A Behavioral Test Battery for the Repeated Assessment of Motor Skills, Mood, and Cognition in Mice
Instructor Prep
concepts
Student Protocol
All methods described here have been approved by the Committee on the Use of Live Animals in Teaching and Research (CULATR), the University of Hong Kong.
1. General protocol
NOTE: This section is based on Deacon19.
- Behavioral room setup
- Get rid of unrelated stimulation/distraction, including direct bright light on the experimental apparatus, odor, noise, and other irrelevant animals, in the behavioral room (which should be about 10 m2 with adjustable lighting and, preferably, have an anteroom).
NOTE: Since the mouse is a nocturnal animal, lighting under 15 lux in the open-field test, novel object recognition test, and social interaction test could minimize the interference/stress from the light and help the mouse to focus on the test. - Set the camera for video recording at least 1.5 m above the floor, ensuring that it is out of the sight of the testing mouse.
- Get rid of unrelated stimulation/distraction, including direct bright light on the experimental apparatus, odor, noise, and other irrelevant animals, in the behavioral room (which should be about 10 m2 with adjustable lighting and, preferably, have an anteroom).
- Housing and habituation
- Group-house the mice in an animal unit under observation (e.g., group-housing no more than four adult mice).
NOTE: Here, 3-month-old male C57BL/6N mice were used and housed in a 1144B cage. Rule out sick, injured, or severely stressed mice. Experiences of starvation, thirst, or being bullied may affect the performance of the mouse. - Arrange for the same animal handler to conduct all the behavioral tests, to diminish variability. Perform the transportation, handling, and experiment during the light cycle from 7:00 a.m. to 7:00 p.m. If possible, arrange all the other handling, such as the administration of any drug/toxin (e.g., intranasal instillation of silica nanoparticles) or cage-cleaning, after the test.
- Relabel the cages with random numbers to blind the experimenter before each experiment. Habituate the mice to the experimental environment in the behavioral room for 15 to 30 min in their home cages. Keep the home cages in the behavioral room during the entire experiment.
- Before starting the experiment, put a nonexperimental C57BL/6N mouse in the apparatus so that the experimental condition for the first mouse is the same as the rest. Then, clean the apparatus as follows: remove the urine and feces with a clean paper towel, clean the experimental device with tap water, and then, cover the odor left by the mouse by wiping the apparatus with a paper towel lightly sprayed with 70% ethanol.
- To minimize the distraction caused by the experimenter, ask the experimenter to leave the behavioral room during video recording, or stay behind the curtain during the Morris water maze test.
- Group-house the mice in an animal unit under observation (e.g., group-housing no more than four adult mice).
- Behavioral test arrangement
- Arrange the behavioral test in the order as shown in Figure 1A. Plan to perform the tests spaced out by 24 h, except for the elevated plus maze test.
NOTE: Since the entire procedure requires up to 2 weeks, simplify the battery by choosing among similar tests before application in an acute or short-term study.
CAUTION: The 2 day open-field test ensures proper habituation for the novel object recognition test. Test the forced swimming test last as it may cause stress in C57BL/6N mice.
- Arrange the behavioral test in the order as shown in Figure 1A. Plan to perform the tests spaced out by 24 h, except for the elevated plus maze test.
2. BehavioralTest Protocol
- Open-field test8,18,19,20
- Perform the open-field test in a 60 cm (length [L]) x 60 cm (width [W]) x 40 cm (height [H]) nontransparent white plastic arena.
NOTE: Using multiple arenas can increase the throughput of the test. - Start the camera recording and gently put the mouse next to the middle of a wall of the arena, facing that same wall. Record the behavior of the mouse for 10 min before returning it to the home cage. Clean the apparatus as described in step 1.2.4.
NOTE: The start point in the open-field test can also be the center of the arena. Be consistent among the mice. - Repeat until all the mice finish the protocol. Counterbalance the testing order between groups.
- Perform the data analysis as follows.
- Divide the arena into four squares by four squares (imaginary grid) on the computer screen. To assess locomotor function, count the number of lines crossed by the mouse in the arena19.
NOTE: The definition of “crossing a line” is when both hind limbs cross it. This definition also applies to the elevated plus maze test. - Measure the time spent in the central area as an indicator of anxiety. The central area is the four squares in the center of the arena.
NOTE: Additional parameters, such as rearing (both front paws off the ground, with front paws against a wall or standing), latency to the first rear, and grooming and freezing indicate the emotionality of the mouse. - Alternatively, use a tracking software, as described in detail by Seibenhener and Wooten8, to measure the distance traveled, the speed, and the time spent in the center area.
- Divide the arena into four squares by four squares (imaginary grid) on the computer screen. To assess locomotor function, count the number of lines crossed by the mouse in the arena19.
- Perform the open-field test in a 60 cm (length [L]) x 60 cm (width [W]) x 40 cm (height [H]) nontransparent white plastic arena.
- Accelerating rotarod test18
- Perform the 3 day training of the accelerating rotarod test before any treatment of drugs or toxin/modeling/onset of disease and test the motor function for 1 day, as planned in Figure 1A.
NOTE: The mouse receives three trials per day during the training and testing. Each trial starts with the rotation of the rod and ends with the drop of the mouse. - Place the rotarod apparatus on the bench in the behavioral room. Avoid direct lighting to the equipment. Program the equipment as starting from 4 rpm and accelerating to 40 rpm within 5 min.
- In each trial, put the mouse on the static rod, facing the wall of the machine. Start the device when the mouse is settled. Stop the device once the mouse drops and record the time the mouse spent on the rod. Immediately repeat for another two trials before returning the mouse back to the home cage.
- Repeat the procedure on the other mice.
- Measure the average time spent on the rod of three trials during testing to estimate motor function.
NOTE: The average time on the rod during the third day of training is the baseline of motor function.
- Perform the 3 day training of the accelerating rotarod test before any treatment of drugs or toxin/modeling/onset of disease and test the motor function for 1 day, as planned in Figure 1A.
- Social interaction test18
- For the social interaction test, use an open-field arena with two identical transparent chambers (8 cm [L] x 6 cm [W] x 12 cm [H]) with holes on the surface—and a novel mouse (helper) which is a same-sex juvenile conspecific that has had no previous contact with the subject mouse. Figure 1B shows the scheme of the procedure. Clean the arena and the chambers as described in step 1.2.4.
NOTE: The novel mouse cannot be a littermate or cage mate of the subject mouse. It is group-housed and healthy. Habituate the novel mouse to the behavioral room for 15 to 30 min as described in step 1.2.3. - Separately place the two chambers in the middle of two opposite walls of the arena. Introduce the subject mouse into the arena as described in step 2.1.2 and shown in Figure 1B, for a 3 min exploration. Return the subject mouse to the home cage and remove any urine or feces in the arena.
- Put the helper in one of the chambers. Reintroduce the subject mouse to the arena and record for 3 min. Afterward, return both mice to each of their own home cages. Repeat the procedures with other subject mice as described above.
NOTE: Counterbalance the side of the helper or randomly assign it within the group. - From the video, estimate the parameter describing the social interaction activity of the mice as thelper/tempty, which means the ratio of time interacting with the helper chamber (thelper) and the empty one (tempty), or use the recognition index thelper/(thelper + tempty).
NOTE: An interaction between the subject mouse and the chamber is defined as when the mouse’s nose is within 2 cm of the chamber and pointing toward it.
- For the social interaction test, use an open-field arena with two identical transparent chambers (8 cm [L] x 6 cm [W] x 12 cm [H]) with holes on the surface—and a novel mouse (helper) which is a same-sex juvenile conspecific that has had no previous contact with the subject mouse. Figure 1B shows the scheme of the procedure. Clean the arena and the chambers as described in step 1.2.4.
- Elevated plus maze test10
- Conduct the elevated plus maze test on the same day after all mice are tested in the open-field test. Clean the apparatus as described in step 1.2.4.
NOTE: The configuration of the elevated plus maze is a “+”-shape. It has two open arms (30 cm x 5 cm x 0.5 cm) across from each other and perpendicular to two closed arms (30 x 5 x 16 cm) with a center platform (5 cm x 5 cm x 0.5 cm). The maze is elevated 40 cm from the ground. - Place the mouse at the junction of the open and closed arms, facing the open arm that is opposite to the experimenter (Figure 1C). Record the behavior for 5 min before returning the mouse to the home cage. Repeat till all mice are tested.
NOTE: Entering the maze with its face to the open arm could increase the mouse’s exploration of the open arm. - Measure the time the mouse spent in the open arms (topen) and in the closed arms (tclose) based on the video: topen/tclose indicates the level of anxiety.
- Conduct the elevated plus maze test on the same day after all mice are tested in the open-field test. Clean the apparatus as described in step 1.2.4.
- Forced swim test11
- The apparatus of the forced swim test is a cylindrical tank that is 30 cm high and 20 cm in diameter. Fill the tank up to 15 cm high with tap water at room temperature (23 – 25 °C).
NOTE: Use fresh water for each mouse. - Start the video recording and gently put the mouse in the water, in the center of the apparatus. Record the video for 6 min before putting the mouse back in its home cage under infrared light.
NOTE: Do not disturb the mouse by drowning it or twisting its tail. - Measure the immobility time in the last 5 min of the recorded video. Mobility means any movements other than those required to balance the body and to keep the head above the water.
- The apparatus of the forced swim test is a cylindrical tank that is 30 cm high and 20 cm in diameter. Fill the tank up to 15 cm high with tap water at room temperature (23 – 25 °C).
- Novel object recognition test17,18
- Set up the novel object recognition test to include 2 days of habituation, 1 day of familiarization, and 1 day of testing (Figure 1D); each session is 10 min per mouse, and the intersession interval is 24 h.
NOTE: The habituation using the open-field test is performed as described in section 2.1. The mouse interacts with two identical objects (old objects) in familiarization. In the test, the mouse interacts with one of the old objects and a new object, both placed in the same place as the objects in familiarization. The apparatus of the novel object recognition test includes an open-field arena and two sets of objects. Each set contains two identical objects (objects A and A and objects B and B). Objects A and B are similar in size but different in texture (glass/plastic/paper), shape (round/cubic), and color (bright/dark). The objects should be odor-free and big enough for the mouse to explore within 10 min. The appropriate size for adult C57BL/6N mice is 8 cm tall and 5 cm wide/in diameter. - Mark the positions of the two objects in familiarization and test, which are 5 cm away from the side and 7 cm away from the top of the arena.
NOTE: Mark the position on the evening before the familiarization to avoid the smell of the marker. - In the familiarization, the mouse interacts with one set of identical objects. Clean the arena and objects as described in step 1.2.4 before placing the mouse in the arena, facing the middle of the wall as shown in Figure 1D. Record for 10 min before returning the mouse to the home cage. Repeat until all the mice are finished and return all the cages to the animal unit.
NOTE: Counterbalance the objects used in familiarization within the group to diminish bias (e.g., mice No. 1 and 2 explore objects A and A, and mice No. 3 and 4 explore objects B and B; in this way, the novel object is object B for mice No. 1 and 2 and object A for mice No. 3 and 4). - Perform the test 24 h after the familiarization. Use the same procedure as for the familiarization, except replace one of the objects with one from another set (Figure 1D). Repeat until all the mice have performed the test and, afterward, return all the cages to the animal unit.
NOTE: Counterbalance the side of the new object within the group to diminish bias (e.g., introduce mice No. 1 and 3 to objects A and B, and mice No. 2 and 4 to objects B and A). In this way, the novel object shows at the right side for mice No. 1 and 4, and at the left side for mice No. 2 and 3. Here, the left side is the left side of the experimenter when facing the arena. - Measure the time that each mouse interacts with the new object (tnew) and the old object (told) separately, from the video footage in the test phase. An interaction between the animal and the object is described in the note following step 2.3.4. Calculate the memory of the mouse as the preference to the novel object = tnew/told; or tnew/(tnew + told).
NOTE: tnew/told equals to 1 or tnew/(tnew + told) equals to 0.5 means the mouse has no preference for the novel object (i.e., memory impairment). The time interacting with objects in the familiarization can serve as a control of the experiment. The total time indicates the exploration activity of the mouse, and tleft/tright suggests spatial bias.
- Set up the novel object recognition test to include 2 days of habituation, 1 day of familiarization, and 1 day of testing (Figure 1D); each session is 10 min per mouse, and the intersession interval is 24 h.
- Morris water maze test16
- Set up the apparatus as follows.
- Put the water maze, a circular pool (of 120 cm in diameter and 60 cm deep), in the center of a behavioral room, and mark the position of the maze to ensure the position remains the same during the entire experiment.
- Divide the maze into four equal imaginary quadrants. Hang the visual cues (e.g., circle, square, triangle, and pentagon) in the center of each quadrant, 130 cm above the floor and 53 cm away from the wall of the maze.
NOTE: The maze and the cue must stay in the same position during the entire test, so the mouse can form accurate spatial memory. - Place a platform 25 cm away from the wall, in the center of the fourth quadrant, and mark the position. The platform for the mouse is 10 cm in diameter.
NOTE: The position and diameter of the platform determine the difficulty of the task. The nearer it is to the wall of the maze, or the bigger the platform, the easier the task. - Fill the water maze with water (with a temperature of 23 to 25 °C, colored into white and made opaque by milk powder/food whitening powder) until the water level is 1 cm higher than the platform. Bring the mice into the behavioral room for 15 to 30 min of habituation as shown in step 1.2.3 and turn on the infrared light above the cages, which will be used to dry the mice.
NOTE: Cover the top of the platform with white cloth and net so that the mouse can easily climb onto it. Make sure there is no direct lighting above the water.
- Conduct the training phase as follows.
- The training phase takes 5 days, four trials per day. Semi-randomly arrange the starting points on each day as demonstrated in literature16. This effort prevents the mouse from establishing associative memory, which is the most common way to “cheat” in the test.
- At the beginning of each trial, start the video recording and gently put the mouse into the maze.
NOTE: Do not drop the mouse into the tank or twist its tail, which may cause extra stress and disorientation. - Ask the experimenter to stay out of sight of the mouse and to return to take the mouse back to its home cage when any of the following happens: (i) the mouse cannot locate the platform within 60 s; (ii) the mouse finds the platform within 60 s and stays on it for 10 s. In circumstance (i), ask the experimenter to place the mouse on the platform and let it stay there for 10 s.
NOTE: Point (ii) means the mouse successfully located the platform. - Stop the video and put the mouse back in the home cage under infrared light.
NOTE: Maintaining the mice’s body temperature is critical for their performance because hypothermia stresses mice and may affect the following tests. - Repeat the procedure with another mouse.
- Based on the video, record the escape latency, which is the duration of the period the mouse spends in the maze, from entering the maze till the moment it successfully locates the platform. If the mouse cannot find the platform or stays there for less than 10 s in 60 s, the escape latency counts as 60 s. Plot the learning curve against the training days with the average escape latency per day.
NOTE: The escape latency does not include the 10 s spend on the platform.
- Perform the probe phase as follows.
- On the sixth day of the Morris water maze test, set up the apparatus as described in step 2.7.1., take a picture of the maze to record the position of the platform, and then remove the platform from the tank.
- Start the video recording, and gently put the mouse into the maze in the quadrant diagonally opposite to the target quadrant.
- Ask the experimenter to stay out of sight of the mouse during the 1 min video recording. Afterward, have the experimenter take the mouse out of the maze and put it back in the home cage.
- Use the image taken at step 2.7.3.1. as a reference to measure the duration of any platform crossing. Time the duration the mouse stays in the target quadrant (ttarget), according to the video. The total time is ttotal. Measure the preference to the target quadrant as ttarget /ttotal.
- Set up the apparatus as follows.
A Behavioral Test Battery for the Repeated Assessment of Motor Skills, Mood, and Cognition in Mice
Learning Objectives
This behavioral test battery was designed for the comprehensive and valid behavioral analysis of motor, mood, and cognition, which are commonly affected in neurodegeneration5. We have applied this battery to study the behavioral changes in young adult C57BL/6N mice after respiratory exposure to silica nanoparticles for 1 month and 2 months18. The results revealed that C57BL/6N mice exposed to silica nanoparticles showed various behavioral changes after different exposure times18. Briefly, results in the open-field test (Figure 2A) and the accelerating rotarod test (Figure 2B) demonstrated that silica nanoparticles exposure did not affect the locomotor or motor function in mice, indicating a full capability of accomplishing the other tests. Social interaction activity was affected after a 1 month exposure to silica nanoparticles (Figure 2C). Considering anxiety or depression would also decrease sociability, we analyzed data of the open-field test, elevated plus maze test (Figure 2D,E), and the forced swim test (Figure 2F), which did not indicate any comorbidity of anxiety nor depression at the 1 month time point. A 2 month exposure to silica nanoparticles resulted in anxiety according to the results in the elevated plus maze test (Figure 2E). A similar trend was shown in the central area duration in the open-field test (Figure 2D). Cognitive impairment was also detected in the Morris water maze test and novel object recognition test after a 2 month exposure (Figure 3). It should be noted that the protocol was slightly different in the two trials of the Morris water maze test. An additional lamp was added in the second trial, so all the mice always stayed under the lamp to keep warm. Hence, no nonperformer was shown in the second trial, whilst two out of eight mice became nonperformers in the probe test in the first trial.
We adjusted the protocol so that most of the tests in the battery can be repeatedly tested. The key is to maintain the motivation of the tests. Tests like social interaction test, novel object recognition test, and elevated plus maze test are motivated by novelty (i.e., a novel juvenile helper, novel objects, and a novel environment, respectively). By maintaining the novelty in the protocol, the young adult C57BL/6N mice showed a consistent performance when tested again after 1 month. According to our data, when introduced to two different helpers in the two trials, mice consistently showed a preference greater than ten-fold to the helper than to the empty chamber in the social interaction test (Figure 4A). In the novel object recognition test, the normal mice consistently preferred the novel object to the old object (Figure 4B). However, in the elevated plus maze test, when tested again in the same environment after 1 month, the exploration dropped by half (Figure 4C)10. Theoretically, young adult C57BL/6 mice can be tested repeatedly in these tests as long as the experimental condition, including the novelty and the status of the mice, remains the same. We have repeated these tests every month for up to three times in our lab. Noteworthy, the Morris water maze test cannot be tested repeatedly in the same group of young adult C57BL/6N mice as the experience significantly interferes with the performance when repeatedly tested. According to our data, the mice still remembered the platform even after 1 month, showing correct and long-term spatial memory. When changing the position of the platform, the experienced mice learned faster than naïve mice, as they had learned the rules and searching strategy from the prior training (Figure 4D).
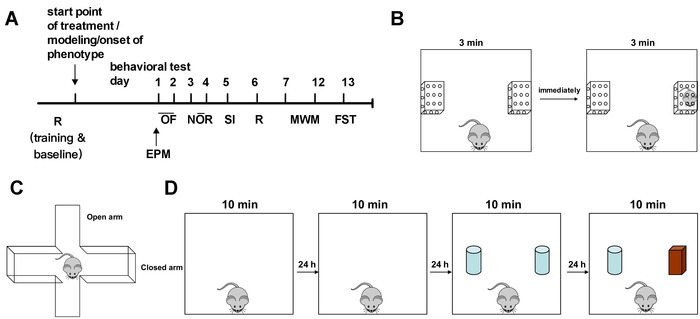
Figure 1: Schematics. (A) Arrangement of the behavioral test battery and the schematic plots of (B) the social interaction test, (C) the elevated plus maze test, and (D) the novel object recognition test. Abbreviations: R = accelerating rotarod test; OF = open-field test; EPM = elevated plus maze test; NOR = novel object recognition test; SI = social interaction test; MWM = Morris water maze test; FST = forced swimming test. The starting point of the mouse in the test is shown by the mouse in the scheme. Please click here to view a larger version of this figure.
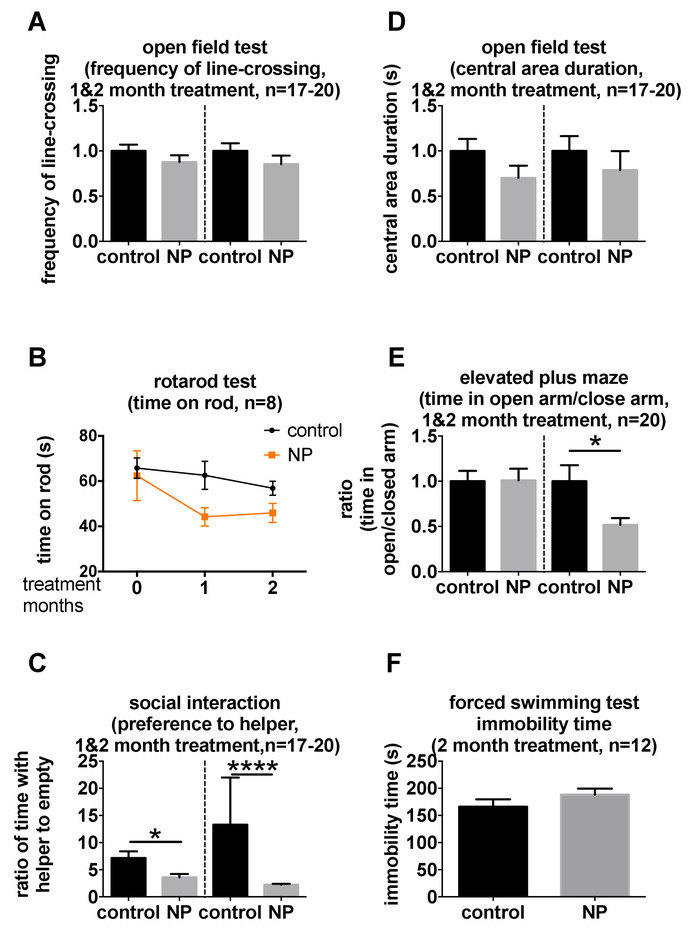
Figure 2: Changes in motor and mood in mice exposed to silica nanoparticles for 1 month or 2 months, detected by the behavioral test battery. Mice were tested in (A and D) the open field test, (B) the rotarod test, (C) the social interaction test, (E) the elevated plus maze test, and (F) the forced swimming test comprised in the battery. N = 8, 12, or 20, which means each group had 8, 12, or 20 mice, respectively, as demonstrated in each figure. N = 17 – 20 means each group had 20 mice, except for a control group at 1 month, which consisted of 17 mice. In panels A, D, and E, data were first normalized to control at each time point and, then, were analyzed with two-tailed Student's t-test. The data in panel B were analyzed by repeated measures two-way ANOVA. The data in panels C and F were analyzed with two-tailed Student's t-test. All data is shown as mean ±S.E.M. * and **** mean p < 0.05 and 0.0001, respectively. These data have been published previously by You et al.18. Please click here to view a larger version of this figure.
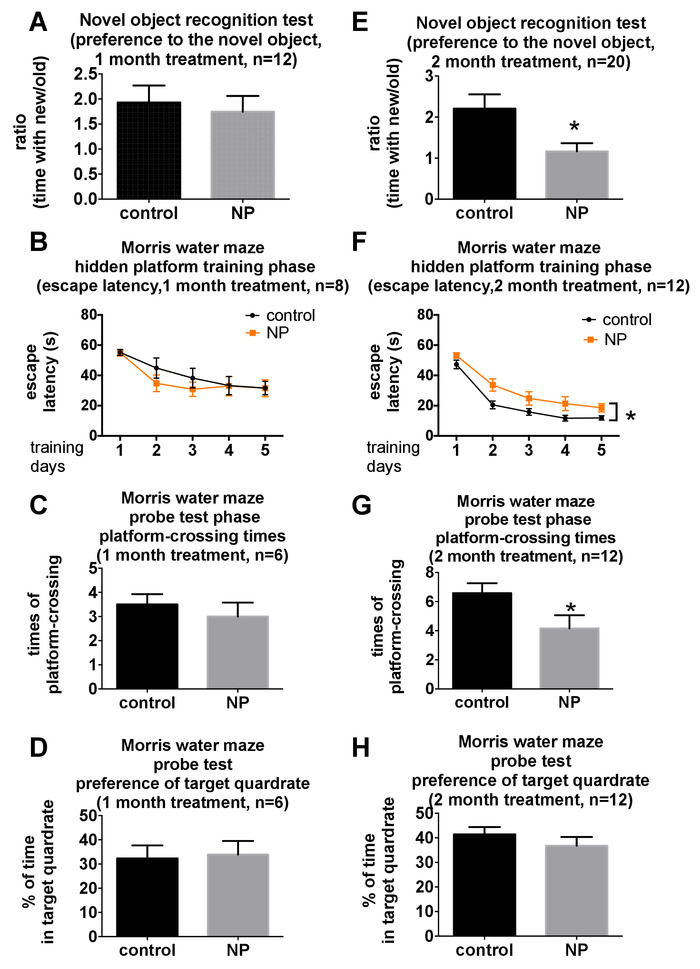
Figure 3: Changes in cognition after being exposed to silica nanoparticles for 1 month or 2 months. Changes in the mice's cognition after being exposed to silica nanoparticles for (A) 1 month or (E) 2 months, detected by the novel object recognition test. Changes in the mice's cognition after being exposed to silica nanoparticles for (B – D) 1 month or (F – H) 2 months, detected by the Morris water maze test. Mice were repeatedly tested in the novel object recognition test. A different batch of mice was tested in the Morris water maze test at different time points. N = 6, 8, 12, or 20, which means each group had 6, 8, 12, or 20 mice, respectively, as demonstrated in each figure. In panels A, C, D, E, G, and H, data were analyzed with two-tailed Student's t-test. The data in panels B and F were analyzed by repeated measures two-way ANOVA. All data are shown as mean ±S.E.M. * means p < 0.05. These data have been published previously by You et al.18. Please click here to view a larger version of this figure.
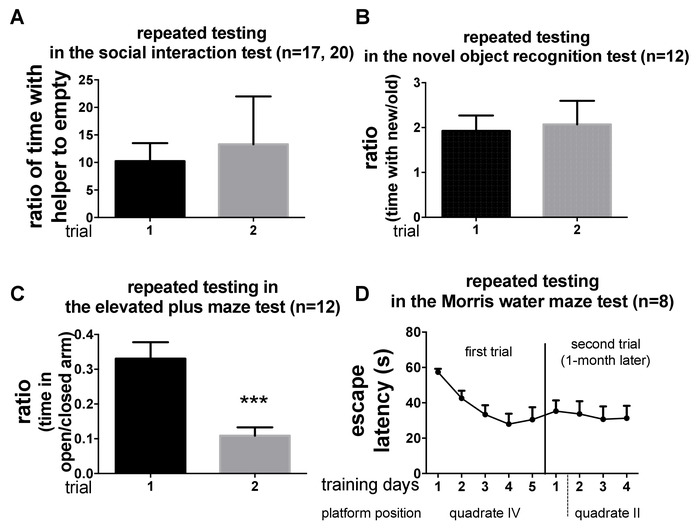
Figure 4: Representative data in tests. Representative data in tests, including (A) the social interaction test, (B) the novel object recognition test, and (C) the elevated plus maze test, tested in naïve mice (trial 1) and repeatedly tested in the same batch of mice (trial 2). (D) Representative data of the Morris water maze test when repeatedly tested. This figure has been modified from You et al.18. All data are shown as mean ±S.E.M. and analyzed with unpaired Student's t-test. P < 0.001, compared to trial 1. Please click here to view a larger version of this figure.
List of Materials
| chambers in social interaction test | home made | (8 cm (L) x 6 cm (W) x 12 cm (H)), transparant with holes, plastic | |
| cylindrical tanks used in forced swimming test | home made | 30 cm height, 20 cm diameters, glass | |
| elevated plus maze | home made | open arms (30 x 5 x 0.5 cm) ,closed arms (30 x 5 x 16 cm), center platform (5 x 5 x 0.5 cm), 40 cm tall. Plastic, nontransparant | |
| IITC Roto-Rod Apparatus | IITC life science Inc. | 755, series 8 | |
| open field arena | home made | 60 cm (L) x 60 cm (W) x 40 cm (H), plastic, nontransparant | |
| water maze | home made | 120 cm in diameter, 60 cm deep, steel |
Lab Prep
Pharmacological and toxicological studies in neurodegeneration require comprehensive behavioral analysis in mice because motor dysfunctions and dysfunctions in mood and cognition are common and often shared symptoms in neurodegenerative diseases. Shown here is a behavioral test battery for motor, mood, and cognition, which can be repeatedly tested in a longitudinal study. This battery assesses the overall behavioral phenotype in mice by examining each domain of behavior with at least two independent well-accepted tests (i.e., open-field test and rotarod test for motor function, social interaction test, elevated plus maze test, and forced swim test for emotional function, and Morris water maze test and novel object recognition test for cognitive function). Therefore, this sensitive and comprehensive test battery is a powerful tool for the study of behavioral alternation in neurodegeneration.
Pharmacological and toxicological studies in neurodegeneration require comprehensive behavioral analysis in mice because motor dysfunctions and dysfunctions in mood and cognition are common and often shared symptoms in neurodegenerative diseases. Shown here is a behavioral test battery for motor, mood, and cognition, which can be repeatedly tested in a longitudinal study. This battery assesses the overall behavioral phenotype in mice by examining each domain of behavior with at least two independent well-accepted tests (i.e., open-field test and rotarod test for motor function, social interaction test, elevated plus maze test, and forced swim test for emotional function, and Morris water maze test and novel object recognition test for cognitive function). Therefore, this sensitive and comprehensive test battery is a powerful tool for the study of behavioral alternation in neurodegeneration.
Procedure
Pharmacological and toxicological studies in neurodegeneration require comprehensive behavioral analysis in mice because motor dysfunctions and dysfunctions in mood and cognition are common and often shared symptoms in neurodegenerative diseases. Shown here is a behavioral test battery for motor, mood, and cognition, which can be repeatedly tested in a longitudinal study. This battery assesses the overall behavioral phenotype in mice by examining each domain of behavior with at least two independent well-accepted tests (i.e., open-field test and rotarod test for motor function, social interaction test, elevated plus maze test, and forced swim test for emotional function, and Morris water maze test and novel object recognition test for cognitive function). Therefore, this sensitive and comprehensive test battery is a powerful tool for the study of behavioral alternation in neurodegeneration.
