或更多组分), 单相材料在那里构型熵贡献 () 对吉布斯自由能 () 是重要的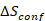
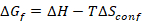 足以推动形成单相固体溶液17。合成多组分 ESOs, 其中阳离子构型紊乱是在阳离子点观察, 需要精确控制的组成, 温度, 沉积速率, 淬火速率和淬火温度7,16.该方法旨在使从业者能够合成相纯和化学均质的熵稳定氧化物陶瓷小球和相纯, 单晶, 平面薄膜的期望化学计量。可以用大于90% 的理论密度合成大块材料, 从而对电子、磁性和结构特性进行研究, 或用作薄膜物理气相沉积 (PVD) 技术的来源。由于在这里考虑的熵稳定氧化物有五阳离子, 薄膜 PVD 技术, 采用五来源, 如分子束外延 (外延) 或共溅射, 将提出的挑战, 沉积化学均匀薄膜流量漂移。该协议的结果是在化学上均质, 单晶, 扁平 (方根) 粗糙度的 0.15 nm 的熵稳定氧化物薄膜从单一的材料来源, 这表明具有名义化学成分。该薄膜合成协议可通过包含原位电子或光学特性技术来增强, 用于实时监测合成和精制质量控制。这种方法的预期限制来源于激光能量漂移, 这可能会限制高质量薄膜厚度低于1微米。
足以推动形成单相固体溶液17。合成多组分 ESOs, 其中阳离子构型紊乱是在阳离子点观察, 需要精确控制的组成, 温度, 沉积速率, 淬火速率和淬火温度7,16.该方法旨在使从业者能够合成相纯和化学均质的熵稳定氧化物陶瓷小球和相纯, 单晶, 平面薄膜的期望化学计量。可以用大于90% 的理论密度合成大块材料, 从而对电子、磁性和结构特性进行研究, 或用作薄膜物理气相沉积 (PVD) 技术的来源。由于在这里考虑的熵稳定氧化物有五阳离子, 薄膜 PVD 技术, 采用五来源, 如分子束外延 (外延) 或共溅射, 将提出的挑战, 沉积化学均匀薄膜流量漂移。该协议的结果是在化学上均质, 单晶, 扁平 (方根) 粗糙度的 0.15 nm 的熵稳定氧化物薄膜从单一的材料来源, 这表明具有名义化学成分。该薄膜合成协议可通过包含原位电子或光学特性技术来增强, 用于实时监测合成和精制质量控制。这种方法的预期限制来源于激光能量漂移, 这可能会限制高质量薄膜厚度低于1微米。
尽管薄膜氧化物材料的生长和表征有显著的进步10,18,19,20,21, 立体与氧化物中的电子结构可以导致在最终材料中产生显著的差异, 这是由看似微不足道的方法论差异引起的。此外, 多组分熵稳定氧化物的领域是相当新生的, 仅二个当前报告薄膜综合在文献7,16。ESOs 对这个过程特别好, 规避了化学气相沉积和分子束外延所带来的挑战。在这里, 我们提供了一个详细的综合协议的散装和薄膜 ESOs (图 1), 以尽量减少材料处理困难, 意外的财产变化, 并提高发现在外地的加速。
Protocol
Representative Results
Discussion
我们已经描述并显示了一个合成散装和高质量的协议, 单晶薄膜 (镁0.25 (1 x)Cox镍0.25 (1 x)铜0.25 (1 x)锌0.25 (1 x)) O (x = 0.20, 0.27、0.33) 和 (Mg0.25 (1-x)Co0.25 (1 x)Ni0.25 (1 x)铜x锌0.25 (1 x)) O (x = 0.11, 0.27) 熵稳定氧化物。我们期望这些合成技术适用于广泛的熵稳定氧化物组成, 更多的发现, 在发展和扩大的领域。此外, 组分变化的熵?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
这项工作是由国家科学基金会资助的一部分。DMR-0420785 (XPS)。我们感谢密歇根大学的密歇根材料鉴定中心 (MC)2, 为其提供 XPS 和密歇根大学 Van Vlack 实验室的 XRD。我们还要感谢托马斯. Kratofil 对散装材料的准备工作的协助。
Materials
| MAGNESIUM OXIDE 99.95% | Fisher | AA1468422 | |
| COBALT(II) OXIDE, 99.995% | Fisher | AA4435414 | |
| NICKEL(II) OXIDE 99.998% | Fisher | AA1081914 | |
| COPPER(II) OXIDE 99.995% | Fisher | AA1070014 | |
| ZINC OXIDE 99.99% | Fisher | AA8781230 | |
| TRICHLROETHLENE SEMICNDTR 9 | Fisher | AA39744K7 | |
| ACETONE SEMICNDTR GRD 99.5% | Fisher | AA19392K7 | |
| 2-PROPANOL ACS 99.5% | Fisher | A416S4 | |
| Mineral oil, pure | Acros Organics | AC415080010 | |
| alumina crucible | MTI Corporation | eq-ca-l50w40h20 | |
| ZIRCONIA (YSZ) GRINDING MEDIA | Inframat Advanced Materials | 4039GM-S010 | |
| SiC paper 320/600/800/1200 | South Bay Technology | SDA08032-25 | |
| MgO (100) substrate, 5x5x0.5 mm, 1SP | MTI Corporation | MGa050505S1 | |
| OXYGEN COMPRESSED ULTRA HIGH PURITY GRADE, 99.999% | Cryogenic Gases | OXYUHP | |
| NITROGEN COMPRESSED EXTRA DRY GRADE | Cryogenic Gases | NITEX |
References
- Tsai, M. H., Yeh, J. W. High-Entropy Alloys: A Critical Review. Mater Res Lett. 2 (3), 107-123 (2014).
- Yeh, J. W., et al. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv Eng Mater. 6 (5), 299-303 (2004).
- Gao, M. C., Carney, C. S., Dogan, N., Jablonksi, P. D., Hawk, J. A., Alman, D. E. Design of Refractory High-Entropy Alloys. Jom. 67 (11), 2653-2669 (2015).
- Gludovatz, B., Hohenwarter, A., Catoor, D., Chang, E. H., George, E. P., Ritchie, R. O. A fracture-resistant high-entropy alloy for cryogenic applications. Science. 345 (6201), 1153-1158 (2014).
- Zou, Y., Ma, H., Spolenak, R. Ultrastrong ductile and stable high-entropy alloys at small scales. Nat Commun. 6, 7748 (2015).
- Poulia, A., Georgatis, E., Lekatou, A., Karantzalis, A. E. Microstructure and wear behavior of a refractory high entropy alloy. Int J Refract Met Hard Mater. 57, 50-63 (2016).
- Rost, C. M., et al. Entropy-stabilized oxides. Nat Commun. 6, 8485 (2015).
- Jiang, S., et al. A new class of high-entropy perovskite oxides. Scripta Mater. 142, 116-120 (2018).
- Gild, J., et al. High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics. Sci Rep. 6 (October), 37946 (2016).
- Schlom, D. G. others Strain Tuning of Ferroelectric Thin Films. Annu Rev Mater Res. 37, 589-626 (2007).
- Zhao, T., et al. Electrical control of antiferromagnetic domains in multiferroic BiFeO3 films at room temperature. Nat Mater. 5 (10), 823-829 (2006).
- Borisov, P., Hochstrat, A., Chen, X., Kleemann, W., Binek, C. Magnetoelectric Switching of Exchange Bias. Phys Rev Lett. 94 (11), 117203 (2005).
- Weidenkaff, A., Robert, R., Aguirre, M., Bocher, L., Lippert, T., Canulescu, S. Development of thermoelectric oxides for renewable energy conversion technologies. Renew Energy. 33 (2), 342-347 (2008).
- Pickett, W. E. Electronic structure of the high-temperature oxide superconductors. Rev Mod Phys. 61 (2), 433-512 (1989).
- Berardan, D., Franger, S., Dragoe, D., Meena, A. K., Dragoe, N. Colossal dielectric constant in high entropy oxides. Phys Status Solidi – Rapid Res Lett. 10 (4), 328-333 (2016).
- Meisenheimer, P. B., Kratofil, T. J., Heron, J. T. Giant Enhancement of Exchange Coupling in Entropy-Stabilized Oxide Heterostructures. Sci Rep. 7 (1), 13344 (2017).
- Miracle, D. B. High-Entropy Alloys: A Current Evaluation of Founding Ideas and Core Effects and Exploring "Nonlinear Alloys.". Jom. , 1-7 (2017).
- Mannhart, J., Schlom, D. G. Oxide Interfaces-An Opportunity for Electronics. Science. 327 (5973), 1607-1611 (2010).
- Mundy, J. A., et al. Atomically engineered ferroic layers yield a room-temperature magnetoelectric multiferroic. Nature. 537 (7621), 523-527 (2016).
- Martin, L. W., Chu, Y. H., Ramesh, R. Advances in the growth and characterization of magnetic, ferroelectric, and multiferroic oxide thin films. Mater Sci Eng R Rep. 68 (4), 89-133 (2010).
- Saremi, S., et al. Enhanced Electrical Resistivity and Properties via Ion Bombardment of Ferroelectric Thin Films. Adv Mater. 28 (48), 10750-10756 (2016).
- Cullity, B. D., Weymouth, J. W. Elements of X-ray Diffraction. Am J Phys. 25 (6), 394-395 (1957).
- Rijnders, G. J. H. M., Koster, G., Blank, D. H. A., Rogalla, H. In situ monitoring during pulsed laser deposition of complex oxides using reflection high energy electron diffraction under high oxygen pressure. Appl Phys Lett. 70 (14), 1888-1890 (1997).
- Sullivan, M. C., et al. Complex oxide growth using simultaneous in situ reflection high-energy electron diffraction and x-ray reflectivity: When is one layer complete?. Appl Phys Lett. 106 (3), 031604 (2015).
- Eres, G., et al. Time-resolved study of SrTiO3 homoepitaxial pulsed-laser deposition using surface x-ray diffraction. Appl Phys Lett. 80 (18), 3379-3381 (2002).
- Fleet, A., Dale, D., Suzuki, Y., Brock, J. D. Observed Effects of a Changing Step-Edge Density on Thin-Film Growth Dynamics. Phys Rev Lett. 94 (3), 036102 (2005).
- Luca, G. D., Strkalj, N., Manz, S., Bouillet, C., Fiebig, M., Trassin, M. Nanoscale design of polarization in ultrathin ferroelectric heterostructures. Nat Commun. 8 (1), 1419 (2017).
- De Luca, G., Rossell, M. D., Schaab, J., Viart, N., Fiebig, M., Trassin, M. Domain Wall Architecture in Tetragonal Ferroelectric Thin Films. Adv Mater. 29 (7), (2017).
- Gruenewald, J. H., Nichols, J., Seo, S. S. A. Pulsed laser deposition with simultaneous in situ real-time monitoring of optical spectroscopic ellipsometry and reflection high-energy electron diffraction. Rev Sci Instrum. 84 (4), 043902 (2013).
- . MDC Vacuum Products | Vacuum Components, Chambers, Valves, Flanges & Fittings Available from: https://mdcvacuum.com/DisplayContentPageFull.aspx?cc=b8ca254a-cdc0-4b71-8603-af10ce18bbcb (2018)
- Dijkkamp, D., et al. Preparation of Y-Ba-Cu oxide superconductor thin films using pulsed laser evaporation from high Tc bulk material. Appl Phys Lett. 51 (8), 619-621 (1987).
- Biegalski, M. D., et al. Relaxor ferroelectricity in strained epitaxial SrTiO3 thin films on DyScO3 substrates. Appl Phys Lett. 88 (19), 192907 (2006).
- Schlom, D. G., Chen, L. Q., Pan, X., Schmehl, A., Zurbuchen, M. A. A Thin Film Approach to Engineering Functionality into Oxides. J Am Ceram Soc. 91 (8), 2429-2454 (2008).
- Damodaran, A. R., Breckenfeld, E., Chen, Z., Lee, S., Martin, L. W. Enhancement of Ferroelectric Curie Temperature in BaTiO3 Films via Strain-Induced Defect Dipole Alignment. Adv Mater. 26 (36), 6341-6347 (2014).

